Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.408
Peer-review started: September 10, 2022
First decision: November 2, 2022
Revised: November 20, 2022
Accepted: December 5, 2022
Article in press: December 5, 2022
Published online: January 16, 2023
Processing time: 123 Days and 20.3 Hours
Anti-leucine-rich glioma inactivated protein 1 (anti-LGI1) encephalitis is an infrequent type of autoimmune encephalitis (AE) characterized by acute or subacute cognitive and psychiatric disturbance, facio-brachial dystonic seizures (FBDSs), and hyponatremia. Anti-LGI1 AE has increasingly been considered a primary form of AE. Early identification and treatment of this disease are clearly very important.
Here, we report that a male patient developed severe anti-LGI1 encephalitis, which was initially misdiagnosed as a sleep disturbance. He was hospitalized for epileptic seizures and typical FBDSs half a month after he developed sleep disturbances. LGI1 antibodies were detected in his cerebrospinal fluid and serum (1:100 and 1:3.2, respectively), which led to the diagnosis of classic anti-LGI1 AE. No obvious abnormality was observed on brain computed tomography images. T2-weighted fluid-attenuated inversion recovery and T2-weighted scans of brain magnetic resonance imaging (MRI) showed slightly elevated signals within the left basal ganglia area. No tumor was detected within the brain of this patient using MRI. After hormone and antiepileptic drug treatment, the patient’s symp
Anti-LGI1 antibody-associated encephalitis has characteristic clinical manifestations, such as cognitive impairment, psychiatric symptoms, seizures, sleep disorders, hyponatremia, and FBDSs. LGI1 antibodies are present in the serum and/or cerebrospinal fluid, but their production is sensitive to immunosuppressants, and this disease has a relatively good prognosis. In particular, we should be aware of the possibility of anti-LGI1 antibody-associated encephalitis in adolescents with sleep disorders to avoid missed diagnoses and misdiagnoses.
Core Tip: Anti-leucine-rich glioma inactivated protein 1 (anti-LGI1) encephalitis is a rare autoimmune encephalitis (AE) characterized by acute or subacute cognitive impairment, facio-brachial dystonic seizures, psychiatric disturbances and hyponatremia. Herein, we report that a male patient developed severe anti-LGI1 encephalitis, which was initially misdiagnosed as sleep disturbance. He had antibodies targeting LGI1 both in his cerebrospinal fluid and serum, which led to the diagnosis of typical anti-LGI1 AE. The case indicated that we should be aware of the possibility of LGI1 antibody-associated encephalitis to avoid missed diagnoses and misdiagnoses especially in adolescents with sleep disorders.
- Citation: Kong DL. Anti-leucine-rich glioma inactivated protein 1 encephalitis with sleep disturbance as the first symptom: A case report and review of literature. World J Clin Cases 2023; 11(2): 408-416
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/408.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.408
In general, the phrase autoimmune encephalitis (AE) is defined as diseases caused by antigen-antibody reactions of the immune system to the central nervous system[1]. The main clinical manifestations of AE are acute or subacute epileptic seizures, facio-brachial dystonic seizures (FBDSs), cognitive disturbances, and mental disorders.
Sleep dysfunction in patients with AE has received little attention and is most likely neglected because clinicians pay more attention to neurological and psychiatric symptoms. Nevertheless, sleep disorders are very common in AE patients and often persist beyond the acute stage, which seriously affects patients’ quality of life. All patterns of somnipathy can arise in AE patients due to the influence of the disease on an extensive number of brain networks participating in sleep initiation and regulation. Anti-IgLON5 and anti-N-methyl-d-aspartate (NMDA) receptor encephalitis are two representative diseases in which sleep disturbances are common and serious. Somnipathy varies according to the disease stage in anti-NMDA receptor encephalitis, and the core symptom in anti-IgLON5 disease is sleep disorders[2].
However, few reports described sleep disorders associated with anti-leucine-rich glioma inactivated protein 1 (anti-LGI1) antibody encephalitis. Anti-LGI1 antibody-associated encephalitis is a type of AE that is characterized by epilepsy, a recent memory decline, and mental and behavioral abnormalities as its main clinical manifestations. Since anti-LGI1 encephalitis is a recently identified disease, limited data are available on its clinical manifestation, especially in patients presenting with sleep disturbances as the initial symptom. Here, we report a patient who developed severe anti-LGI1 encephalitis, which was initially misdiagnosed as a sleep disorder. The patient was hospitalized for epileptic seizures and typical FBDSs half a month after he developed the sleep disturbance.
Sleep disorders, sudden limb convulsions with unconsciousness.
