Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.417
Peer-review started: October 7, 2022
First decision: November 4, 2022
Revised: November 16, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 16, 2023
Processing time: 96 Days and 21.9 Hours
Angiomyolipoma (AML), the most common benign tumor of the kidney, is usually composed of dysmorphic blood vessels, smooth muscle, and mature adipose tissue. To our knowledge, AML with cystic degeneration has rarely been documented. Cystic degeneration, hemorrhage, and a lack of fat bring great challenges to the diagnosis.
A 60-year-old man with hypertension presented with a 5-year history of cystic mass in his left kidney. He fell 2 mo ago. A preoperative computed tomography (CT) scan showed a mixed-density cystic lesion without macroscopic fat density, the size of which had increased compared with before, probably due to hemorrhage caused by a trauma. Radical nephrectomy was performed. Histopathological studies revealed that the lesion mainly consisted of tortuous, ectatic, and thick-walled blood vessels, mature adipose tissue, and smooth muscle-like spindle cells arranged around the abnormal blood vessels. The tumor cells exhibited positivity for human melanoma black-45, Melan-A, smooth muscle actin, calponin, S-100, and neuron-specific enolase, rather than estrogen receptor, progesterone receptor, CD68, and cytokeratin. The Ki-67 labeling index was less than 5%. The final diagnosis was a fat-poor renal AML (RAML) with prominent cystic degeneration.
When confronting a large renal cystic mass, RAML should be included in the differential diagnosis.
Core Tip: Angiomyolipoma (AML) is a clinically common benign kidney tumor. The majority of classic AMLs can be diagnosed preoperatively through radiological technology because of the appearance of an adipose component. We report a rare case of a fat-poor renal AML (RAML) with prominent cystic degeneration. The establishment of RAML diagnosis is challenging because of the lack of specificity of imaging features. Histopathological and immunohistochemical examinations show that the three classic components express AML markers, supporting the final diagnosis.
- Citation: Lu SQ, Lv W, Liu YJ, Deng H. Fat-poor renal angiomyolipoma with prominent cystic degeneration: A case report and review of the literature. World J Clin Cases 2023; 11(2): 417-425
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/417.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.417
Angiomyolipoma (AML), also called hamartoma, is the most common benign kidney tumor. Fischer first described this tumor in 1911 with distinctive pathologic features, including dysmorphic blood vessels, smooth muscle, and mature adipose tissue[1]. The histogenesis of AML is still a matter of debate because hard evidence is lacking. Perivascular epithelioid cells (PECs) are traditionally considered as the principal cellular source of AML. Thus, it is also considered as a part of the PEComa family. AML can occur sporadically or in association with the tuberous sclerosis complex or, more rarely, sporadic lymphangioleiomyomatosis[2]. On the pathological basis of the elastin-poor, tortuousand ectatic vascular structures that readily tend to rupture, the most dangerous complication of renal AML (RAML) is hemorrhage (Wunderlich’s syndrome[3]), occurring spontaneously or induced by trauma. The majority of classic AMLs can be diagnosed preoperatively by using radiological technology because of the appearance of an adipose component. However, when adipose tissue is absent or hemorrhage occurs, the diagnosis may become challenging. We present a case of RAML with prominent cystic degeneration and little fat tissue, which has rarely been documented. The histopathological and immunohistochemical results support a hypothesis about the pathogenesis of this neoplasm.
A 60-year-old Chinese man presented to the Department of Urology with a 5-year history of a cystic mass in the left kidney, the size of which had increased after his fall 2 mo ago.
The patient who was annually receiving medical examinations given by his employer was told in 2017 that he had a cystic mass in the left kidney. This lesion occurred as a painless mass, approximately 3 cm in the greatest diameter. He denied any obvious clinical symptoms except occasional mild distending feelings and soreness in the left loin. He fell 2 mo ago. A computed tomography (CT) scan of the abdomen at a local hospital showed a growing mass (measuring 6 cm in diameter) in the kidney.
The patient had suffered from hypertension for more than 10 years. He denied any typical symptoms of tuberous sclerosis such as facial sebaceous adenoma, epilepsy, or intellectual disability. There was no clinical imaging showing sporadic lymphangioleiomyomatosis like pneumothorax, chylous pleural effusions, or cystic lung disease. He denied any eye symptoms, heart disease, pulmonary abnormalities, or bone disease.
The patient denied any family history of renal diseases, including renal masses, renal cell carcinoma (RCC), AML, and tuberous sclerosis.
On physical examination, the vital signs were as follows: Body temperature, 36.0 °C; blood pressure, 137/87 mmHg; heart rate, 73 beats per min; respiratory rate, 20 breaths per min. The physical examination revealed no abnormalities.
Laboratory tests after admission were as follows: White blood cell count (3.36 × 109/L; normal range: 4.0-10.0), red blood cell count (3.41 × 1012/L; normal range: 3.50-5.50), and platelet count (188 × 109/L; normal range: 90-300). The results of biochemistry tests were: Alanine aminotransferase (26 U/L; normal range: 0-40), aspartate aminotransferase (68 U/L; normal range: 0-40), total albumin (82.7 g/L; normal range: 64-82), glucose (6.24 mmol/L; normal range: 3.9-6.1), triglyceride (2.39 mmol/L; normal range: 0.4-1.8), and potassium (3.27 mmol/L; normal range: 3.5-5.1).
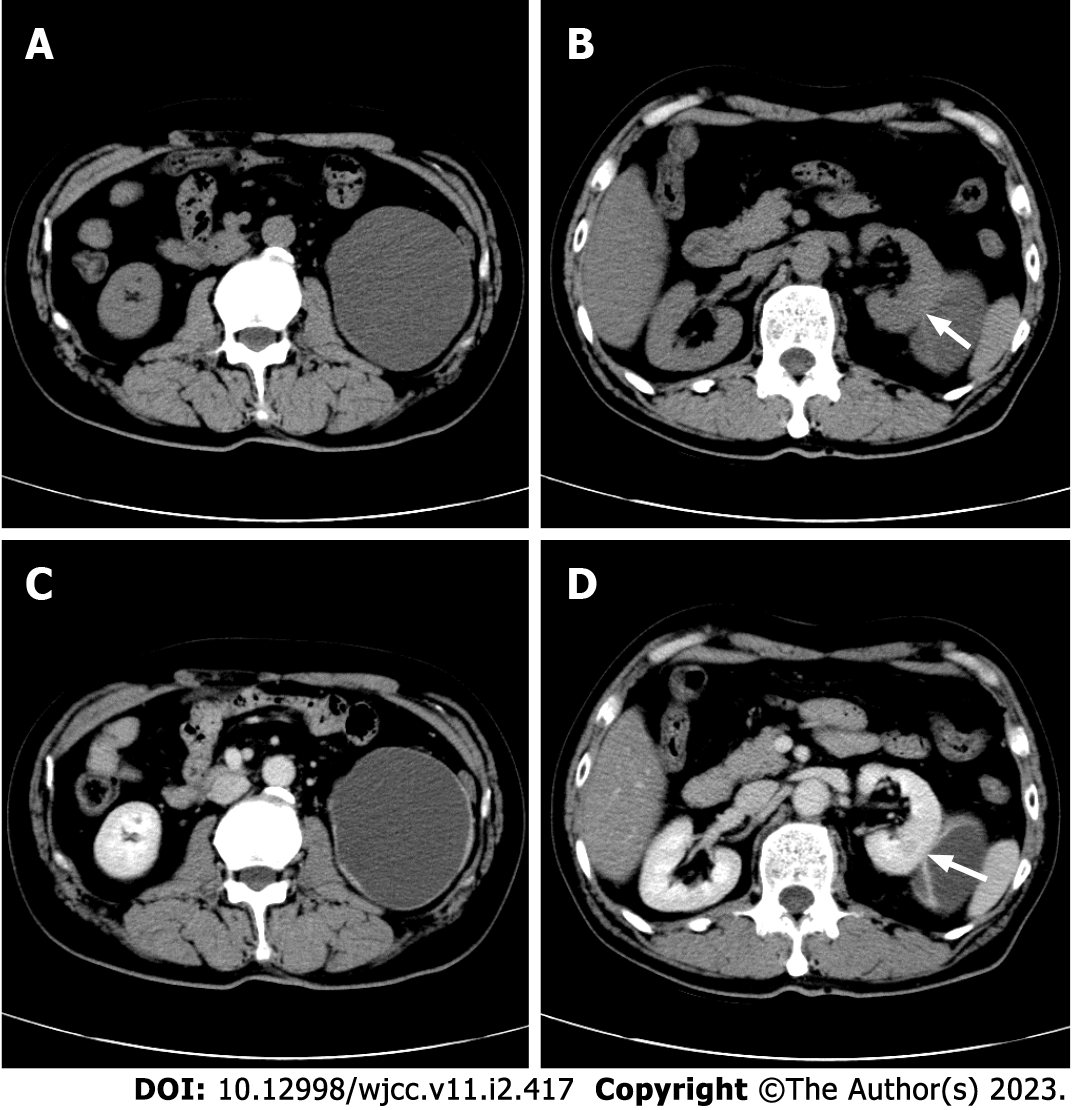
A CT scan at our hospital discovered an 8.6 cm × 7.4 cm, oval to round, mixed hypodense and isodense, cystic exophytic lesion with mainly liquid density in the middle-lower pole of the left kidney (Figure 1A). The left kidney was compressed and shifted upward. The lesion was well demarcated, encircled by an asymmetrical, irregular wall thickened in the areas adjacent to the renal parenchyma. Small mural nodules, a smooth linear septum, and an inconspicuous patch inside the lesion could be seen (Figure 1B). These features were slightly enhanced in the cortical phase and gradually washed out in the late phase (Figures 1C and D). The central liquid area remained unenhanced. There was no macroscopic fat density. The CT attenuation value varied from -8 Hounsfield units (HU) to 32 HU. Regional lymph node metastasis and intravascular extension were not observed.
Fat-poor RAML with prominent cystic degeneration.
A laparoscopic unroofing operation for the renal cyst was initially performed. The wall of the cystic mass was broken during the operation, producing dull red liquid. The mass was clearly demarcated from the normal tissues with a capsule. A disordered form, visible errhysis, and coagula were found at the bottom of the mass, around which there was hemosiderosis. With concern for misdiagnosis of malignancy and bleeding risk with percutaneous biopsy, intraoperative frozen examination was performed. This was unable to rule out cystic RCC.
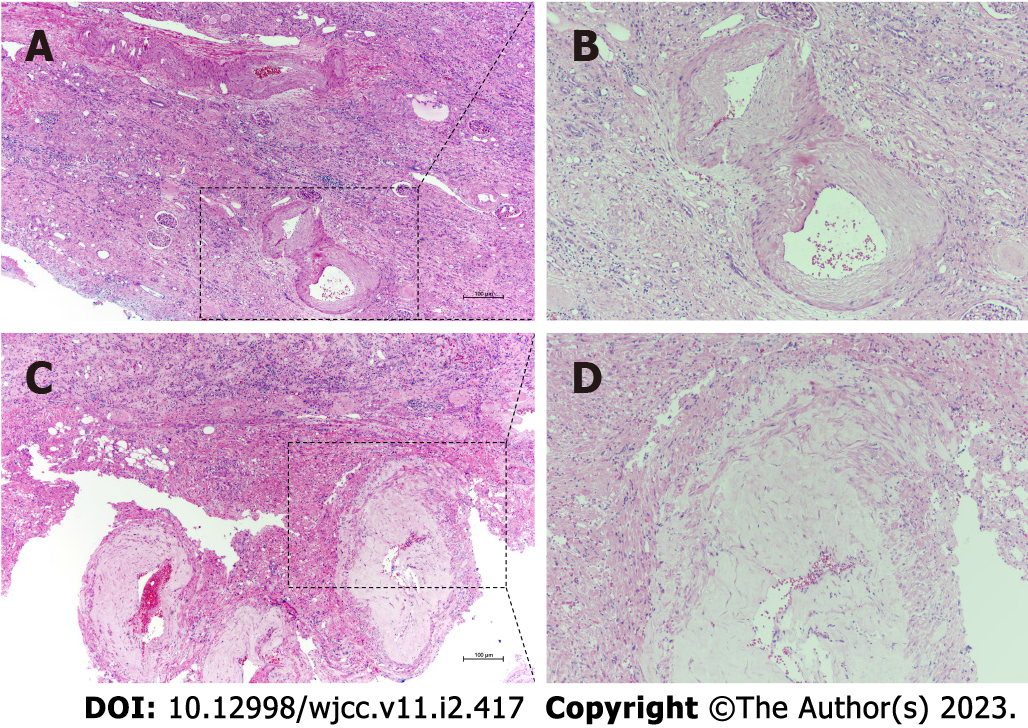
Finally, the patient underwent radical nephrectomy. The excised mass was fixed in formalin and embedded in paraffin. Hematoxylin and eosin staining and immunohistochemistry were performed to establish a definitive diagnosis. Pathologically, the remnant of the cystic lesion mainly consisted of tortuous, ectatic, and partly hyalinized blood vessels and mature adipose tissue, which were organized sporadically in a sheet-like pattern among the abnormal blood vessels (Figure 2A). The smooth muscle-like spindle cells, whose nuclei differed in size, were arranged randomly as short fascicles with a focal radial configuration. Hemorrhage, slight inflammatory cell infiltration, and inconspicuous necrotic foci were observed. Epithelioid cells were absent (Figure 2B).
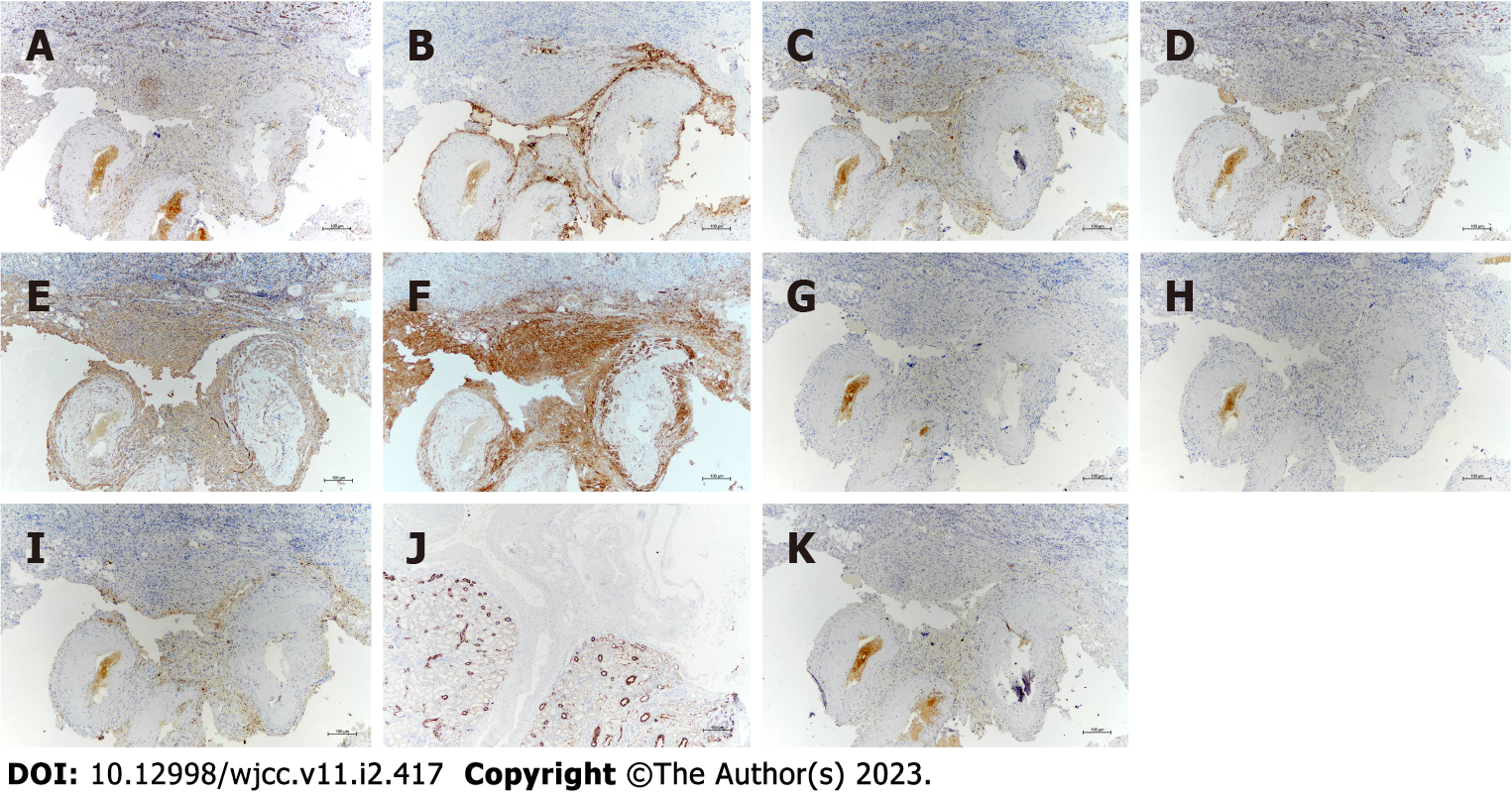
Immunostaining showed that, in addition to neuron-specific enolase (Figure 3A), the tumor cells exhibited positivity for melanosome-associated proteins, including human melanoma black-45 (HMB-45) (Figure 3B) and Melan-A (Figure 3C), S-100 (Figure 3D), and smooth muscle proteins, including smooth muscle actin (SMA) (Figure 3E) and calponin (Figure 3F), which were strongly stained in the spindle cells. In contrast, staining for estrogen receptor (Figure 3G), progesterone receptor (Figure 3H), CD68 (Figure 3I), and cytokeratin (CK) (Figure 3J) was negative. The Ki-67 labeling index (Figure 3K) was less than 5%.
The patient underwent radical nephrectomy. No complications were noted during or after surgery. Electrocardiogram and chest X-ray examination were performed half a year after operation, and no obvious abnormality was found.
AML is a well-known tumor that is composed of dysmorphic blood vessels, smooth muscle, and mature adipose tissue in variable proportions. Immunohistochemically, positivity for HMB-45 and Melan-A, two melanocytic markers, is often observed in spindle tumor cells. Smooth muscle markers such as SMA are also positive. Meanwhile, the mass does not express CKs and other epithelial markers[2]. Imaging technology plays an important role in the AML diagnosis, with the significant identification of the macroscopic fat component, which appears as a homogeneous hyperechoic mass on B-mode ultrasound and results in the loss of signal intensity on fat suppression imaging. CT has excellent sensitivity, specificity, positive predictive value (PPV), and negative predictive value in identifying AML[4].
Histologically, classic RAML usually presents as a well-delineated, isodense and hypodense mixed renal cortical mass, containing various proportions of visible fat with attenuation < -10 HU on unenhanced CT (UECT) images. Thin-slice multidetector row CT[5] and double-echo gradient-echo chemical shift magnetic resonance imaging (MRI)[6] can be used to differentiate AML with minimal fat from other renal neoplasms. However, the imaging diagnosis of AML will become indeterminate in two situations. First, the fat component of “minimal-fat” or “fat-poor” AML (defined as fat cells < 25% per high-power field[7-9]) is invisible. Second, hemorrhage, necrosis, or cystic degeneration may obscure the underlying fat[10].
To our knowledge, AML with cystic degeneration has rarely been documented and only accounts for less than 1% of RAMLs. In addition, most cystic AMLs consist of multiple small cysts with or without grossly depicted large cysts[11]. In this case, we reported a patient with a cyst-prominent RAML containing little fat tissue and only one septum on UECT. Based on the clinical and laboratory data, we propose a possible pathophysiologic mechanism: Trauma from the patient’s fall ruined the fragile vascular wall of the tumor and resulted in rupture. As hemorrhage occurred, the size of the tumor slowly increased. The patient did not suffer any serious clinical symptoms, perhaps owing to the cystic capsule, composed of tumor tissues and adjacent fibrous texture, which prevented the exudate from approaching the peritoneum. The central area of the tumor grew faster and was correspondingly lacking blood supply, thus resulting in local necrosis and liquefaction. Additionally, the poor blood supply was exaggerated with abnormal blood vessels, resulting in the enlargement of the necrotic region. Meanwhile, a part of the nephric tubules was likely physically compressed by the tumor tissues, leading to hydronephrosis and an increase in tumor size. The apparent synergy between bleeding, necrosis, liquefaction, and potential hydronephrosis supports the formation of an internal and fluid-filled prominent cystic mass with few residual lesions in the periphery. Over time, the degradation of blood and liquefied necrotic tumor produced the hypodensity on CT scan.
The absence of fat density, cystic appearance, and heterogeneous enhancement on contrast enhanced CT (CECT) raise a broad differential diagnosis, including cystic clear cell RCC, multilocular cystic RCC (MCRCC), papillary RCC (PRCC), oncocytomas, cystic nephroma (CN) or mixed epithelial and stromal tumors (MEST), and complex renal cysts. On ultrasound, the presence of an anechoic rim or intratumoral cysts suggests RCC, and shadowing suggests AML[12,13]. Doppler ultrasound also improves the ability to diagnose AML[14]. Cystic lesions of RCC generally display an irregular wall and are thicker than those of common cystic diseases. Compared with cystic RAML, the enhancement of mural nodules, septa, and solid composition in the cyst cavity of RCC is more obvious. Moreover, calcification is commonly found in RCC, but not in AML[15,16].
Homogeneous attenuation on UECT and enhancement on CECT images indicate that AML contains abundant muscle and minimal fat[7]. The early dark cortical band sign can be observed in up to 60% of clear cell RCC cases, facilitating the differential diagnosis from fat-poor AML with high specificity and PPV[17]. Some studies demonstrate that the combination of quantitative data obtained by specific region of interest in corticomedullary phase[18], convention-radiomics CT nomogram[19,20], and circularity index on CECT[21] help distinguish fat-poor AML from clear cell RCC. Magnetic resonance parameters may be of value in evaluating RCCs[5,22]. The immunoprofile of clear cell RCC is identical to other epithelial tumors which exhibit strong cytoplasmic expression of CK and epithelial membrane antigen[23,24].
Historically, MCRCC is considered to be a subtype of RCC[25]. The 2004 World Health Organization classification of kidney tumors categorized MCRCC as a separate entity with a good prognosis[26]. The diagnostic criteria for MCRCC include a grossly multilocular cystic appearance, a yellowish solid component limited to small areas with no expansive nodules and no tumor necrosis, and a microscopically low grade[27]. Hemorrhage, necrosis, and cystic degeneration are also common in PRCC and oncocytomas[26,28]. PRCC has variable proportions of papillae and may be bilateral or multifocal[26]. On imaging, PRCC is distinguished by the low level of enhancement and shows progressive enhancement when evaluated in the arterial (50-60 HU) and venous phases (65-75 HU)[24,29]. Meanwhile, PRCC is hypointense on T2-weighted images[5,24]. Oncocytomas display a central stellate scar that is hypodense on CT. The intense enhancement peaks in the nephrographic phase and rapidly washes out[30,31]. Sharing a similar presentation with MCRCC on imaging, CN is a benign neoplasm belonging to the family of MEST of the kidney, which usually shows multilocular, thick-walled cystic lesions with numerous thick, smooth, and contrast-enhanced septations[29]. MEST normally appears as well-margined, multifocal cystic masses with septa and nodular components on CT. Spindle cells resembling ovarian stroma as well as the epithelium lining the cystic structures are typical components of MEST[32,33]. Complex renal cysts are believed to undergo rupture, hemorrhage, or an acute infection. The features of MEST on CT include high attenuation values, the presence of thick or calcified walls, and septations with or without nodules[29].
After the diagnosis of RAML, treatments aimed at preserving renal function, relieving clinical symptoms, and reducing bleeding risk should be carried out. Active monitoring is often proposed as the preferred strategy for asymptomatic masses smaller than 4 cm in diameter[34]. Direct clinical interventions are employed for patients with RAML as follows: Those with clinical symptoms, the largest diameter is greater than 4 cm, those suspected of having malignant transformation, and women of childbearing age[34-36]. Emergency patients or cases with aneurysms larger than 5 cm, tuberous sclerosis complex (TSC)-associated AML, and who cannot insist on follow-up should also be included[35,37]. The tumor volume of sporadic AML and TSC-associated AML both increases with time, while the sporadic type is usually asymptomatic and relatively slow in growth[38]. Therefore, the imaging follow-up interval for RAML should be determined according to the clinical situation of the patient.
Transcatheter arterial embolization (TAE), which is capable of shrinking tumor, hemostasis, and protecting normal renal tissue, can be performed safely without permanent impairment[39,40]. TAE is recommended as a first-line choice for bleeding AML[41]. Surgical resection is still the most effective treatment for AML with operation indications, including suspicion of malignancy, symptoms, and a high risk of hemorrhage. Compared with nephrectomy, partial nephrectomy (PN) can better preserve renal function and reduce mortality. Currently, the treatment of RCC is more likely to preserve nephron, which is also applicable to the treatment of AML[42]. PN, whether open surgical, laparoscopic, or robotic assisted, has become a common surgical procedure[39]. Dong et al[43] reported an off-clamp retroperitoneoscopic tumor evacuation, which is feasible, safe, and effective for treating complex sporadic RAMLs.
Mammilian target of rapamycin (mTOR) inhibitors, such as sirolimus or everolimus, a new targeted drug, can be used to treat patients with TSC and sporadic AML. These medications result in tumor shrinkage via inhibition of the mTOR pathway and subsequent tumor cell proliferation. Low-dose everolimus maintenance therapy represents an effective and tolerated approach to achieve TSC-associated AML control[44-46].
We present a rare case of AML with cystic degeneration as the main imaging clue, which easily raises a complex differential diagnosis. The clinical data and histopathological results further support a new possible subtype for RAML and explicate the pathogenesis. However, more cases and insights into underlying molecular mechanisms are required to confirm this conclusion.
In general, imaging is able to diagnose AML given its typical appearance. It is advisable to combine imaging performance on ultrasonography, CT, and MRI when diagnosing AML. In this case, we describe an atypical presentation of AML. When faced with a large cystic mass of the kidney, diagnosis is more complicated with a broad differential beyond AML. As imaging features in this context lack specificity, an accurate diagnosis relies on pathological examination. Various proportions of the three classic components detected microscopically along with immunohistochemical staining can provide a confident diagnosis of AML. Considering the risk of hemorrhage, early diagnosis and suitable treatments are very important. In clinical work, routine pathological examination should be considered. Furthermore, percutaneous biopsy can be an option to avoid potentially unnecessary surgery[24].
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Limaiem F, Tunisia; Moreno-Gómez-Toledano R, Spain; Swanson KJ, United States S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Fischer W. Die nierentumoren bei der tuberosen hirnsklerose. Zeigl Beitr Pathol Anat Allg Pathol. 1911;50:235. |
| 2. | Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, Perry KT, Nadler RB. Update on the Diagnosis and Management of Renal Angiomyolipoma. J Urol. 2016;195:834-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Chesa Ponce N, Artiles Hernández JL, Ponce Socorro JM, del Rosario Medina J, Castro López-Torrella V, Betancort de León R. [Wunderlich's syndrome as the first manifestation of a renal angiomyolipoma]. Arch Esp Urol. 1995;48:305-308. [PubMed] |
| 4. | Davenport MS, Neville AM, Ellis JH, Cohan RH, Chaudhry HS, Leder RA. Diagnosis of renal angiomyolipoma with hounsfield unit thresholds: effect of size of region of interest and nephrographic phase imaging. Radiology. 2011;260:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Thiravit S, Teerasamit W, Thiravit P. The different faces of renal angiomyolipomas on radiologic imaging: a pictorial review. Br J Radiol. 2018;91:20170533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Kim JK, Kim SH, Jang YJ, Ahn H, Kim CS, Park H, Lee JW, Kim S, Cho KS. Renal angiomyolipoma with minimal fat: differentiation from other neoplasms at double-echo chemical shift FLASH MR imaging. Radiology. 2006;239:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Jinzaki M, Tanimoto A, Narimatsu Y, Ohkuma K, Kurata T, Shinmoto H, Hiramatsu K, Mukai M, Murai M. Angiomyolipoma: imaging findings in lesions with minimal fat. Radiology. 1997;205:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 218] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Simpson E, Patel U. Diagnosis of angiomyolipoma using computed tomography-region of interest < or =-10 HU or 4 adjacent pixels < or =-10 HU are recommended as the diagnostic thresholds. Clin Radiol. 2006;61:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. 2014;39:588-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 10. | Schieda N, Kielar AZ, Al Dandan O, McInnes MD, Flood TA. Ten uncommon and unusual variants of renal angiomyolipoma (AML): radiologic-pathologic correlation. Clin Radiol. 2015;70:206-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Chung YE, Oh YT, Choi YD. Cystic angiomyolipoma mimicking cystic renal cell carcinoma. J Urol. 2011;185:1098-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Yamashita Y, Ueno S, Makita O, Ogata I, Hatanaka Y, Watanabe O, Takahashi M. Hyperechoic renal tumors: anechoic rim and intratumoral cysts in US differentiation of renal cell carcinoma from angiomyolipoma. Radiology. 1993;188:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Siegel CL, Middleton WD, Teefey SA, McClennan BL. Angiomyolipoma and renal cell carcinoma: US differentiation. Radiology. 1996;198:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Jinzaki M, Ohkuma K, Tanimoto A, Mukai M, Hiramatsu K, Murai M, Hata J. Small solid renal lesions: usefulness of power Doppler US. Radiology. 1998;209:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Merran S, Vieillefond A, Peyromaure M, Dupuy C. Renal angiomyolipoma with calcification: CT-pathology correlation. Br J Radiol. 2004;77:782-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Takahashi N, Leng S, Kitajima K, Gomez-Cardona D, Thapa P, Carter RE, Leibovich BC, Sasiwimonphan K, Sasaguri K, Kawashima A. Small (< 4 cm) Renal Masses: Differentiation of Angiomyolipoma Without Visible Fat From Renal Cell Carcinoma Using Unenhanced and Contrast-Enhanced CT. AJR Am J Roentgenol. 2015;205:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Ogawa Y, Morita S, Takagi T, Yoshida K, Tanabe K, Nagashima Y, Nishina Y, Sakai S. Early dark cortical band sign on CT for differentiating clear cell renal cell carcinoma from fat poor angiomyolipoma and detecting peritumoral pseudocapsule. Eur Radiol. 2021;31:5990-5997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Wang X, Song G, Jiang H. Differentiation of renal angiomyolipoma without visible fat from small clear cell renal cell carcinoma by using specific region of interest on contrast-enhanced CT: a new combination of quantitative tools. Cancer Imaging. 2021;21:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Ma Y, Ma W, Xu X, Guan Z, Pang P. A convention-radiomics CT nomogram for differentiating fat-poor angiomyolipoma from clear cell renal cell carcinoma. Sci Rep. 2021;11:4644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Nie P, Yang G, Wang Z, Yan L, Miao W, Hao D, Wu J, Zhao Y, Gong A, Cui J, Jia Y, Niu H. A CT-based radiomics nomogram for differentiation of renal angiomyolipoma without visible fat from homogeneous clear cell renal cell carcinoma. Eur Radiol. 2020;30:1274-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Kang HS, Park JJ. Circularity Index on Contrast-Enhanced Computed Tomography Helps Distinguish Fat-Poor Angiomyolipoma from Renal Cell Carcinoma: Retrospective Analyses of Histologically Proven 257 Small Renal Tumors Less Than 4 cm. Korean J Radiol. 2021;22:735-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A. Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology. 2012;263:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 23. | Grignon DJ, Che M. Clear cell renal cell carcinoma. Clin Lab Med. 2005;25:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Gordetsky J, Zarzour J. Correlating Preoperative Imaging with Histologic Subtypes of Renal Cell Carcinoma and Common Mimickers. Curr Urol Rep. 2016;17:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Delahunt B, Eble JN. History of the development of the classification of renal cell neoplasia. Clin Lab Med. 2005;25:231-246, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 621] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 27. | You D, Shim M, Jeong IG, Song C, Kim JK, Ro JY, Hong JH, Ahn H, Kim CS. Multilocular cystic renal cell carcinoma: clinicopathological features and preoperative prediction using multiphase computed tomography. BJU Int. 2011;108:1444-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Antonopoulos P, Drossos C, Triantopoulou C, Picramenos D, Dalamarinis C, Costacopoulos A. Complications of renal angiomyolipomas: CT evaluation. Abdom Imaging. 1996;21:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Hélénon O, Crosnier A, Verkarre V, Merran S, Méjean A, Correas JM. Simple and complex renal cysts in adults: Classification system for renal cystic masses. Diagn Interv Imaging. 2018;99:189-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Rosenkrantz AB, Hindman N, Fitzgerald EF, Niver BE, Melamed J, Babb JS. MRI features of renal oncocytoma and chromophobe renal cell carcinoma. AJR Am J Roentgenol. 2010;195:W421-W427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 31. | Razik A, Das CJ, Sharma S. Angiomyolipoma of the Kidneys: Current Perspectives and Challenges in Diagnostic Imaging and Image-Guided Therapy. Curr Probl Diagn Radiol. 2019;48:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Michal M, Hes O, Bisceglia M, Simpson RH, Spagnolo DV, Parma A, Boudova L, Hora M, Zachoval R, Suster S. Mixed epithelial and stromal tumors of the kidney. A report of 22 cases. Virchows Arch. 2004;445:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Sahni VA, Mortele KJ, Glickman J, Silverman SG. Mixed epithelial and stromal tumour of the kidney: imaging features. BJU Int. 2010;105:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Oesterling JE, Fishman EK, Goldman SM, Marshall FF. The management of renal angiomyolipoma. J Urol. 1986;135:1121-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 338] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol. 2002;168:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 127] [Reference Citation Analysis (0)] |
| 36. | Preece P, Mees B, Norris B, Christie M, Wagner T, Dundee P. Surgical management of haemorrhaging renal angiomyolipoma in pregnancy. Int J Surg Case Rep. 2015;7C:89-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Christiano AP, Yang X, Gerber GS. Malignant transformation of renal angiomyolipoma. J Urol. 1999;161:1900-1901. [PubMed] |
| 38. | Steiner MS, Goldman SM, Fishman EK, Marshall FF. The natural history of renal angiomyolipoma. J Urol. 1993;150:1782-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 269] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 39. | Hatano T, Egawa S. Renal angiomyolipoma with tuberous sclerosis complex: How it differs from sporadic angiomyolipoma in both management and care. Asian J Surg. 2020;43:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Lee S, Park HS, Hyun D, Cho SK, Park KB, Shin SW, Choo SW, Do YS. Radiologic and clinical results of transarterial ethanol embolization for renal angiomyolipoma. Eur Radiol. 2021;31:6568-6577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 41. | Krueger DA, Northrup H; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:255-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 580] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 42. | Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, Blute ML. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468-71; discussion 472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 500] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 43. | Dong K, Shen M, Ju G, Han S, Wang Z, Lu K, Xu D. Off-clamp Retroperitoneoscopic Tumour Evacuation for Sporadic Renal Angiomyolipomas with High RENAL Nephrometry Scores: A Novel Surgical Technique and Its Outcomes. Eur Urol. 2021;79:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Guo G, Gu L, Zhang X. Everolimus in Invasive Malignant Renal Epithelioid Angiomyolipoma. Front Oncol. 2020;10:610858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Hatano T, Endo K, Tamari M. Efficacy and safety of low-dose everolimus treatment for renal angiomyolipoma associated with tuberous sclerosis complex. Int J Clin Oncol. 2021;26:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Luo C, Ye WR, Zu XB, Chen MF, Qi L, Li YL, Cai Y. Low-Dose Everolimus Maintenance Therapy for Renal Angiomyolipoma Associated With Tuberous Sclerosis Complex. Front Med (Lausanne). 2021;8:744050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |