Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.385
Peer-review started: November 15, 2022
First decision: November 30, 2022
Revised: December 15, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 16, 2023
Processing time: 57 Days and 19.8 Hours
Quantitative studies on the changes in inflammation-related content in tears, especially the effect of diabetes, are lacking. In this study, we measured the preoperative and postoperative tear inflammatory mediator levels in cataract patients, focusing on the expression of inflammatory factors in postoperative cataracts in the diabetic, and investigated the effect of drugs on the control of postoperative inflammation.
To study the expression of inflammatory factors in elderly people with type 2 diabetes after cataract surgery.
Patients with a mean age of 70.3 ± 6.3 years were divided into group A (composed of elderly patients with cataracts and type 2 diabetes, n = 20 eyes) and group B (patients with age-related cataract, n = 20 eyes). Their tears were collected before each operation and on days 1 and 3, and weeks 1, 2, 3, and 4 post-surgery. Saline (150 μL) was dropped into the conjunctival sac of the surgical eye, followed by oculogyration in four directions. The fluid in the conjunctival sac was extracted using a sterile syringe and stored in Eppendorf tubes at -80 °C until measurement. The expression levels of matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2, interleukin-6 (IL-6), and IL-20 in tear fluid were measured using enzyme-linked immunosorbent assays.
The postoperative expression levels of MMP-2, MMP-9, TIMP-2, IL-6, and IL-20 in group A were significantly higher than those in group B, whereas the concentration of TIMP-1 in group A remained lower than that in group B. The levels of MMP-2 and IL-6 in both groups continuously increased until the peak in the first postoperative week, and then gradually decreased over the next three weeks. Ultimately, MMP-2 declined to a lower level than that preoperatively at week 4, but IL-6 decreased to the same level as that preoperatively. The level of MMP-9 peaked in the first two weeks postoperative and then returned to the same level as 1-day post-operation. The concentration of TIMP-1 post-operation remained constant at a lower level than before surgery, and TIMP-2 Levels remained stable in both groups. IL-20 content started to increase in the third week after surgery.
Inflammatory factor levels in tears fluctuated before and post-operation, which indicated more severe postoperative inflammation in the first two weeks.
Core Tip: In this study, we compared the expression of inflammatory factors in postoperative tears of cataract patients and found that postoperative inflammation was more severe in elderly patients with cataract combined with type 2 diabetes; moreover, the level of postoperative inflammatory factors fluctuated greatly, and the inflammation was more severe in the first two weeks after surgery.
- Citation: Lv J, Cao CJ, Li W, Li SL, Zheng J, Yang XL. Tear inflammation related indexes after cataract surgery in elderly patients with type 2 diabetes mellitus. World J Clin Cases 2023; 11(2): 385-393
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/385.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.385
Cataracts are the main cause of blindness and affect millions of people worldwide[1]. Diabetes is one of the most prevalent chronic diseases in the world. Patients with type 2 diabetes have a higher risk of cataracts than those without diabetes and require surgery more urgently[2]. Cataract patients with diabetes are also at a higher risk of delayed incisional healing and postoperative complications, such as dry eye, corneal epithelial defects or erosions, persistent inflammatory reactions, and infections[3]. Currently, China is rapidly becoming an aging society, with an increasing proportion of the aged population. Changes in tear composition in elderly patients resulting from loss of the meibomian gland gradually aggravate with age. Additionally, abnormal diabetes-induced variations in tear components might cause postoperative inflammatory reactions in patients with type 2 diabetes[4]. Xerophthalmia was observed significantly more frequently in diabetic patients than in non-diabetics 7 d after phacoemulsification[5]. Another retrospective clinical study confirmed that the risk of complications in patients with diabetes was highest in the first 2 wk after cataract surgery[6].
The development of postoperative inflammation may be significantly affected by these inflammation-related mediators, but quantitative studies on inflammatory-related content changes in tears, particularly the effect of diabetes mellitus, are still lacking. This study focused on the postoperative expression of inflammatory factors in elderly diabetic cataracts to discuss the effects of drugs on the control of postoperative inflammation.
Patients diagnosed with age-related cataracts and treated with cataract surgery in our hospital between December 2021 and January 2022 were divided into group A (cataract with combined type 2 diabetes mellitus, n = 20 eyes) and Group B (elderly patients with cataracts but no diabetes, n = 20 eyes).
The inclusion criteria were as follows: patients with cataract with or without a confirmed history of type 2 diabetes mellitus, eligibility for geriatric cataract surgery, clear state of consciousness, and ability to cooperate with relevant examinations.
Exclusion criteria: Patients with previous/current ocular/systemic inflammation, fever, immunological diseases, history of ocular surgery or trauma, intraoperative complications, or inability to cooperate with examinations.
General clinical parameters, such as age, sex, body temperature, height, and weight, and detailed medical history were acquired, measured, and recorded. Hemanalysis and measurement of indicators were performed for all patients, including blood glucose, triglycerides (TG), total cholesterol (TC), glycated hemoglobin (HbA1c), glycated albumin (GA), tear matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2, interleukin-6 (IL-6), and IL-20. All patients underwent ophthalmic observations and examinations, including visual acuity (before and posto
Tears were collected before surgery and on days 1 and 3 and weeks 1, 2, 3, and 4 post-operation. Saline (150 μL) was dropped into the conjunctival sac of the surgical eye, followed by oculogyration in four directions. The fluid in the conjunctival sac was extracted using a sterile syringe and stored in Eppendorf tubes at -80 °C until measurement. The concentrations of MMP-2, MMP-9, TIMP-1, TIMP-2, IL-6, and IL-20 in the tear fluid were measured using enzyme-linked immunosorbent assay.
All patients were administered pranoprofen eye drops 3 times/d and levofloxacin eye drops 3 times/d, three days before surgery. All procedures were performed by the same surgeon. Mydriasis was induced with compound tropicamide 30 min before surgery and surface anesthesia with oxybuprocaine hydrochloride drops before surgery. A main incision was made on the temporal side of the transparent cornea, and a secondary incision was made on the inferior temporal (left eye) or superior temporal (right eye) side of the transparent cornea. Continuous circular capsulorhexis was performed through the injection of viscoelastic agents, the nucleus was emulsified after hydro-dissection and hydro-delineation, followed by aspiration of the cortex, and the intraocular lens was implanted into the polished capsular bag. Surgery was completed after irrigation of the anterior chamber, aspiration of viscoelastic agents, and closure of the conjunctiva with solution. After surgery, all patients were administered tobramycin and dexamethasone eye drops three times/d for one month, pranoprofen eye drops three times/d for two weeks, and levofloxacin eye drops three times/d for two weeks.
Differences in inflammatory factor expression (indicated as mean and standard deviation) between diabetic and non-diabetic elderly patients with cataract were determined by performing repeated-measures and Analysis of Variance using SPSS 26.0. Differences in age, intraocular pressure (IOP), HbA1c, GA, TG, and TC between the two groups were verified using Student’s test in SPSS 26.0. Differences in sex between the two groups were determined using the χ2 test. Statistical significance was set at P < 0.05.
A comparison was performed with 19 eyes of 19 males (47.5%) and 21 eyes of 21 females (52.5%), whose mean age was (70.3 ± 6.3) years, and the mean disease course duration of diabetes in group A was (6.8 ± 2.2) years. Patients were further grouped based on their preoperative visual acuity as ≤ 0.1, 0.1-0.3, and ≥ 0.3. The composition of sex and age, visual acuity, IOP, TG, and TC between the groups was not significantly different, while significant differences were detected in HbA1c and GA (Table 1).
| Groups | Age1 (yr) | Gender2 (M/F) | Visual acuity (BCVA)3 | Intraocular pressure1 (mmHg) | HbA1c1 (%) | GA1 (%) | TG1 (mmol/L) | TC1 (mmol/L) | ||
| ≤ 0.1 | 0.1-0.3 | ≥ 0.3 | ||||||||
| Group A | 69.3 ± 6.6 | 9/11 | 9 | 8 | 3 | 15.3 ± 2.28 | 8.2 ± 0.6 | 25.1 ± 4.8 | 2.0 ± 0.3 | 5.7 ± 0.4 |
| Group B | 71.0 ± 5.0 | 10/10 | 8 | 10 | 2 | 15.8 ± 2.76 | 5.4 ± 0.1 | 14.0 ± 1.5 | 1.7 ± 0.3 | 5.6 ± 0.4 |
| χ2/F value | 1.196 | 0.100 | 0.481 | 0.225 | 8.197 | 8.700 | 0.238 | 0.749 | ||
| P value | 0.557 | 0.752 | 0.829 | 0.575 | 0.002 | 0.020 | 0.458 | 0.142 | ||
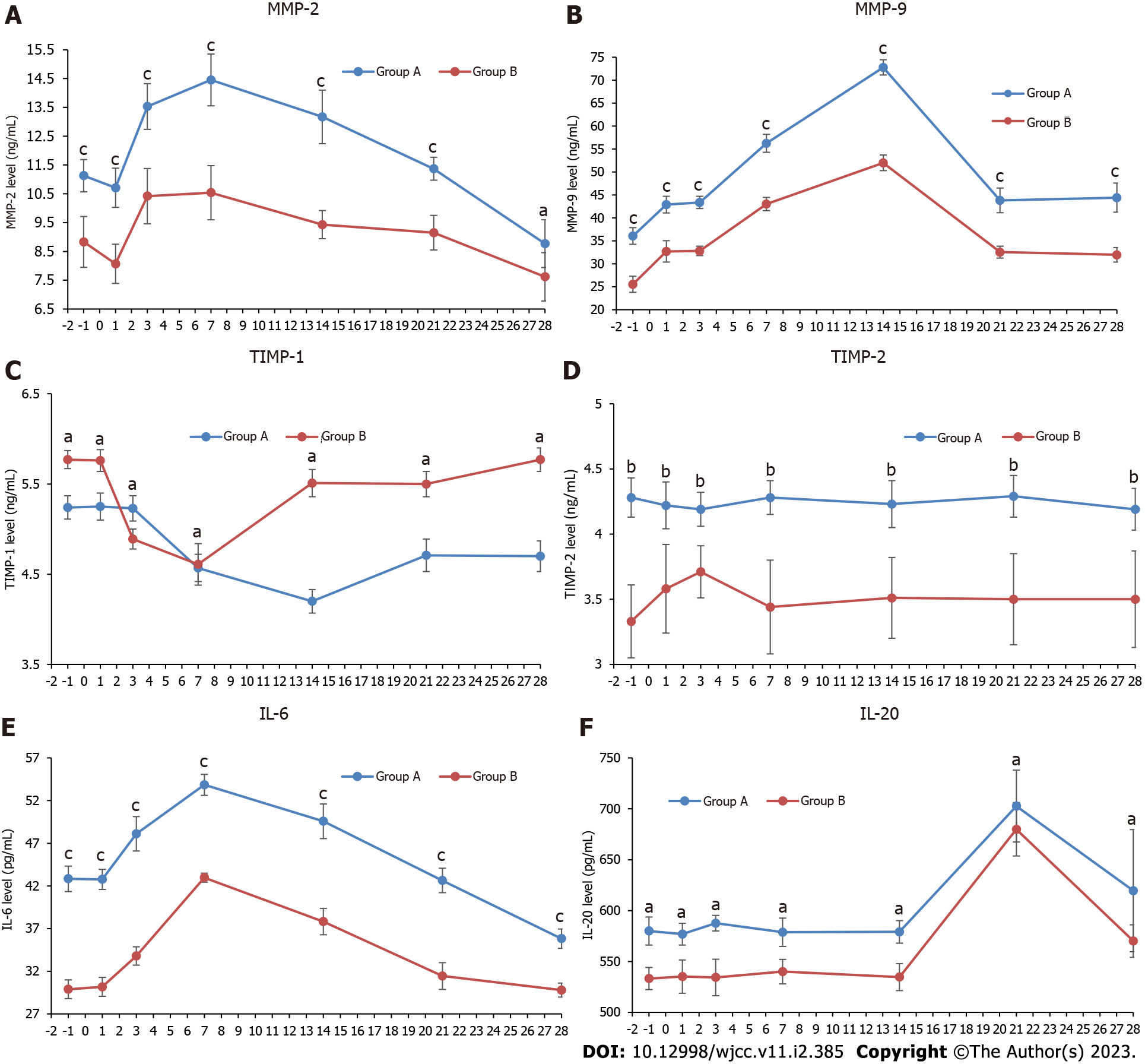
The level of MMP-2 in both groups continuously increased until it peaked in the first week postoperatively and then gradually decreased over the next three weeks, ultimately declining to a level lower than the preoperative level at week 4. The level of MMP-9 peaked in the first two weeks postoperative and then returned to the same level as 1-day post-operation. The expression levels of MMP-2 and MMP-9 in group A were significantly higher than those in group B at all time points (Table 2, Figure 1A and B; P < 0.001).
| Time | MMP-2 (ng/mL) | MMP-9 (ng/mL) | ||||||
| Group A | Group B | t value | P value | Group A | Group B | t value | P value | |
| Preoperative | 11.13 ± 0.56 | 8.83 ± 0.88 | 11.65 | 0.000 | 36.07 ± 1.82 | 25.55 ± 1.74 | 13.22 | 0.000 |
| 1 d | 10.71 ± 0.68 | 8.07 ± 0.68 | 10.54 | 0.000 | 42.90 ± 1.82 | 32.69 ± 2.33 | 10.96 | 0.000 |
| 3 d | 13.53 ± 0.79 | 10.42 ± 0.96 | 11.06 | 0.000 | 43.37 ± 1.33 | 32.80 ± 1.02 | 18.09 | 0.000 |
| 1 wk | 14.45 ± 0.9 | 10.54 ± 0.94 | 8.22 | 0.000 | 56.25 ± 1.96 | 43.02 ± 1.45 | 20.45 | 0.000 |
| 2 wk | 13.17 ± 0.93 | 9.43 ± 0.49 | 12.29 | 0.000 | 72.78 ± 1.66 | 51.99 ± 1.71 | 41.48 | 0.000 |
| 3 wk | 11.37 ± 0.40 | 9.15 ± 0.60 | 9.99 | 0.000 | 43.81 ± 2.68 | 32.55 ± 1.3 | 14.70 | 0.000 |
| 4 wk | 8.77 ± 0.83 | 7.62 ± 0.84 | 2.63 | 0.017 | 44.41 ± 3.15 | 31.97 ± 1.58 | 13.79 | 0.000 |
After a decline in the first two postoperative weeks and an increase from the third week, the concentration of TIMP-1 in group A was still lower than that before surgery at four weeks post-operation. The expression level of TIMP-1 in group A was lower than that in group B (Figure 1C, P < 0.05). The level of tear TIMP-2 in group A was higher than that in group B before and after operation (Table 3, Figure 1D; P < 0.01).
| Time | TIMP-1 (ng/mL) | TIMP-2 (ng/mL) | ||||||
| Group A | Group B | t value | P value | Group A | Group B | t value | P value | |
| Preoperative | 5.24 ± 0.13 | 5.77 ± 0.10 | 2.34 | 0.032 | 4.28 ± 0.15 | 3.33 ± 0.28 | 6.13 | 0.004 |
| 1 d | 5.25 ± 0.15 | 5.76 ± 0.12 | 2.06 | 0.028 | 4.22 ± 0.18 | 3.58 ± 0.34 | 6.01 | 0.003 |
| 3 d | 5.23 ± 0.14 | 4.89 ± 0.11 | 2.83 | 0.027 | 4.19 ± 0.13 | 3.71 ± 0.2 | 5.41 | 0.007 |
| 1 wk | 4.57 ± 0.15 | 4.61 ± 0.23 | 0.45 | 0.060 | 4.28 ± 0.13 | 3.44 ± 0.36 | 5.08 | 0.006 |
| 2 wk | 4.20 ± 0.13 | 5.51 ± 0.15 | 2.75 | 0.021 | 4.23 ± 0.18 | 3.51 ± 0.31 | 6.51 | 0.002 |
| 3 wk | 4.71 ± 0.18 | 5.50 ± 0.14 | 5.75 | 0.005 | 4.29 ± 0.16 | 3.50 ± 0.35 | 6.60 | 0.004 |
| 4 wk | 4.70 ± 0.17 | 5.77 ± 0.13 | 7.34 | 0.003 | 4.19 ± 0.16 | 3.50 ± 0.37 | 6.64 | 0.003 |
After surgery, IL-6 Levels in both groups increased in the first week, but remained at a higher level in group A than in group B (Figure 1E, P < 0.001). Similar trends in IL-20 Levels were observed in the two groups, which were also higher in group A than in group B (P < 0.05). Its concentration remained constant before the third week after operation, surged to a peak in the third week post-operation, and then started to slump in the fourth week (Table 4, Figure 1F).
| Time | IL-6 (pg/mL) | IL-20 (pg/mL) | ||||||
| Group A | Group B | t value | P value | Group A | Group B | t value | P value | |
| Preoperative | 42.84 ± 1.49 | 29.89 ± 1.09 | 22.33 | 0.000 | 579.90 ± 13.89 | 533.15 ± 10.9 | 2.78 | 0.021 |
| 1 d | 42.77 ± 1.18 | 30.17 ± 1.11 | 24.65 | 0.000 | 576.82 ± 10.67 | 535.13 ± 16.38 | 2.39 | 0.024 |
| 3 d | 48.11 ± 2.01 | 33.79 ± 1.08 | 19.90 | 0.000 | 587.52 ± 7.62 | 534.28 ± 17.92 | 2.91 | 0.037 |
| 1 wk | 53.85 ± 1.24 | 42.97 ± 0.52 | 25.76 | 0.000 | 578.75 ± 13.9 | 539.97 ± 11.95 | 2.50 | 0.038 |
| 2 wk | 49.58 ± 2.02 | 37.82 ± 1.55 | 14.63 | 0.000 | 579.08 ± 11.15 | 534.64 ± 13.27 | 2.67 | 0.035 |
| 3 wk | 42.64 ± 1.43 | 31.44 ± 1.57 | 16.73 | 0.000 | 702.67 ± 35.3 | 679.85 ± 26.2 | 2.62 | 0.032 |
| 4 wk | 35.82 ± 1.14 | 29.79 ± 0.81 | 13.70 | 0.000 | 619.55 ± 60.04 | 570.05 ± 15.94 | 2.43 | 0.036 |
Hyperglycemia contributes to impaired corneal sensitivity, reduces nerve fiber density, and delays epithelial wound healing. Due to reduced corneal sensitivity, reflex-induced tear secretion decreases together with the blink rate in diabetic patients, which ultimately leads to increased tear evaporation[7]. Corneal incision accompanied by nerve amputation and microscopic light illumination in cataract surgeries, use of anesthetics, mydriatic drops, and postoperative antibiotics and hormones increases the risk of postoperative complications in diabetic patients. In summary, patients with type-2-diabetes with cataracts are at a higher risk of postoperative complications and have more difficulty in epithelial wound healing than cataracts in patients with normal blood glucose levels, which suggests that more attention should be paid to their treatment.
MMPs are a highly conserved family of proteinases that can degrade various extracellular matrix components[8]. The expression levels of MMPs are extremely low under normal physiological conditions and can be significantly upregulated by inflammatory factors, growth factors, and pathological conditions such as high glucose and oxidative stress. TIMPs are active in many tissues and body fluids as endogenous inhibitors of MMPs[9]. It was confirmed both in vitro and in vivo that upregulated expression levels of MMP-2 and MMP-9 in wound healing of high glucose cultured corneal epithelial cells and corneal epithelial cells from diabetic rats can lead to xerophthalmia, defects, and erosions of corneal epithelial and ocular inflammation[10]. Increased MMP-9 expression in ocular tissues has also been observed in recurrent corneal erosion, skin ulcers, and diabetic retinopathy[11]. Tears containing levels of MMP-2, MMP-9, and TIMP-2 before and post-operation, were estimated to be higher in patients with diabetes than in elderly patients with cataracts but no diabetes. It is thought to be a response to the stimulation of the ocular surface by long-term high blood glucose concentrations and chronic inflammation. In addition, the gradual increase in MMP-2/9 Levels in the first two postoperative weeks suggested that severe inflammatory responses occurred in the first two weeks post cataract surgery. TIMP-1 expression was suppressed after surgery in both groups and was more significant in group A. This suppression works in concert with the upregulated expression of MMPs and ultimately causes severe inflammation in patients with diabetes.
IL-6 is a pleiotropic cytokine that affects various cell types, including pro-inflammatory and anti-inflammatory cytokines[12]. Dysregulation of IL-6 signaling is associated with the pathogenesis of several autoimmune and inflammatory diseases, including type 2 diabetes[13]. The causality between chronic low-grade inflammation, indicated by elevated circulating levels of inflammatory cytokines
The interaction between IL-20 and its receptor may have pro-inflammatory, angiogenic, and chemo-attractive effects in chronic inflammatory diseases, especially atherosclerosis and rheumatoid arthritis. This may also have a certain degree of impact on type 2 diabetes. We also detected the expression of IL-20 and related receptors in corneal epithelial cells, dendritic cells, and monocytes of wild-type mice. By promoting the aggregation and activation of T-cells in the injured cornea, IL-20 exerts anti-inflammatory effects without increasing neutrophil chemotaxis or promoting corneal epithelialization and wound healing[16]. This process of corneal re-epithelialization can be inhibited by the absence of neutrophils or T cells. In this study, IL-6 Levels gradually increased to a peak on days 1 and 3; and on week 1 post-operation, and then gradually decreased at weeks 2, 3, and 4 post-operation. This might be related to the gradual aggravation of early inflammation, which could induce the expression of IL-6 to further promote anti-inflammatory effects after cataract surgery. The increase in IL-20 in the third week after cataract surgery might be caused by the decreased release of inflammatory factors in the third week after cataract surgery, which could promote IL-20 expression and further contribute to corneal wound healing.
In this study, as there was a trend of correlated changes in postoperative inflammatory factor expression when the same ophthalmic medication was applied pre and postoperatively to the eyes of both groups, it was speculated that the application of anti-inflammatory and infection-preventive ophthalmic drugs before and after surgery had an effect on postoperative healing. Meanwhile, both the pre and postoperative levels of relevant inflammatory factors were higher in the test group than in the control group, indicating that the postoperative inflammatory response was higher in the test group based on the application of the same dosages of ophthalmic drugs. Therefore, it was considered clinically that within one week after cataract surgery, the frequency and duration of relevant ophthalmic drugs could be increased to reduce the postoperative inflammatory response in patients with combined diabetes and cataracts. Another study found that the use of ultrasound emulsification combined with IOL implantation based on routine glycemic control, IOP control, and anti-inflammation in patients with cataracts combined with diabetes, reduced the levels of inflammatory factors in the atrial fluid and oxidative stress indicators in such patients[17].
Our study has several limitations. First, it was a small sample; second, there was a lack of information about the patients' blood glucose levels and the duration of their disease, and some patients may have been undiagnosed or were untreated for diabetes before surgery; third, the number of preoperative tears and tear volume in patients was inadequate.
Comparison between inflammatory indices at different time points before and after surgery revealed more severe postoperative inflammation in patients with Type 2 diabetes with cataracts than in elderly patients with cataracts but without diabetes. Postoperative levels of inflammatory factors in tears were fluid, particularly compared to levels before-the operation. The expression of most inflammatory factors peaked in the first two weeks after surgery, when patients were considered most vulnerable to inflammatory complications. Therefore, the increased use of anti-inflammatory drugs in the first two postoperative weeks was proposed based on our observations.
Quantitative studies on the changes in inflammation-related content in tears, especially the effect of diabetes, are lacking. In this study, we measured the preoperative and postoperative tear inflammatory mediator levels in cataract patients, focusing on the expression of inflammatory factors in postoperative diabetic cataracts in the elderly, and investigated the effect of drugs on the control of postoperative inflammation.
Postoperative inflammation is more severe in diabetic patients with cataracts than in elderly cataract patients who are not diabetic, and the level of inflammatory factors in the postoperative tears is also higher in the former. Therefore, this strengthened the recommendation for the use of anti-inflammatory drugs in the first two postoperative weeks, that was proposed based on our observations.
This study studies the expression of inflammatory factors in elderly people with type 2 diabetes after cataract surgery. This may provide a basis for the timing and duration of anti-inflammatory medication use in patients undergoing cataract surgery.
This study was an observational study. The patients were divided into two groups. Group A (patients with cataracts with combined type 2 diabetes) and group B (patients with cataracts without combined type 2 diabetes). Their tears were collected before each operation and on days 1 and 3 and weeks 1, 2, 3, and 4 post-surgery, and an enzyme-linked immunosorbent assay was used to detect the level of inflammatory mediators in tear fluid.
The expression levels of matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of metalloproteinase-2 (TIMP-2), interleukin-6 (IL-6), and IL-20 in group A were significantly higher than those in group B after surgery, whereas the expression level of TIMP-1 in group A was always lower than that in group B.
Postoperative tear inflammation is more severe in cataract patients with diabetes than in elderly patients. Inflammatory factor levels in tears fluctuated before and post-operation, which indicated more severe postoperative inflammation in the first two weeks.
Future studies should expand the sample size, standardize inclusion criteria for cataract patients with or without type 2 diabetes, measure their blood glucose levels before surgery, and investigate other disease characteristics to reduce confounding factors and increase the number of preoperative tear collections and tear volumes for patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karalliedde J, United Kingdom; Meyhofer S, Germany S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 245] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 2. | Grzybowski A, Kanclerz P, Huerva V, Ascaso FJ, Tuuminen R. Diabetes and Phacoemulsification Cataract Surgery: Difficulties, Risks and Potential Complications. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Simpson RG, Moshirfar M, Edmonds JN, Christiansen SM. Laser in-situ keratomileusis in patients with diabetes mellitus: a review of the literature. Clin Ophthalmol. 2012;6:1665-1674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Yoo TK, Oh E. Diabetes mellitus is associated with dry eye syndrome: a meta-analysis. Int Ophthalmol. 2019;39:2611-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Jiang D, Xiao X, Fu T, Mashaghi A, Liu Q, Hong J. Transient Tear Film Dysfunction after Cataract Surgery in Diabetic Patients. PLoS One. 2016;11:e0146752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Gemensky-Metzler AJ, Sheahan JE, Rajala-Schultz PJ, Wilkie DA, Harrington J. Retrospective study of the prevalence of keratoconjunctivitis sicca in diabetic and nondiabetic dogs after phacoemulsification. Vet Ophthalmol. 2015;18:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1844] [Cited by in RCA: 2177] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 8. | Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 494] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44-46:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 505] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 10. | Sakimoto T, Shoji J, Yamada A, Sawa M. Upregulation of matrix metalloproteinase in tear fluid of patients with recurrent corneal erosion. Jpn J Ophthalmol. 2007;51:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 750] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 12. | Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1740] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 13. | Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 345] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med. 2013;71:174-187. [PubMed] |
| 15. | Sakimoto T, Sawa M. Metalloproteinases in corneal diseases: degradation and processing. Cornea. 2012;31 Suppl 1:S50-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Zhang W, Magadi S, Li Z, Smith CW, Burns AR. IL-20 promotes epithelial healing of the injured mouse cornea. Exp Eye Res. 2017;154:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Gao X, Hao L, Wang J, Ma G, Zhang T. Effect of Phacoemulsification Combined with Intraocular Lens Implantation on Inflammatory Factors, Oxidative Stress Response and Hemorheology in Diabetic Cataract Patients. J Coll Physicians Surg Pak. 2018;28:762-765. [PubMed] |