This patient initially visited the doctor because he suffered from suddenly persistent insomnia (with difficulties initiating and especially maintaining sleep). Dream enactment and somniloquy occurred during his sleep, and he felt fatigue and weakness after waking in the morning. The doctor prescribed some sleeping pills. No significant improvement was observed after taking the medicine for several days. Half a month later, his right hand twitched involuntarily in the evening before admission when he had his hair cut; this symptom lasted for approximately 3 s and was not given much attention. After waking the next morning, he had two other attacks with intervals of approximately half an hour, lasting approximately 3–5 s each. Then, secondary limb convulsions appeared as follows: Flexion of both upper limbs, ankylosis of both lower limbs, unconsciousness, eyes turning up, crown closure, and mouth foaming, which lasted for approximately five minutes. Immediately, the patient's consciousness became lucid, and after waking, he could not recall the course of the disease and experienced slight dizziness and headache
Then, he went to the emergency department of our hospital. He was administered an intravenous injection of "mannitol, acetylglutamine and sodium acetate ringer" after brain computed tomography (CT) scan, which showed no obvious abnormality. Then, intermittent involuntary twitching of the right hand was still present, which occurred once in approximately 1-2 h and lasted 3-5 seconds each time. The patient was admitted to our hospital for further diagnosis and treatment.
He had previously been healthy.
There is no history of familial genetic diseases.
The neurological examination showed no obvious abnormality.
The white blood cell count (11.23 × 109 cells/L) and neutrophil percentage (89%) increased significantly in the full blood count. All biochemical indexes and thyroid function were normal or negative.
Cerebrospinal fluid (CSF): A routine CSF examination displayed acellular fluid. No bacterial growth or abnormal biochemistry was observed in the CSF. The opening pressure was not abnormal. CSF cytology showed that the percentage of leukocytes in multiple nuclei increased by 50% (reference value 0%–6%), and the other indexes were normal.
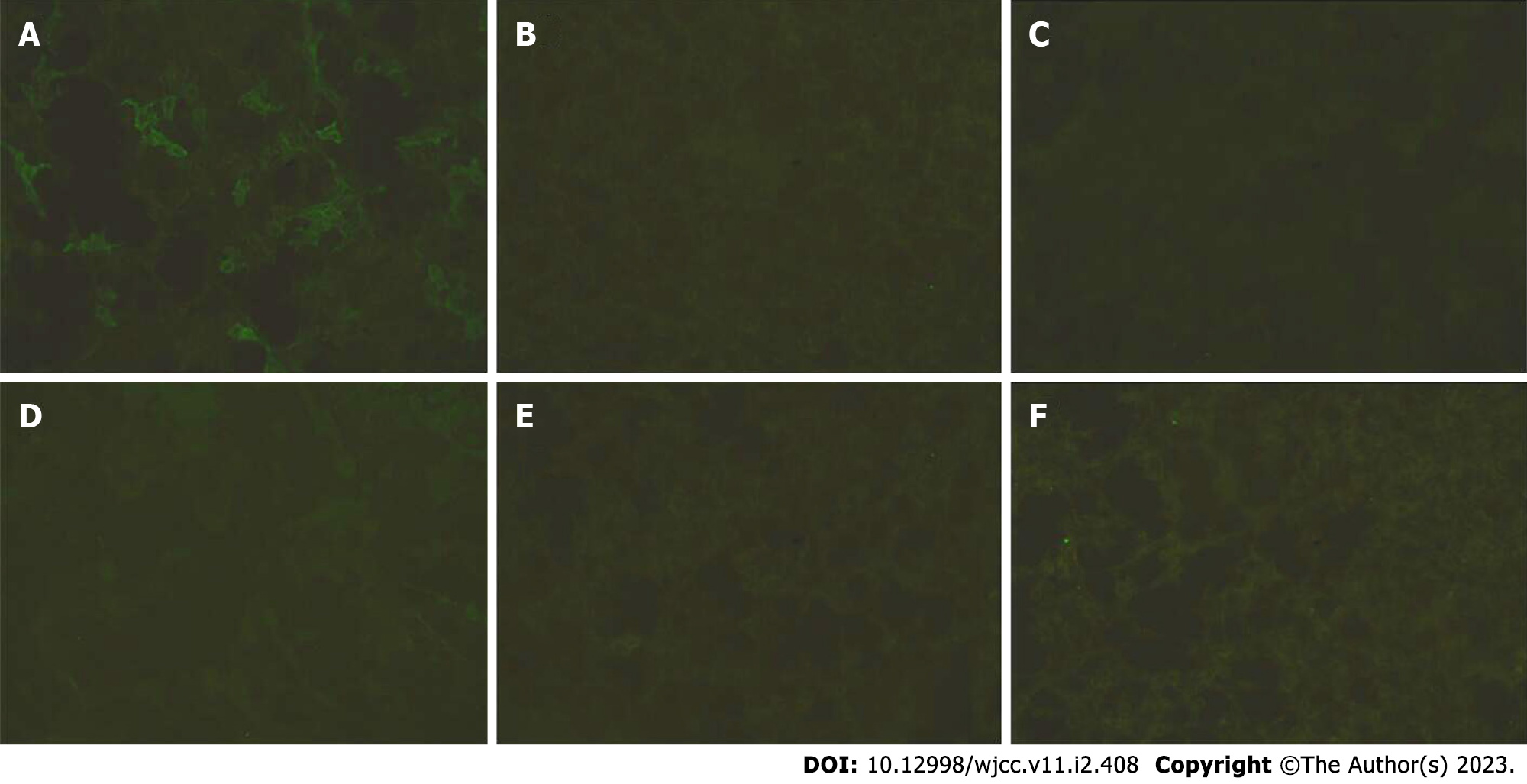
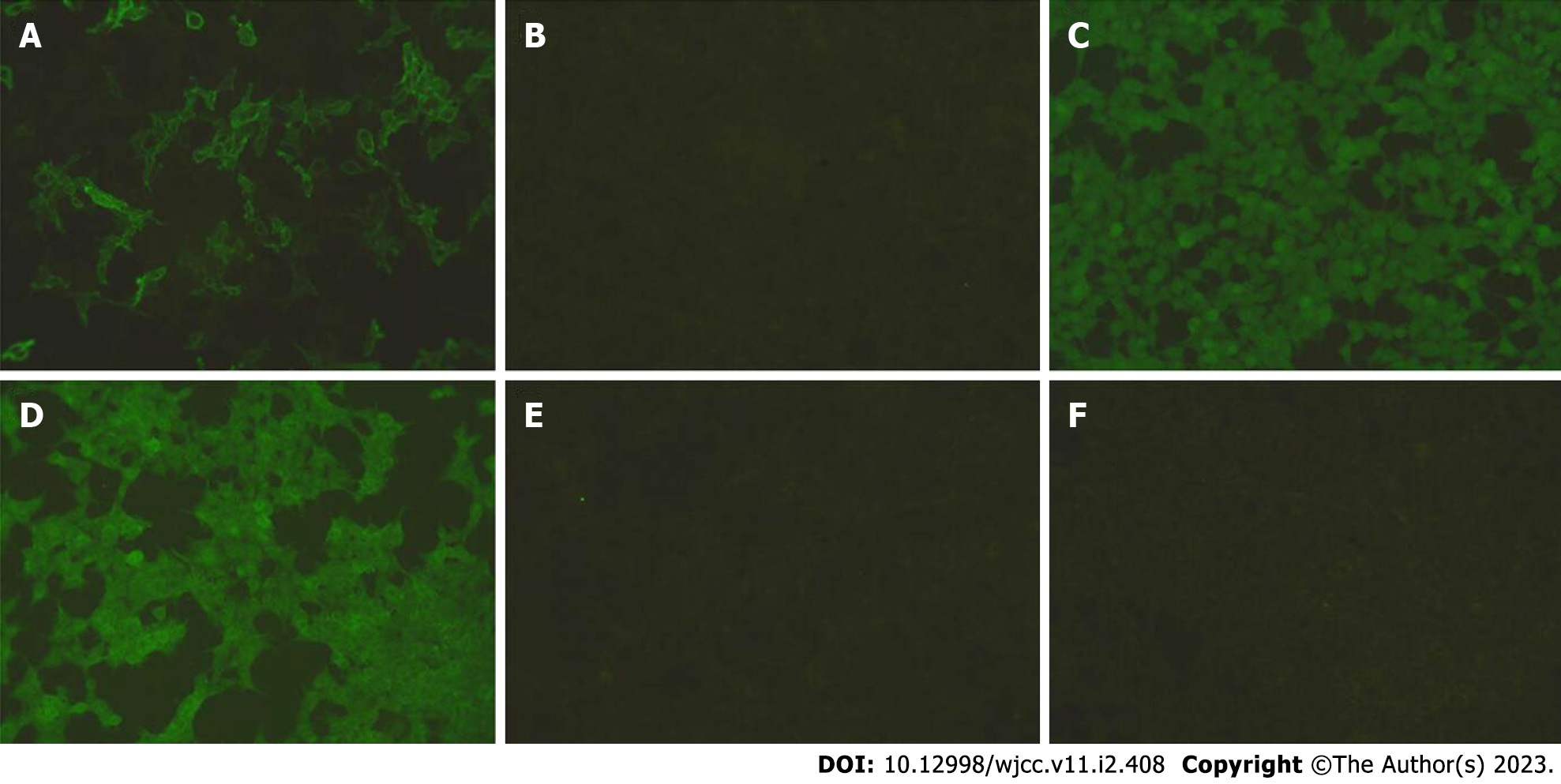
Detection of autoimmune antibodies: LGI1 antibodies were examined and were positive in both the serum and cerebrospinal fluid (1:100 and 1:3.2, respectively), and other antibodies (contactin-associated protein 2 (CASPR2), N-methyl-D-aspartate receptor (NMDAR), glutamic acid decarboxylase (GAD65), GABA, and AMPA1) were negative (Figures 1 and 2), which confirmed the diagnosis.
No abnormalities in hepatitis B surface antibody test results. Human immunodeficiency virus antibodies and Treponema pallidum-specific antibodies were normal or negative.
A computed tomography (CT) scan of the thorax showed inflammation of the right lower lobe of the lung. No abnormality was detected on the brain CT scan.
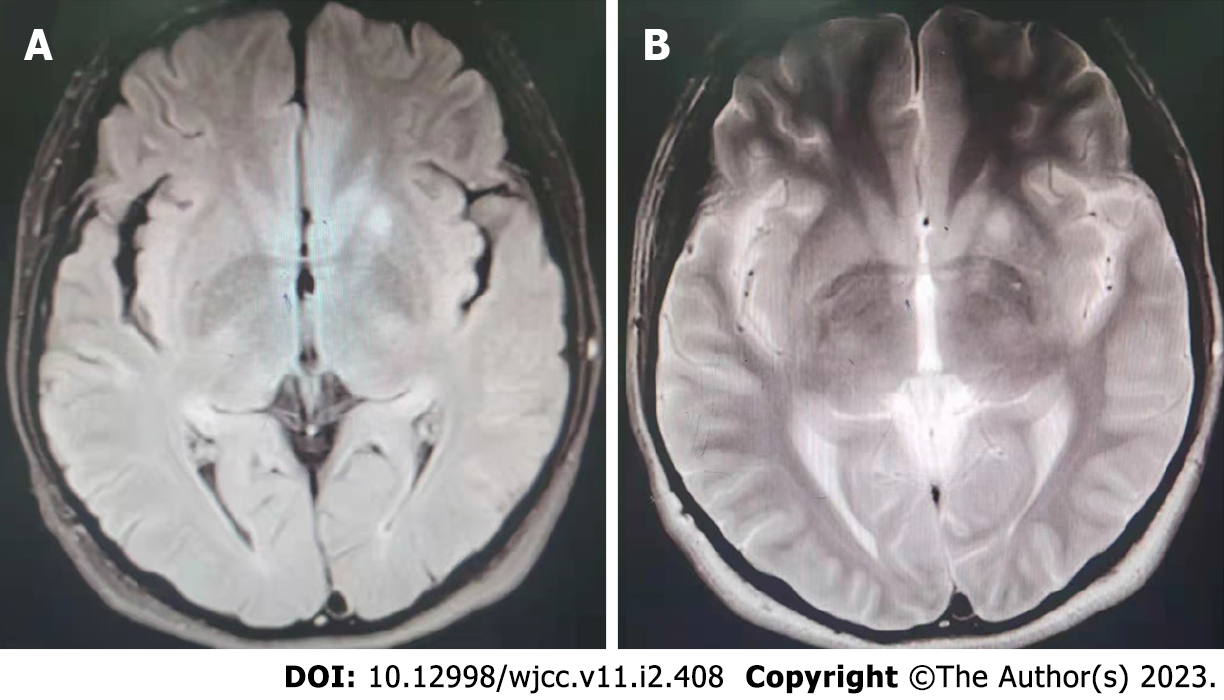
Brain magnetic resonance imaging (MRI) (Figure 3) results: An abnormal shadow signal was observed in the left basal ganglia area, which displayed a high signal in T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI and T2-weighted MRI.
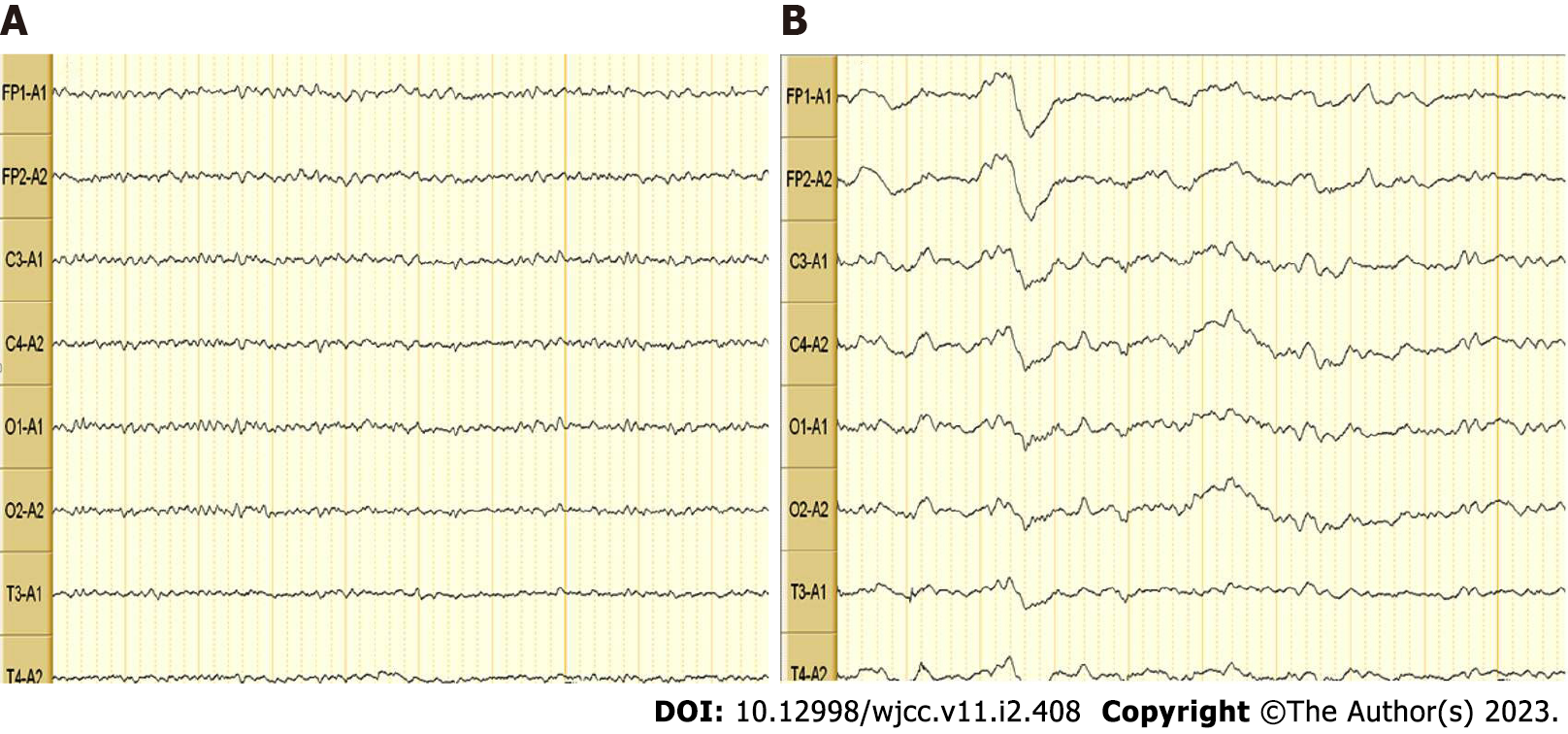
Video electroencephalogram (EEG) (Figure 4): The patient’s EEG was mildly abnormal. Multiple slow wave bursts were observed during wakefulness. Interference artifacts may have been present.
Clinicians must determine the correct diagnosis as early as possible, and excluding the presence of an underlying malignancy is certainly worthwhile because it may be associated with this pathology[3]. In this case, no signs suggested the presence of neoplasia.
In this case, a series of clinical manifestations, such as sleep disorders, seizures, and typical FBDCs, combined with abnormal signals of brain MRI and positive LGI1 antibodies in blood and cerebrospinal fluid prove that this case conforms to the diagnosis of anti-LGI1 autoimmune encephalitis.
Immunotherapy was initiated with dexamethasone, and a continuous intravenous infusion of sodium valproate was administered to control the seizures.
No major seizures occurred during hospitalization, but intermittent FBDSs persisted during the first several days after hospitalization. One week later, FBDSs did not appear. Two weeks later, the patient's symptoms improved significantly, and thus he was discharged and was told to gradually reduce the dose of prednisone after discharge. During hospitalization, the patient's sleep disturbances gradually decreased, and the patient's sleep completely returned to normal at discharge. The patent was discharged with antiepileptic therapy and corticosteroid. He is followed up every three months and symptoms have not recurred.
The incidence and mortality rates of encephalitis are 8%-18.45%[4-7]. The disease has been recognized by an increasing number of clinicians since the first case of teratoma-related anti-NMDA receptor encephalitis was reported in 2007[8].
Dalmau et al[9] were the first to report that anti-NMDAR encephalitis has a close relationship with teratoma in 2007. Lai et al[10] first discovered anti-AMPA receptor encephalitis in 2009, and Lancaster et al[11] identified anti-GABABR encephalitis in 2010. Anti-LGI1 antibody encephalitis and anti-CASPR2 antibody encephalitis were first discovered by Lai et al[12] in 2010. Anti-LGI1 encephalitis is infrequently associated with tumors, and most patients recovered after treatment with steroids or other immunotherapies[13].
A survey discovered that AE substantially affected the patients’ quality of life[14]. The diagnosis of AE is very difficult due to its sophisticated clinical symptoms[15]. The key to the diagnosis of AE is a neuronal autoimmune antibody, and a close relationship has been observed between the difference in the antibody titer and its clinical course[16].
The main manifestations of anti-LGI1 encephalitis are epilepsy, cognitive and mental disorders, hyponatremia, and sleep disorders, and explanations are provided below.
Among the patients positive for LGI antibodies, twenty percent-forty percent[17] have FBDSs, which are short, frequent, and unconscious seizures, together with dystonia, simple upper limb spasm and contraction, and ipsilateral face twitch (not long, 3 s, several times a day). Some scholars[18] postulate that FBDSs might be dystonic. However, Irani et al[19] and other scholars proposed that FBDSs were a type of epileptic seizure.
Previous studies have discovered that ninety percent of patients have seizures that primarily present in the following three types: FBDS, focal to bilateral tonic-clonic seizure and mesial temporal lobe epilepsy -like seizure[20-26]. In a recent study, patients with anti-LGI1 AE were divided into the following three groups according to the epilepsy symptomatology: FBDS alone (FBDS-only), epileptic seizures without FBDS [non-FBDS], and coexistence of FBDS and other seizures (FBDS+)[27]. Researchers found that FBDSs were significantly decreased and even vanished after treatment with oral steroids[28]. Basal ganglia lesions are present in patients with FBDSs[1].
FBDSs are the particular seizure type experienced by anti-LGI1 AE patients, and probably half of the patients experience FBDSs[20,21,22,19,29]. FBDSs are easy to identify and diagnose; however, EEG shows abnormalities during onset in only a few patients[19,29]. The origin of FBDSs remains controversial. Cortical, subcortical, and cortical-subcortical origins have been shownby distinct studies[27,30,31]. A study on anti-LGI1 AE[18] concluded that anti-LGI1 antibodyassociated encephalitis commonly damaged the hippocampus and basal ganglia, which was slightly different from a previous study that discovered that the motor cortex and hippocampus may be two main targets in anti-LGI1 AE[24,20]. In addition, immunotherapy reduces epileptic seizures and prevents complications[32]. A study[33] reported high T2 and FLAIR signals in the bilateral temporal lobe and hippocampus on brain MRI[18]. This finding is consistent with the report that LGI1 is primarily expressed in the temporal cortex and hippocampus.
The seizure form experienced by this patient FBDS+, and the symptoms were obviously relieved after treatment with hormone and antiepileptic drugs, consistent with the characteristics of anti-LGI1 encephalitis.
Previous studies discovered that approximately ninety-five percent of patients suffered from cognitive dysfunctions. Majoie et al[34] discovered that eighty-nine percent of LE patients had dysmnesia. Malter et al[17] identified relationships between cognitive impairment and the disease course before immunotherapy.
The main manifestations of mental/behavioral disturbances are individual and behavioral abnormalities, such as prone to anger, anxiety, impulsive behavior, and hallucinations[18,35]. Serum anti-LGI1 antibodies may remain detectable after full clinical recovery[36]. A similar mechanism[35] can be hypothesized in anti-LGI1 encephalitis because the LGI1-ADAM22-AMPAR interaction is proposed to influence long-term depression (LTD)[37,38]. As LTD is also essential for spatial memory, disruption of this process might explain the spatial disorientation observed in patients with anti-LGI1 encephalitis.
Hyponatraemia: As reported, hyponatraemia occurs in sixty percent of patients with LGI1 AE[21,18]. The main reason was regarded as abnormal secretion of antidiuretic hormones, which will be correlated with simultaneous LGI1 expression in the hypothalamus and kidney.
Brain MRI: Most LE patients presented abnormal T2 and FLAIR signals in bilateral temporal lobe regions on brain MRI, and a small proportion of patients showed abnormal signals in one side of the hippocampus. Furthermore, the lesions often involve the temporal lobe and basal ganglia[39]. In some patients with FBDSs, high T1/T2 are detected signals on brain MRI[40] and high FDG-PET metabolism has been observed in the basal ganglia[19].
In our case, FLAIR and T2-weighted scans showed slightly elevated signals within the left basal ganglia area, consistent with the characteristics of anti-LGI1 encephalitis detected using MRI.
Sleep disturbances: Sleep disturbances are also common in patients with AE[41]. Sleep dysfunctions have also been described in association with various neuron-specific antibody biomarkers, including IgLON5, LGI1, CASPR2, NMDA receptor, and Ma2. There are four forms of sleep disorders: rapid eye movement sleep behavior disorder, hypersomnia, fragmented sleep, and sleep-disordered breathing. New sleep complaints (e.g., gasping and snoring) were reported by seventy-three percent of AE patients in one study[42].
LGI1 is a glycoprotein located in the synapse and primarily expressed in the neocortex and hippocampus[43]. A recent study of PSG revealed that sleep efficiency, total sleep time, N3 sleep and REM sleep decreased significantly in anti-LGI1 encephalitis patients[44]. Another study[45] demon
However, other authors[50,51] found that the chief immunological targets of anti-LGI1 encephalitis are the motor cortex, limbic system, brainstem and striatum thalamus, consistent with the findings that LGI1 is broadly expressed in neurons and some axonal terminals throughout the Central Nervous System.
In this case, the manifestations of sleep disorders were persistent insomnia (with difficulties initiating and especially maintaining sleep), dream enactment and somniloquy, which lasted for half a month before the seizures began. Furthermore, sleep disorders responded poorly to general sleeping pills, and the symptoms were relieved rapidly after immunotherapy, consistent with the characteristics of sleep disorders in AE.
In conclusion, sleep disturbance, marked by symptoms including sleep fragmentation, dream enactment behaviors and ambiguous or total loss of physiological sleep rhythms, could be a visible and inherent characteristic of anti-LGI1 encephalitis.
Improving the detection of sleep disorders is conducive to the early detection of anti-LGI1 AE, especially in patients presenting with sleep disorders as the initial symptoms; this approach may prevent missed diagnoses and misdiagnoses. Additionally, this approach may allow patients to receive treatment as soon as possible and promote the early recovery of patients.
EEG: Usually, a specific change in EEG is not observed in patients with anti-LGI1 AE. The abnormal EEG for FBDSs is probably caused by a deeply located or highly localized epileptogenic zone[21,23].
The case report illustrates the importance of antibody testing and early recognition of sleep disturbances in identifying this condition, which is often undiagnosed. Early recognition and initiation of therapy are important in the management of patients with anti-LGI1 AE and their prognosis and may both prevent perpetual neurological impairment and improve long-term outcomes. Unfortunately, polysomnography and FDG-PET were not completed due to the limitations of our hospital’s facilities. In a future study, we will try to collect these data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kirkik D, Turkey; Velnar T, Slovenia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2660] [Article Influence: 295.6] [Reference Citation Analysis (0)] |
| 2. | Muñoz-Lopetegi A, Graus F, Dalmau J, Santamaria J. Sleep disorders in autoimmune encephalitis. Lancet Neurol. 2020;19:1010-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | van Sonderen A, Petit-Pedrol M, Dalmau J, Titulaer MJ. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat Rev Neurol. 2017;13:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 4. | Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, Stahl JP, Mailles A, Drebot M, Rupprecht CE, Yoder J, Cope JR, Wilson MR, Whitley RJ, Sullivan J, Granerod J, Jones C, Eastwood K, Ward KN, Durrheim DN, Solbrig MV, Guo-Dong L, Glaser CA; International Encephalitis Consortium. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 756] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 5. | Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 872] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 6. | Mailles A, Stahl JP; Steering Committee and Investigators Group. Infectious encephalitis in france in 2007: a national prospective study. Clin Infect Dis. 2009;49:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 276] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Thakur KT, Motta M, Asemota AO, Kirsch HL, Benavides DR, Schneider EB, McArthur JC, Geocadin RG, Venkatesan A. Predictors of outcome in acute encephalitis. Neurology. 2013;81:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Sansing LH, Tüzün E, Ko MW, Baccon J, Lynch DR, Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3:291-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2002] [Cited by in RCA: 1720] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 10. | Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, Matà S, Kremens D, Vitaliani R, Geschwind MD, Bataller L, Kalb RG, Davis R, Graus F, Lynch DR, Balice-Gordon R, Dalmau J. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 527] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 11. | Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura A, Ohta K, Iizuka T, Guzman M, Graus F, Moss SJ, Balice-Gordon R, Dalmau J. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 618] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 12. | Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, Cowell JK, Dalmau J. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 724] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 13. | Tumminelli G, Battisti C, Cioni C, Mignarri A, Annunziata P, Federico A. Demyelinating polyneuropathy in a case of anti-LGI1 encephalitis. Muscle Nerve. 2017;56:E2-E3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Cohen J, Sotoca J, Gandhi S, Yeshokumar AK, Gordon-Lipkin E, Geocadin RG, Frick KD, Probasco JC, Venkatesan A. Autoimmune encephalitis: A costly condition. Neurology. 2019;92:e964-e972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Vollmer TL, McCarthy M. Autoimmune encephalitis: A more treatable tragedy if diagnosed early. Neurology. 2016;86:1655-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, Gleichman AJ, Balice-Gordon R, Rosenfeld MR, Lynch D, Graus F, Dalmau J. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 676] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 17. | Malter MP, Frisch C, Schoene-Bake JC, Helmstaedter C, Wandinger KP, Stoecker W, Urbach H, Surges R, Elger CE, Vincent AV, Bien CG. Outcome of limbic encephalitis with VGKC-complex antibodies: relation to antigenic specificity. J Neurol. 2014;261:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Wang M, Cao X, Liu Q, Ma W, Guo X, Liu X. Clinical features of limbic encephalitis with LGI1 antibody. Neuropsychiatr Dis Treat. 2017;13:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, Schott JM, Armstrong RJ, S Zagami A, Bleasel A, Somerville ER, Smith SM, Vincent A. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 589] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 20. | Bastiaansen AEM, van Sonderen A, Titulaer MJ. Autoimmune encephalitis with anti-leucine-rich glioma-inactivated 1 or anti-contactin-associated protein-like 2 antibodies (formerly called voltage-gated potassium channel-complex antibodies). Curr Opin Neurol. 2017;30:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | van Sonderen A, Schreurs MW, Wirtz PW, Sillevis Smitt PA, Titulaer MJ. From VGKC to LGI1 and Caspr2 encephalitis: The evolution of a disease entity over time. Autoimmun Rev. 2016;15:970-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol. 2017;30:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 23. | Gao L, Liu A, Zhan S, Wang L, Li L, Guan L, Zhao X, Zhang X, Wang Y. Clinical characterization of autoimmune LGI1 antibody limbic encephalitis. Epilepsy Behav. 2016;56:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Li LH, Ma CC, Zhang HF, Lian YJ. Clinical and electrographic characteristics of seizures in LGI1-antibody encephalitis. Epilepsy Behav. 2018;88:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | de Bruijn MAAM, van Sonderen A, van Coevorden-Hameete MH, Bastiaansen AEM, Schreurs MWJ, Rouhl RPW, van Donselaar CA, Majoie MHJM, Neuteboom RF, Sillevis Smitt PAE, Thijs RD, Titulaer MJ. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABA(B)R encephalitis. Neurology. 2019;92:e2185-e2196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 26. | Zhang W, Wang X, Shao N, Ma R, Meng H. Seizure characteristics, treatment, and outcome in autoimmune synaptic encephalitis: A long-term study. Epilepsy Behav. 2019;94:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Chen C, Wang X, Zhang C, Cui T, Shi WX, Guan HZ, Ren HT, Shao XQ. Seizure semiology in leucine-rich glioma-inactivated protein 1 antibody-associated limbic encephalitis. Epilepsy Behav. 2017;77:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Gastaldi M, Thouin A, Vincent A. Antibody-Mediated Autoimmune Encephalopathies and Immunotherapies. Neurotherapeutics. 2016;13:147-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Andrade DM, Tai P, Dalmau J, Wennberg R. Tonic seizures: a diagnostic clue of anti-LGI1 encephalitis? Neurology. 2011;76:1355-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Striano P. Faciobrachial dystonic attacks: seizures or movement disorder? Ann Neurol. 2011;70:179-80; author reply 180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Boesebeck F, Schwarz O, Dohmen B, Graef U, Vestring T, Kramme C, Bien CG. Faciobrachial dystonic seizures arise from cortico-subcortical abnormal brain areas. J Neurol. 2013;260:1684-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Navarro V, Kas A, Apartis E, Chami L, Rogemond V, Levy P, Psimaras D, Habert MO, Baulac M, Delattre JY, Honnorat J; collaborators. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain. 2016;139:1079-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 33. | Yin D, Chen S, Liu J. Sleep Disturbances in Autoimmune Neurologic Diseases: Manifestation and Pathophysiology. Front Neurosci. 2021;15:687536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Majoie HJ, de Baets M, Renier W, Lang B, Vincent A. Antibodies to voltage-gated potassium and calcium channels in epilepsy. Epilepsy Res. 2006;71:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | van Sonderen A, Thijs RD, Coenders EC, Jiskoot LC, Sanchez E, de Bruijn MA, van Coevorden-Hameete MH, Wirtz PW, Schreurs MW, Sillevis Smitt PA, Titulaer MJ. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology. 2016;87:1449-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 420] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 36. | Ariño H, Armangué T, Petit-Pedrol M, Sabater L, Martinez-Hernandez E, Hara M, Lancaster E, Saiz A, Dalmau J, Graus F. Anti-LGI1-associated cognitive impairment: Presentation and long-term outcome. Neurology. 2016;87:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 37. | Ohkawa T, Fukata Y, Yamasaki M, Miyazaki T, Yokoi N, Takashima H, Watanabe M, Watanabe O, Fukata M. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci. 2013;33:18161-18174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 38. | Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 738] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 39. | Fauser S, Talazko J, Wagner K, Ziyeh S, Jarius S, Vincent A, Schulze-Bonhage A. FDG-PET and MRI in potassium channel antibody-associated non-paraneoplastic limbic encephalitis: correlation with clinical course and neuropsychology. Acta Neurol Scand. 2005;111:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Flanagan EP, Kotsenas AL, Britton JW, McKeon A, Watson RE, Klein CJ, Boeve BF, Lowe V, Ahlskog JE, Shin C, Boes CJ, Crum BA, Laughlin RS, Pittock SJ. Basal ganglia T1 hyperintensity in LGI1-autoantibody faciobrachial dystonic seizures. Neurol Neuroimmunol Neuroinflamm. 2015;2:e161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 41. | Devine MF, St Louis EK. Sleep Disturbances Associated with Neurological Autoimmunity. Neurotherapeutics. 2021;18:181-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Blattner MS, de Bruin GS, Bucelli RC, Day GS. Sleep disturbances are common in patients with autoimmune encephalitis. J Neurol. 2019;266:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133:2734-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1088] [Cited by in RCA: 959] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 44. | Lin N, Hao H, Guan H, Sun H, Liu Q, Lu Q, Jin L, Ren H, Huang Y. Sleep Disorders in Leucine-Rich Glioma-Inactivated Protein 1 and Contactin Protein-Like 2 Antibody-Associated Diseases. Front Neurol. 2020;11:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Liu X, Yang L, Han Y, Xu J, Pang Z, Du Y, Feng Y, Lin Y. Electrophysiological Evaluation in Identifying Unique Sleep Features Among Anti-LGI1 Encephalitis Patients During Active and Recovery Phase. Nat Sci Sleep. 2021;13:527-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 46. | Ayşit-Altuncu N, Ulusoy C, Öztürk G, Tüzün E. Effect of LGI1 antibody-positive IgG on hippocampal neuron survival: a preliminary study. Neuroreport. 2018;29:932-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Herranz-Pérez V, Olucha-Bordonau FE, Morante-Redolat JM, Pérez-Tur J. Regional distribution of the leucine-rich glioma inactivated (LGI) gene family transcripts in the adult mouse brain. Brain Res. 2010;1307:177-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Uchida S, Soya S, Saito YC, Hirano A, Koga K, Tsuda M, Abe M, Sakimura K, Sakurai T. A Discrete Glycinergic Neuronal Population in the Ventromedial Medulla That Induces Muscle Atonia during REM Sleep and Cataplexy in Mice. J Neurosci. 2021;41:1582-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Peter-Derex L, Devic P, Rogemond V, Rheims S, Mauguière F, Honnorat J. Full recovery of agrypnia associated with anti-Lgi1 antibodies encephalitis under immunomodulatory treatment: a case report with sequential polysomnographic assessment. Sleep Med. 2012;13:554-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Liu X, Han Y, Yang L, Wang B, Shao S, Feng Y, Pang Z, Du Y, Lin Y. The exploration of the spectrum of motor manifestations of anti-LGI1 encephalitis beyond FBDS. Seizure. 2020;76:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Irani SR, Pettingill P, Kleopa KA, Schiza N, Waters P, Mazia C, Zuliani L, Watanabe O, Lang B, Buckley C, Vincent A. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72:241-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |