Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.426
Peer-review started: September 22, 2022
First decision: November 6, 2022
Revised: November 16, 2022
Accepted: December 15, 2022
Article in press: December 15, 2022
Published online: January 16, 2023
Processing time: 111 Days and 19.4 Hours
Hepatic perivascular epithelioid cell neoplasms (PEComas) are rare. Diagnostic and treatment experience with hepatic PEComa remains insufficient.
Three hepatic PEComa cases are reported in this paper: One case of primary malignant hepatic PEComa, one case of benign hepatic PEComa, and one case of hepatic PEComa with an ovarian mature cystic teratoma. During preoperative imaging and pathological assessment of intraoperative frozen samples, patients were diagnosed with hepatocellular carcinoma (HCC), while postoperative pathology and immunohistochemistry subsequently revealed hepatic PEComa. Patients with hepatic PEComa which is misdiagnosed as HCC often require a wider surgical resection. It is easy to mistake them for distant metastases of hepatic PEComa and misdiagnosed as HCC, especially when it's combined with tumors in other organs. Three patients eventually underwent partial hepatectomy. After 1-4 years of follow-up, none of the patients experienced recurrence or metastases.
A clear preoperative diagnosis of hepatic PEComa can reduce the scope of resection and prevent unnecessary injuries during surgery.
Core Tip: Herein, we present three cases of hepatic perivascular epithelioid cell neoplasms (PEComas): One case of primary malignant PEComa, one case of benign PEComa, and one case of PEComa with ovarian mature cystic teratoma. The first case of PEComa cooccurred with an ovarian mature cystic teratoma. All three cases were misdiagnosed as liver cancer before surgery. A high rate of misdiagnosis of hepatocellular carcinoma is noted among patients with PEComa. A clear preoperative diagnosis of hepatic PEComa is crucial before deciding on a treatment plan, especially with the extent of surgical treatment in hepatocellular carcinoma.
- Citation: Kou YQ, Yang YP, Ye WX, Yuan WN, Du SS, Nie B. Perivascular epithelioid cell tumors of the liver misdiagnosed as hepatocellular carcinoma: Three case reports. World J Clin Cases 2023; 11(2): 426-433
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/426.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.426
Perivascular epithelioid cell neoplasms (PEComas) are mesenchymal tumors with the histological and immunophenotypic characteristics of perivascular epithelioid cells. PEComas include multiple tumor types, including angiomyolipoma, lymphangioma, lymphangioleiomyomatosis, clear cell sugar tumors, and tumor types not otherwise specified[1]. Only a few cases of PEComa originating in the liver have been reported, and the majority of these cases are benign[2,3]. Malignant hepatic PEComa are extremely rare.
Hepatic PEComas are typically found during routine physicals. Most patients present with a painless mass, and only a handful of these patients experience epigastric pain from mass compression[4]. The diagnostic criteria for hepatic PEComa have not been unambiguously defined and validated to date[5,6]. Given the current lack of global diagnostic standards for imaging and laboratory sciences, it is easy to mistake primary hepatic PEComa for hepatocellular carcinoma (HCC). Nevertheless, necrosis, mitotic activity, pleomorphism, marked nuclear atypia, infiltrative growth, and large size suggest malignant biological behavior. Hematoxylin-eosin staining and positive immunohistochemical staining for human melanoma black 45 (HMB45) and Melan A are the primary diagnostic evidence for PEComas[1,7]. The clinical and therapeutic management of hepatic PEComa is controversial, but surgery appears to be the preferred treatment[8].
The following is a summary of our experience in diagnosing and treating three patients with hepatic PEComas. This study will help form clinical guidelines for hepatic PEComas.
Case 1: A 37-year-old man was admitted to the hospital with abdominal pain for 1 mo.
Case 2: A 70-year-old woman who was admitted due to an asymptomatic hepatic mass for more than half a month.
Case 3: A 30-year-old woman was admitted to hospital with abdominal bloating for 6 mo.
Case 1: A nodular mass was discovered during an assessment of abdominal pain in the right lobe of his liver at another hospital.
Case 2: The asymptomatic hepatic mass was found during a routine physical examination at another hospital a half a month ago.
Case 3: A liver tumor accompanied by an ovarian lesion was discovered during a routine physical examination 16 d ago.
Case 1: The patient had been suffering from nonalcoholic fatty liver disease for over ten years.
Case 2: The patient claimed no history of past illness.
Case 3: The patient claimed no history of past illness.
None of the three patients had any relevant personal or family history.
Physical examination of all three patients revealed no abnormalities.
Case 1: Laboratory studies of liver function were standard, viral hepatitis markers and tumor indicators, such as alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA-199), and carbohydrate antigen 125 (CA-125), were all negative.
Case 2: Routine blood analysis, liver function, hepatitis virus, and specific tumor markers (including AFP, CEA, CA-125, and CA-199) were all normal.
Case 3: The serum tumor markers (AFP, CEA, CA-199) and serology for hepatitis B and C, but not CA-125, were all normal. Tumors of ovarian origin may account for the elevated CA-125 levels.
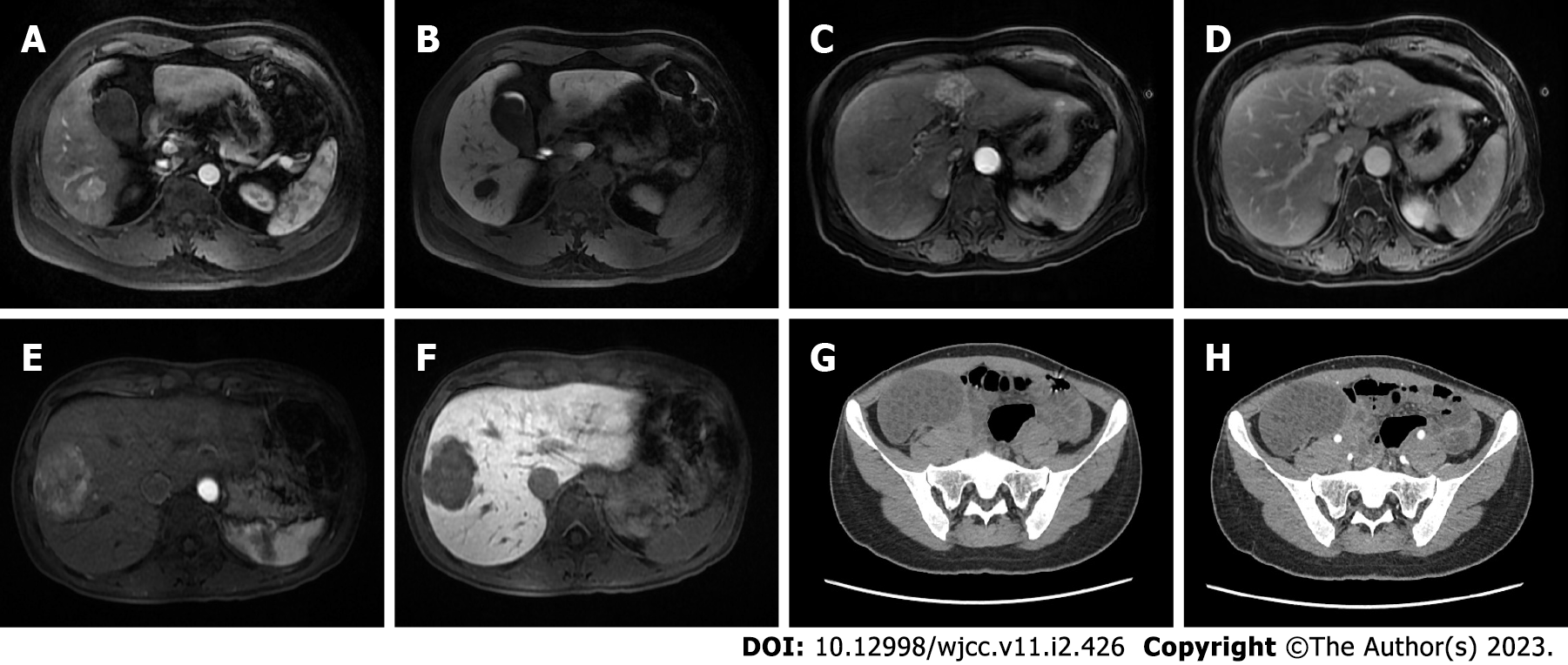
Case 1: Ultrasonography (US) revealed a hypoechoic, irregularly shaped nodule located in the right posterior lobe of the liver. Computed tomography (CT) with 3-phase enhancement of the upper abdomen was performed. The pre contrast CT scan exhibited a flake blur and a low-density shadow in S6 of the liver in the same location noted in the US. In contrast, the lesion is markedly enhanced in the arterial phase of contrast-enhanced CT scans. Gadolinium ethoxybenzyldiethylenetriaminepentaacetic acid-enhanced magnetic resonance imaging (MRI) is the liver-specific contrast enhancement agent currently used for the diagnosis of HCC. This assessment revealed a nodular abnormal signal shadow in S6 of the liver measuring 2.5 cm × 2.0 cm × 2.3 cm with smooth edges and a clear boundary. The lesion presented as hypointensity on T1-weighted images (T1WIs) and heterogeneous hyperintensity on T2-weighted images (T2WIs). The arterial phase revealed significantly enhanced lesions, whereas the portal venous phase exhibited a lower degree of enhancement compared with the liver parenchyma (Figure 1A and B). Based on the imaging findings, we suggested that the lesion was an HCC nodule.
Case 2: The woman presented with an ill-defined mass on US that was internally hypoechoic with a partial hyperechoic area. MRI exhibited a lesion with irregular edges and unclear boundaries that was approximately 5.0 cm × 3.0 cm × 3.1 cm in size located between the hepatic S3 and S4 segments. The lesion showed slight hypointensity on T1WI and heterogeneous hyperintensity on T2WI. In the arterial phase, the lesions displayed obvious heterogeneous enhancement. The enhancement of lesions was significantly weakened in the venous phase, and the neoplasm decayed to a low-signal state in the delayed phase (Figure 1C and D). Based on the imaging findings, the lesion appears to be an HCC nodule.
Case 3: US revealed a slightly heterogeneous hypoechoic nodule in the right anterior lobe of the liver. In a plain CT scan, the lesion (approximately 5.6 cm × 4.7 cm × 5.0 cm) exhibited an undefined mass with heterogeneous density in segment 8 of the liver. On arterial CT, the liver lesion was obviously heterogeneously enhanced. In the portal venous phase, the lesion rapidly returned to an isoattenuating state. Lesion enhancement in the portal and delayed phases decreased rapidly, and the strengthening method showed a rapid in and out pattern. MRI of the abdomen showed heterogeneous hypointensity on T1WI and hyperintensity on T2WI. In enhanced scanning, the lesion showed asymmetrical enhancement in the arterial phase images, and lesion enhancement was significantly weakened in the portal and delayed phase images (Figure 1E and F). Preoperative plain abdominal CT showed a right ovarian lesion measuring approximately 7.8 cm × 4.8 cm × 7.9 cm with multiple cystic low-density shadows scattered throughout. The boundaries of the lesion were clearly defined, and the lesion was not strengthened after undergoing contrast-enhanced computed tomography, which contained some fatty components (Figure 1G and H). According to the imaging findings, the liver lesion appears to be an HCC nodule, and the ovarian lesion is considered to be an ovarian tumor.
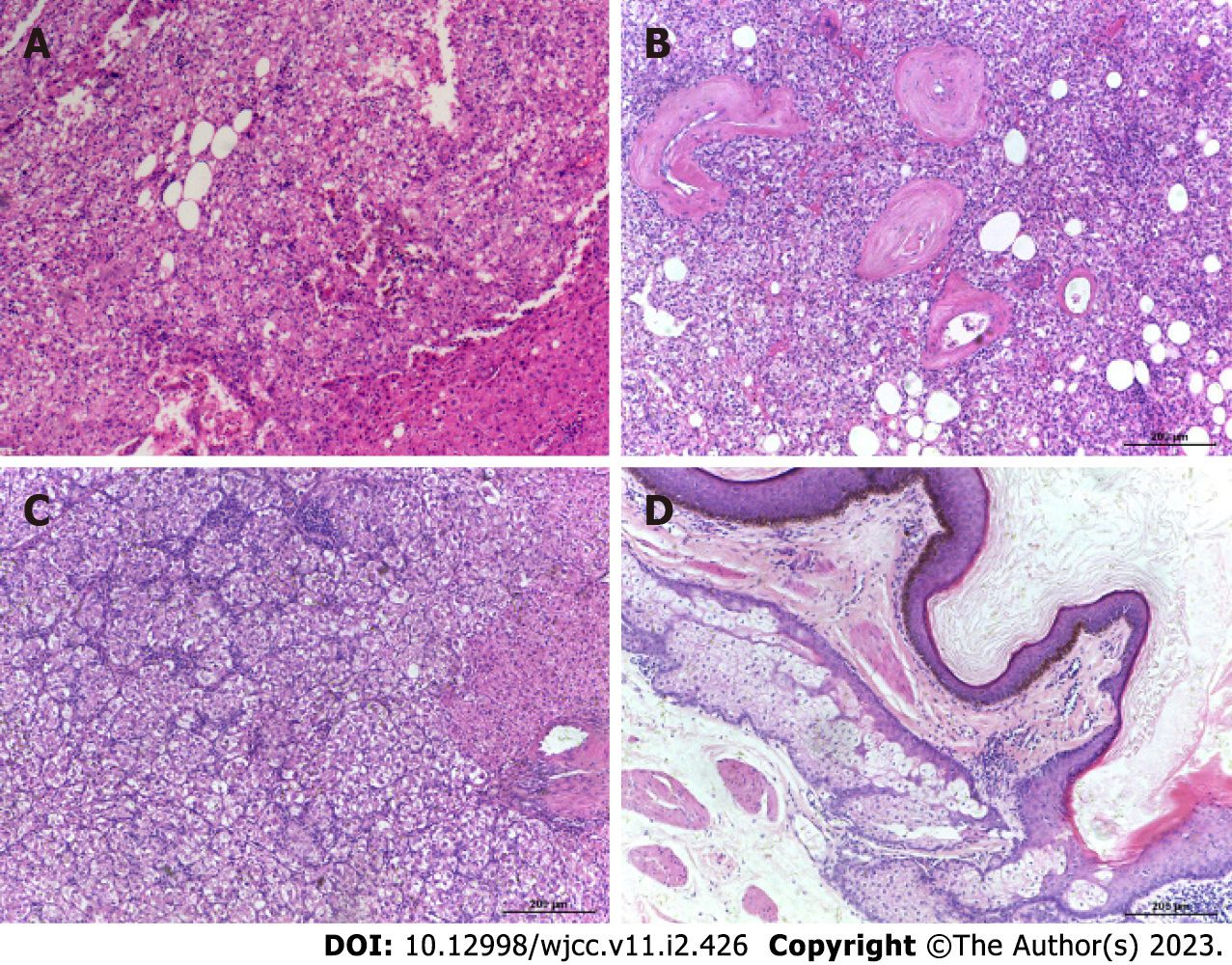
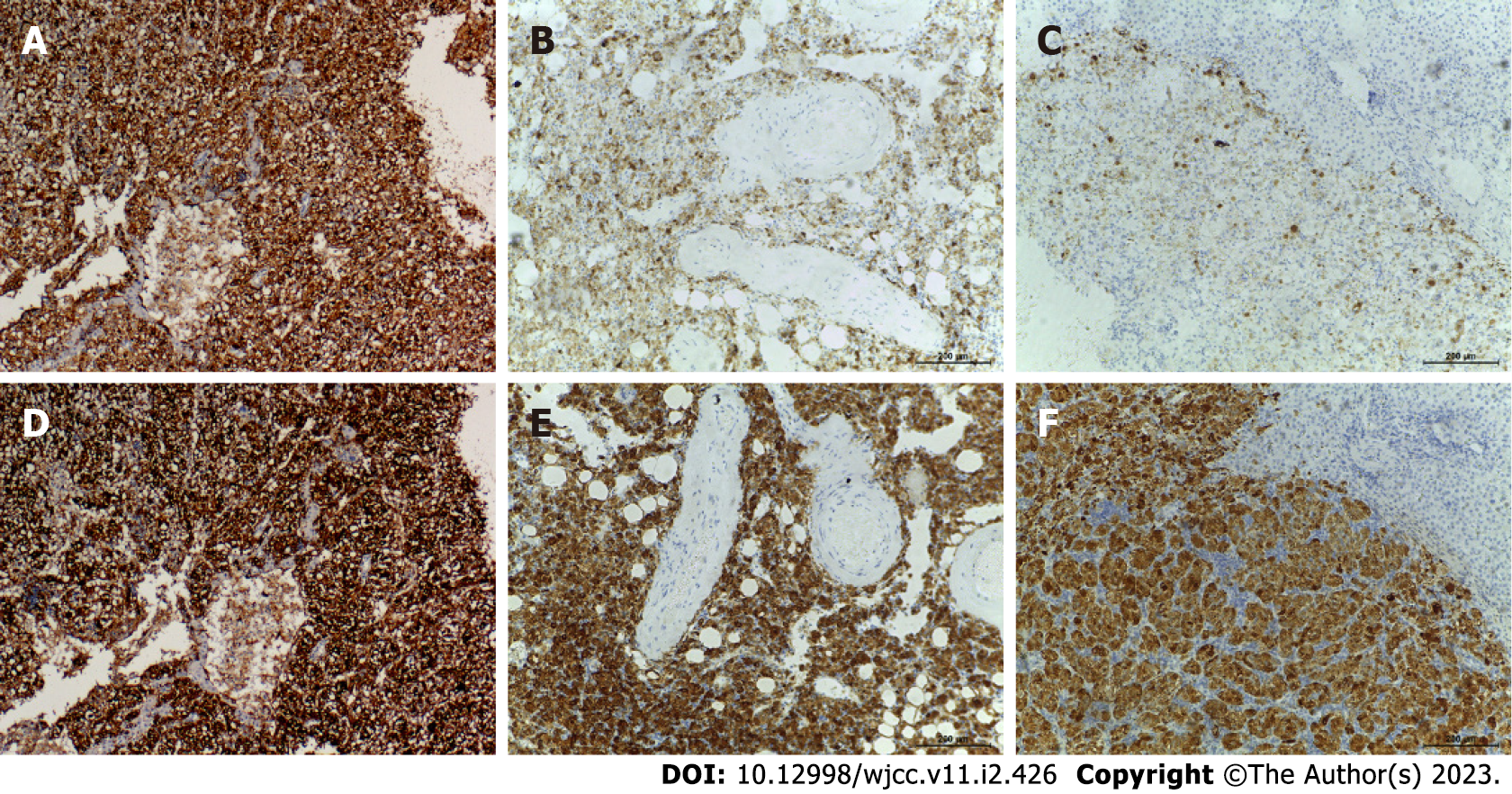
Case 1: Based on intraoperative frozen pathological consultation, HCC was considered to be the most likely diagnosis. Ultimately, the patient underwent partial hepatectomy. Histopathologic results suggest that the tumor tissue is arranged in nests and sheets with rich, slender vascular networks connecting them. Tumor cells are similar in size but irregular in shape. The nucleolus is conspicuous in tumor cells. In addition, the nuclei were irregularly shaped (Figure 2A). Immunohistochemical staining studies showed that some tumor markers were negative, such as hepatocytes, Glypican-3 (GPC-3), cluster of differentiation (CD) 10, and AFP, while others were positive, such as CD34, Melan A and HMB45 (Figure 3A and D). The Ki-67 Labeling index was 10%.
Case 2: Histopathological results revealed that the tumor tissue formed nests and sheets connected by slender, rich vascular networks. Tumor cells were polygonal or round in shape and uniform in size. The nucleolus is conspicuous in tumor cells, and their nucleus is shaped regularly (Figure 2B). The tumor was positive for HMB45, Melan A and CD34 markers with a Ki-67 Labeling index of 1% (Figure 3B and E).
Case 3: Frozen pathological consultation conducted intraoperatively indicated the presence of HCC. The woman underwent surgery as a result of her condition. Histologically, the tumor comprises nests and sheets of large, round to polygonal cells separated by a sinusoidal vascular network with hemorrhage areas. With distinct cell borders, the cells showed abundant cytoplasm, ranging from granular eosinophilia to clear cytoplasm. In tumor cells, the nucleolus is conspicuous, and its shape is regular. Mitoses was rare to absent (Figure 2C). Immunohistochemical analysis showed strong and diffuse expression of HMB45 and Melan-A, whereas markers for hepatocytes, GPC-3, and AFP were negative. The Ki-67 Labeling index was 5% (Figure 3C and F). Histopathologic examination of the ovarian lesion of the 30-year-old woman revealed that it was an ovarian mature cystic teratoma (Figure 2D).
Case 1: A malignant hepatic PEComa was eventually diagnosed based on morphological and immunohistochemical findings.
Case 2: Histopathology and immunohistology revealed that this tumor was a benign hepatic PEComa.
Case 3: Morphologic and immunohistochemical findings suggested the diagnosis of hepatic PEComa and an ovarian mature cystic teratoma was diagnosed by histopathology of the ovarian lesion.
Case 1: The patient underwent partial hepatectomy.
Case 2: Ultimately, she underwent partial hepatectomy.
Case 3: She underwent partial hepatectomy and ovarian teratoma resection.
Case 1: After partial hepatectomy, the patient recovered and was discharged after 1 wk of postoperative care. The patient did not receive any adjuvant neoadjuvant therapy and has remained alive for 12 mo without recurrence or metastases.
Case 2: For the past 35 mo, the patient has not experienced recurrence or metastasis.
Case 3: The patient recovered well and after 46 mo of follow-up without recurrence or metastases.
In these three patients, it was challenging to differentiate between HCC and liver PEComa. Most patients with PEComa of the liver do not exhibit characteristic clinical symptoms, and a small number of patients may suffer from abdominal pain or abdominal discomfort[9]. Hepatic PEComas do not exhibit specific clinical or imaging characteristics, and preoperative imaging and intraoperative frozen pathological examinations as well as uncertainty regarding the optimal surgical margin may lead to misdiagnosis as a primary or metastatic HCC prior to surgery. As a result, the scope of liver resection is further expanded; thus, the minimal level of liver resection required for the trabecular pattern is not achieved.
The differential diagnosis of tumors as hepatic PEComa requires further discussion. PEComas in the liver are typically composed of mature adipose tissues surrounded by thick or thin walls of blood vessels. Epithelioid tumor cells are arranged radially around the vessels, exhibit different degrees of differentiation and are difficult to diagnose histologically[10]. When considering HCC, the significant epithelioid morphology and the trabecular pattern of hepatic PEComa make it extremely easy to misdiagnose it as HCC, particularly based on intraoperative frozen pathological diagnosis. Hepatic PEComa was incorrectly identified as HCC based on frozen pathological analysis in the present cases. Due to morphological similarities, epithelioid tumors, such as HCC, can be easily confused with hepatic PEComa. Thus, it can be challenging to achieve a surgical frozen pathological diagnosis within a short period of time, leading to a high misdiagnosis rate[11,12].
Furthermore, the imaging characteristics of this tumor are related to the histological components; in particular, most tumors are completely devoid of adipose tissue, so fat attenuation is rarely observed on computed tomography and magnetic resonance images[13]. In contrast, the appearance of a hepatic PEComa on CT or MRI is well defined with early enhancement in the arterial phase and nonuniform enhancement in the venous and delayed phases[14]. Lesions with delayed washout may mimic HCC[15]. Consistent with the study results, hepatic PEComa should still be considered whenever a blotchy vascular pattern of the tumor is noted, if there is no evidence of hemorrhage in the tumor, if there is no abnormality in the background parenchyma, when no hepatitis virus markers have been detected, and when liver function tests and tumor markers (AFP, CEA) are normal[16]. Nevertheless, elevated AFP is present in a few cases[17].
Although patients completed US, tomography with three-phase enhancement, enhanced MRI tests and intraoperative frozen pathology, these results suggested HCC nodules until the postoperative pathology and immunohistochemistry confirmed the lesions as primary hepatic PEComas. Its postoperative pathological characteristics include a nest-like arrangement of tumor tissue and a thin vascular network that shuttles between cells. The observation of large clear cells or large cells with eosinophilic condensation around the nucleus alerts the pathologists to the possibility of a PEComa. Importantly, immunohistochemical staining studies confirmed that the lesion is negative for the hepatocyte marker and positive for the specific immunomarkers HMB-45 and Melan-A[18].
To diagnose hepatic PEComa, ultrasound imaging showed clear and specific imaging manifestations of the lesions in the patients. Contrast-enhanced ultrasound (CEUS) could be valuable in differentiating hepatic PEComa from HCC. Hepatic PEComa lesions exhibit a significantly delayed washout compared with other malignant tumors, such as HCC and metastatic liver cancer. Color Doppler flow imaging demonstrated that the larger blood vessels surrounding the lesion may also represent a potential feature of hepatic PEComa[19,20]. Using Sonazoid® contrast agent in CUES may assist in the diagnosis of hepatic PEComa[21]. A mono-typical epithelioid variant of typical hepatic PEComa has been described with some cases exhibiting a pure sinusoidal trabecular pattern that mimics the characteristics of HCC. A pathologist should be familiar with the cytomorphology of hepatic PEComa and its tendency to mimic HCC. If characteristic cell morphologic features or clinical background are not observed, immunostaining of fine needle aspiration cytology or core biopsy must be performed to prevent misdiagnosis[22].
PEComa of the liver is a rare disease with a high probability of misdiagnosis. CEUS may contribute to a more confirmative differential diagnosis of hepatic PEComa. Additionally, immunohistochemistry is currently the only clinical method available to confirm the diagnosis, and surgery is the primary treatment. A clear preoperative diagnosis of hepatic PEComa is essential to determine the extent of surgery that should be performed.
We thank all doctors and nurses for their care of patients, and we also thank the pathology and imaging doctors for their help.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Huang R, China; Yeh CY, Taiwan S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 636] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 2. | Abhirup B, Kaushal K, Sanket M, Ganesh N. Malignant hepatic perivascular epithelioid cell tumor (PEComa) - Case report and a brief review. J Egypt Natl Canc Inst. 2015;27:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Shi H, Bai Y, Guo A. Four cases of primary malignant perivascular epithelioid cell tumour of the liver. Pathology. 2013;45:614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Ma Y, Huang P, Gao H, Zhai W. Hepatic perivascular epithelioid cell tumor (PEComa): analyses of 13 cases and review of the literature. Int J Clin Exp Pathol. 2018;11:2759-2767. [PubMed] |
| 5. | Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 671] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 6. | Anderson WJ, Doyle LA. Updates from the 2020 World Health Organization Classification of Soft Tissue and Bone Tumours. Histopathology. 2021;78:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 7. | Argani P, Aulmann S, Illei PB, Netto GJ, Ro J, Cho HY, Dogan S, Ladanyi M, Martignoni G, Goldblum JR, Weiss SW. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Jung DH, Hwang S, Hong SM, Kim KH, Ahn CS, Moon DB, Alshahrani AA, Lee SG. Clinico-pathological correlation of hepatic angiomyolipoma: a series of 23 resection cases. ANZ J Surg. 2018;88:E60-E65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Klompenhouwer AJ, Verver D, Janki S, Bramer WM, Doukas M, Dwarkasing RS, de Man RA, IJzermans JNM. Management of hepatic angiomyolipoma: A systematic review. Liver Int. 2017;37:1272-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Yang X, Wang Q, Zhou X, Zhou H, Jia W, Hu C, Chu J, Kong L. Retrospective analysis of hepatic perivascular epithelioid cell tumour (PEComa) in a single centre for clinical diagnosis and treatment clinical diagnosis and treatment of hepatic PEComa. Medicine (Baltimore). 2022;101:e29506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Tipirneni KE, Warram JM, Moore LS, Prince AC, de Boer E, Jani AH, Wapnir IL, Liao JC, Bouvet M, Behnke NK, Hawn MT, Poultsides GA, Vahrmeijer AL, Carroll WR, Zinn KR, Rosenthal E. Oncologic Procedures Amenable to Fluorescence-guided Surgery. Ann Surg. 2017;266:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 13. | Ji JS, Lu CY, Wang ZF, Xu M, Song JJ. Epithelioid angiomyolipoma of the liver: CT and MRI features. Abdom Imaging. 2013;38:309-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | O'Malley ME, Chawla TP, Lavelle LP, Cleary S, Fischer S. Primary perivascular epithelioid cell tumors of the liver: CT/MRI findings and clinical outcomes. Abdom Radiol (NY). 2017;42:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Choi HH, Manning MA, Mehrotra AK, Wagner S, Jha RC. Primary Hepatic Neoplasms of Vascular Origin: Key Imaging Features and Differential Diagnoses With Radiology-Pathology Correlation. AJR Am J Roentgenol. 2017;209:W350-W359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Maebayashi T, Abe K, Aizawa T, Sakaguchi M, Ishibashi N, Abe O, Takayama T, Nakayama H, Matsuoka S, Nirei K, Nakamura H, Ogawa M, Sugitani M. Improving recognition of hepatic perivascular epithelioid cell tumor: Case report and literature review. World J Gastroenterol. 2015;21:5432-5441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Yang G, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Hong SM. Clinicopathological correlation and post-resection outcomes of hepatic angiomyolipoma. Ann Hepatobiliary Pancreat Surg. 2021;25:215-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Nie P, Wu J, Wang H, Zhou R, Sun L, Chen J, Yang G. Primary hepatic perivascular epithelioid cell tumors: imaging findings with histopathological correlation. Cancer Imaging. 2019;19:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Gao X, Tang H, Wang J, Yao Q, Wang H, Wang Y, Ma M, Yang W, Yan K, Wu W. Specific imaging features indicate the clinical features of patients with hepatic perivascular epithelioid cell tumor by comparative analysis of CT and ultrasound imaging. Front Oncol. 2022;12:908189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Huang Z, Wu X, Li S, Li K. Contrast-Enhanced Ultrasound Findings and Differential Diagnosis of Hepatic Epithelioid Angiomyolipoma Compared with Hepatocellular Carcinoma. Ultrasound Med Biol. 2020;46:1403-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Huang Z, Xin JY, Li KY. Ultrasound contrast agent Sonazoid for the diagnosis of hepatic epithelioid angiomyolipoma: a case report. BMC Gastroenterol. 2021;21:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Xie L, Jessurun J, Manivel JC, Pambuccian SE. Hepatic epithelioid angiomyolipoma with trabecular growth pattern: a mimic of hepatocellular carcinoma on fine needle aspiration cytology. Diagn Cytopathol. 2012;40:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |