Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.342
Peer-review started: November 5, 2022
First decision: November 22, 2022
Revised: December 3, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 16, 2023
Processing time: 67 Days and 23.2 Hours
Endothelial activation plays an important role in sepsis-mediated inflammation, but the triggering factors have not been fully elucidated. Microvesicles carrying mitochondrial content (mitoMVs) have been implicated in several diseases and shown to induce endothelial activation.
To explore whether mitoMVs constitute a subset of MVs isolated from plasma of patients with sepsis and contribute to endothelial activation.
MVs were isolated from human plasma and characterized by confocal microscopy and flow cytometry. Proinflammatory cytokines, including interleukin (IL)-6, IL-8 and tumour necrosis factor (TNF)-α, and soluble vascular cell adhesion molecule (sVCAM)-1 were detected by ELISA. Human umbilical vein endothelial cells (HUVECs) were stimulated with the circulating MVs to evaluate their effect on endothelial activation.
MitoMVs were observed in plasma from patients with sepsis. Compared with those in healthy controls, expression of MVs, mitoMVs, proinflammatory cytokines and sVCAM-1 was increased. The number of mitoMVs was positively associated with TNF-α and sVCAM-1. In vitro, compared with MVs isolated from the plasma of healthy controls, MVs isolated from the plasma of patients with sepsis induced expression of OAS2, RSAD2, and CXCL10 in HUVECs. MitoMVs were taken up by HUVECs, and sonication of MVs significantly reduced the uptake of mitoMVs by HUVECs and expression of the above three type I IFN-dependent genes.
MitoMVs are increased in the plasma of patients with sepsis, which induces elevated expression of type I IFN-dependent genes. This suggests that circulating mitoMVs activate the type I IFN signalling pathway in endothelial cells and lead to endothelial activation.
Core Tip: Sepsis is a systemic inflammatory response syndrome that can lead to multiple organ dysfunction related to endothelial injury. Increased numbers of circulating microvesicles carrying mitochondrial content (mitoMVs) have been found in patients with systemic lupus erythematosus, which feature inflammation as the pathogenic mechanism. Mitochondrial damage-associated molecular patterns have been shown to induce endothelial activation. Therefore, the presence and function of mitoMVs in sepsis was studied. We found that mitoMVs were increased in plasma of patients with sepsis, and were related to inflammatory markers and induced elevated expression of type-I-IFN-dependent genes in endothelial cells.
- Citation: Zhang HJ, Li JY, Wang C, Zhong GQ. Microvesicles with mitochondrial content are increased in patients with sepsis and associated with inflammatory responses. World J Clin Cases 2023; 11(2): 342-356
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/342.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.342
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated systemic inflammatory response to infection[1], and is characterized by high mortality and substantial morbidity rates[2]. In this dysregulated systemic inflammatory response, endothelial cells (ECs) are increasingly believed to play a crucial role[3]. By shifting to a proinflammatory and pro-adhesive phenotype, activated ECs can amplify the inflammatory response. Moreover, due to the reduced production of NO, activated ECs can impair microcirculatory blood flow, leading to organ injury and even life-threatening organ failure. Therefore, better characterization of the molecular mechanisms of endothelial activation may have diagnostic and therapeutic value for this potentially fatal disease.
Microvesicles (MVs), also called microparticles, are extracellular vesicles that expose phosphati
MVs carrying mitochondrial content (mitoMVs) have been reported in LPS-stimulated monocytes[12]. In addition, increased numbers of circulating mitoMVs have been found in patients with systemic lupus erythematosus[8] and in mouse models of hepatic inflammation[13]. In particular, extracellular mitochondria and mitochondrial damage-associated molecular patterns (DAMPs) are identified as inducers of endothelial activation. On the other hand, sepsis changes the activity of mitochondria, which are essential intracellular regulators of the immune response[14,15]. Therefore, we aimed to test the hypothesis that mitoMVs constitute a subset of MVs isolated from the plasma of patients with sepsis and contribute to endothelial activation.
This study was approved by the Ethics Committee of the First Affiliated Hospital of University of South China. Patients who received medical intensive care for the treatment of sepsis from 2019 to 2020 were recruited. Informed consent was obtained before initiating the study. Adult patients (age 18–85 years) diagnosed with sepsis were recruited. The diagnosis relied on “The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)”[1]. The control group was composed of healthy volunteers who visited the physical examination centre of our hospital during the same period. Patients who were younger than 18 years or older than 85 years, took corticosteroids or immunosuppressive medications prior to arrival, or did not sign the informed consent form were excluded. Information on all subjects enrolled in the study, including their age, sex, medical history, white blood cell count, and some blood parameters, was collected and recorded at the first visit.
Peripheral venous blood samples were drawn and preserved according to previously published protocols. First, after the subjects had fasted for 8 h, whole blood was collected intravenously, but the first tube of blood was discarded. Serum blood tubes and 3.2% trisodium citrate anticoagulant tubes were used to collect the samples, which were placed in an upright position to prevent shaking. These samples were centrifuged at 2000 g for 10 min to prepare serum and platelet-rich plasma. Platelet-rich plasma was then centrifuged again at 2000 g for 10 min to prepare platelet-poor plasma (PPP). PPP and serum samples were stored at -80°C for later use.
Flow cytometry was used to identify and count the MVs of different surface molecules. PPP (250 μL) was mixed with 300 µL of PBS and centrifuged at 2000 g for 10 min at 20°C to remove apoptotic bodies and debris. The upper 500 μL of plasma was transferred to a new tube and centrifuged at room temperature for 30 min at 18000 g to concentrate the plasma MVs. The top 450 μL of plasma was removed, 500 µL PBS was added, and the MVs were recentrifuged. Finally, 400 µL of supernatant was removed, and the remaining 150 μL of MVs was divided into two parts. Fifty microlitres of the remaining MVs was transferred into a flow cytometry tube for the following experiments. The other 100 µL of MVs was stored at -80°C. For in vitro experiments, 2 mL PPP was centrifuged according to the above protocol; however, after the last centrifugation, almost all of the supernatant was discarded, and only 100 µL of the MV pellet was retained and stored at -80°C for later use.
For flow cytometry, to identify the total MV population and determine whether it contained mitochondrial content, a 50 μL MV suspension was incubated with the following fluorescent monoclonal antibodies: APC-labelled anti-tom22 (translocase of the outer mitochondrial membrane 22) (Cat. No. 130-107-698; Miltenyi Biotec, Germany) and FITC-labelled lactadherin (lot no. HH0430-1ML; Haematologic Technologies, United States). In addition, MitoTracker Deep Red (Cat. No. M22426; Invitrogen, Carlsbad, CA, United States) was used to assess the mitochondrial content. After incubation at room temperature for 15 min, FITC- and APC-conjugated isotype control antibodies were used as controls. Calibration beads (Cat. No. F13839; Thermo Fisher Scientific, United States) were used as a size reference. Finally, MVs were identified and counted using a BD Aria II flow cytometer (BD, United States). MV analysis was performed at the second gear flow rate, and light scattering and fluorescence were determined using a logarithmic model. Events that were 0.1-1.0 μm in diameter on FSC-SSC plots and emitted green fluorescence were defined as MVs. FlowJo (Version 7.6.1; BD) was used to obtain and analyse the data. The number of MVs per microlitre of plasma was calculated as previously described[16].
For confocal microscopy, MVs were labelled with MitoTracker Deep Red for 15 min and with lactadherin-FITC for 15 min, followed by washing and mounting on glass slides coated with 50% glycerin. The slides were observed under an LSM 880 confocal microscope (Carl Zeiss, Jena, Germany) in the Medical Instrument and Equipment Technology Laboratory of Hengyang medical college, University of South China.
ELISA was used to detect the serum expression levels of tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, C-reactive protein (CRP) and soluble vascular cell adhesion molecule (sVCAM)-1 (Thermo Fisher Scientific). ELISA plates were coated with the capture antibody at 100 µL/well and incubated overnight at 4°C. The next morning, serum samples were thawed and centrifuged at 1000 g for 15 min before use. Then, 100 µL of the diluted sample was added to the appropriate ELISA plate. The ELISA plate was again incubated overnight at 4°C for maximum sensitivity. On the third morning, 100 µL of the diluted detection antibody was added to each well, followed by incubation at room temperature for 1 h. Subsequently, 100 µL of diluted streptavidin–horseradish peroxidase was added to each well and incubated at room temperature for 30 min. Then, 1´ tetramethylbenzidine substrate solution was added at 100 µL/well and incubated at room temperature for 15 min. Finally, 100 µL of Stop Solution was added to each well, and absorbance was read at 450 nm.
Human umbilical vein endothelial cells (HUVECs) were maintained in endothelial cell medium (ECM, ScienCell, USA), comprising basal medium, 5% foetal bovine serum, 1% endothelial cell growth supplement, and 1% penicillin/streptomycin solution, at 37°C in a humidified 95%:5% (v/v) mixture of air and CO2. When HUVECs reached approximately 80% confluence, they were digested and seeded in a 12-well plate (Nest, Wuxi, China). Before treatment with MVs, the ECM was replaced with FBS-free culture medium, and the MVs were quantified by flow cytometry. The cells were treated with equal numbers of MVs from the plasma of healthy controls (MVcontrol) and MVs from the plasma of patients with sepsis (MVsepsis) for 8 h. Cell-free supernatants were collected for the measurement of IL-8 by ELISA. Total RNA was extracted from the cells, and quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to determine expression of IL-8, CXCR2, RSAD2 and OAS2. The primers for these genes are shown in Table 1.
| Gene | Forward primer | Reverse primer |
| IL-8 | CTCTTGGCAGCCTTCCTGATT | TATGCACTGACATCTAAGTTCTTTAGCA |
| OAS2 | ACGTGACATCCTCGATAAAACTG | GAACCCATCAAGGGACTTCTG |
| RSAD2 | TGAGGTTCTGCAAAGTAGAGTT | GCGAGAATGTCCAAATACTCAC |
| CXCL10 | GTGGCATTCAAGGAGTACCTC | TGATGGCCTTCGATTCTGGATT |
MVs were resuspended and diluted in ECM to the desired concentrations. Sonication of the samples was performed using an ultrasonic processor (VCX130, United States) at 100% power and 6´ 30-s sonication rounds at 1-min intervals.
RNA was isolated from cells by using TRIzol™ Reagent (Cat. No. 15596026; Thermo Fisher Scientific), and cDNA synthesis was performed using a RevertAid First Strand cDNA Synthesis Kit (Cat. No. K1622; Thermo Fisher Scientific). The CFX96 system (Bio-Rad Laboratories) and TB Green® Premix Ex Taq™ II (Cat. No. RR820A; Takara, Tokyo, Japan) were used for real-time PCR-based quantifications. The expression levels of the mRNAs of interest were determined using the delta Ct method and normalized to the GAPDH mRNA level.
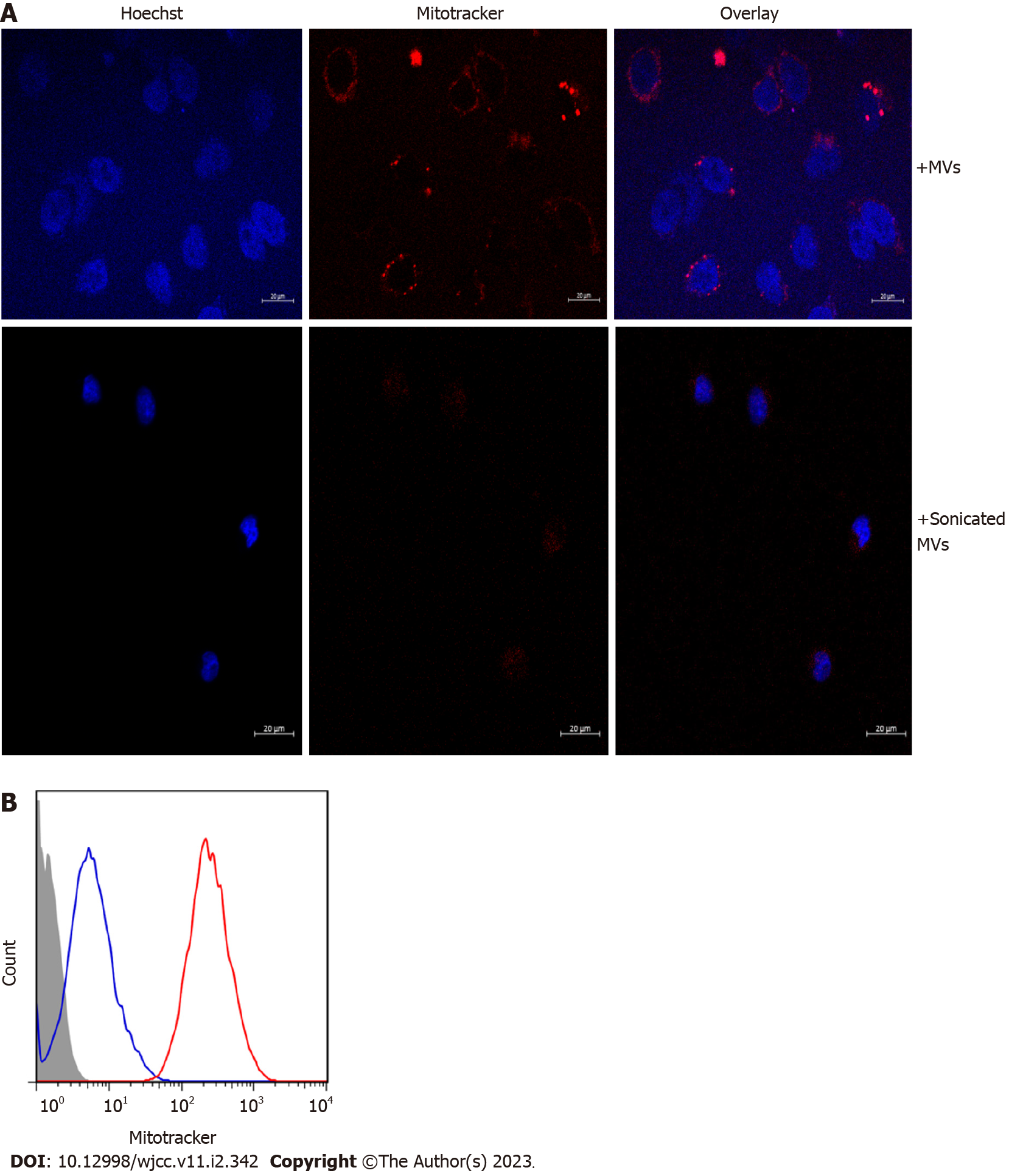
For flow cytometry, HUVECs were seeded in 12-well plates on 12-mm coverslips (Cat. No. 1254580; Fisher). MVs isolated from patients with sepsis were labelled with MitoTracker Deep Red and centrifuged at room temperature for 30 min at 18000 x g. The MVs were washed with PBS and recentrifuged. Before incubation of HUVECs with MVs, the cell culture medium was replaced with fresh FBS-free medium, and the cells were starved for 2 h. MVs were suspended in the same FBS-free medium supplemented with 200 g/mL bovine serum albumin and added to the HUVECs for 2 h at 37°C. Afterwards, the cell supernatant was discarded, and the HUVECs were washed with warm PBS. Subsequently, the coverslips were placed in a single-well Petri dish and processed separately, and the remaining HUVECs in the 12-well plates were detached by trypsinization and fixed with 2% paraformaldehyde (PFA) for flow cytometry.
For immunofluorescence confocal microscopy, HUVECs growing on coverslips were directly fixed with 4% PFA in PBS (pH 7.4) for 15 min at 37°C, quenched with 150 mmol/L Tris pH 8.0 for 5 min, and washed three times with PBS. Nuclei were stained with Hoechst (10 mmol/L in PBS) for 3 min. Coverslips were mounted on concave microscope slides coated with PBS and sealed, and images were acquired on a Zeiss LSM 880 confocal microscope. MitoTracker-labelled MVs were excited with a 543 nm laser and nuclei were excited with a 405 nm laser.
SPSS version 26.0 was used for statistical analysis. Before statistical analyses, data were tested for normal distribution by the Shapiro–Wilk test. Data that fitted the assumption of normal distribution were presented as the mean ± SEM, and Student’s t test was used to compare data from two groups. Non-normally distributed data were presented as the median (first to third interquartile range) and analysed by the Kruskal–Wallis test. Categorical data were analysed by the χ2 test, followed by post hoc Wilcoxon signed-rank test. If the prediction frequency was < 5, Fisher’s exact test was used. Correlations between MVs and cytokines were evaluated with scatter plots and Spearman rank correlation coefficients. P < 0.05 was considered significant. aP < 0.05, bP < 0.01.
The demographic parameters of the patients with sepsis and healthy controls are shown in Table 2. During the study period, on the basis of the inclusion and exclusion criteria, 19 patients with sepsis and 20 control volunteers were finally included. No differences in age, sex, or history of hypertension or diabetes mellitus were observed between the two groups. However, the white blood cell count was significantly higher in the sepsis group (P < 0.001). Microbiological tests were carried out for all patients with sepsis, revealing 13 (68.4%) Gram-negative bacterial infections and 3 (15.8%) Gram-positive infections. In terms of the source of infection, the most common sites of original infection in the sepsis group were the lungs and urine, followed by the abdomen and bloodstream.
| Control (n = 20) | Sepsis (n = 19) | Psepsis vs control | |
| Characteristics | |||
| Agea | 62.5 ± 9.8 | 58.13 ± 9.69 | 0.346 |
| Male sexc | 11 | 7 | 0.256 |
| Hypertensionc | 4 | 6 | 0.480 |
| DMc | 1 | 2 | 0.605 |
| WBC, 109/Lb | 6.18 ± 1.57 | 14.19 ± 8.02 | < 0.001 |
| Site of infection | |||
| Pneumonia | NA | 6 | |
| Urinary | NA | 6 | |
| Abdominal | NA | 5 | |
| Bacteraemia | NA | 2 | |
| Unknown | NA | 3 | |
| Microbial data | |||
| Gram positive | NA | 3 | |
| Gram negative | NA | 13 | |
| Fungi | NA | 1 | |
| Mixed | NA | 2 | |
| Unknown | NA | 4 | |
| Inflammation markers | |||
| TNF-α (pg/mL)b | 6.90 (4.94-9.45) | 26.10 (17.56-35.02) | < 0.001 |
| IL-6 (pg/mL)b | 7.33 (4.68-10.33) | 61.25 (37.54-87.30) | < 0.001 |
| IL-8 (pg/mL)b | 7.30 (5.11–9.00) | 19.59 (14.04-62.19) | < 0.001 |
| CRP (ng/mL)b | 158.62 (67.75-246.42) | 445.07 (382.31-549.73) | < 0.001 |
| sVCAM-1 (ng/mL)b | 89.22 (72.09-99.67) | 164.68 (134.15-198.55) | < 0.001 |
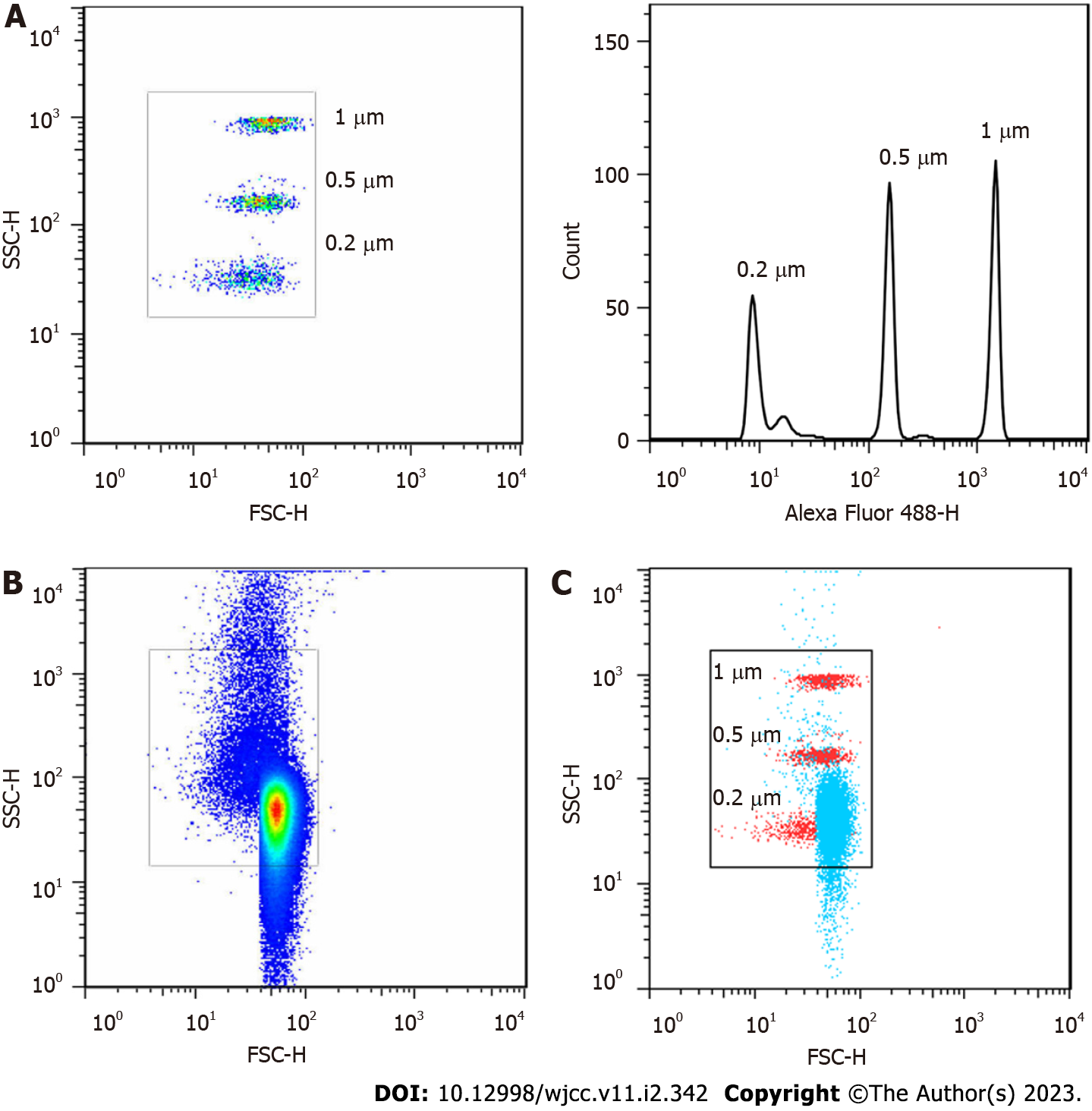
Because MVs are membrane vesicles with diameters ranging from 0.1 to 1 µm, 0.2 µm, 0.5 µm and 1 µm particle standards were used to optimize the flow cytometer before circulating MVs were analysed by flow cytometry. The Aria II flow cytometer with a logical display was capable of distinguishing the above standards (Figure 1A). Most of the MVs separated from plasma were within our delineated range (Figure 1B), and the number of the circulating MV peaks appeared between 0.2 and 0.5 µm (Figure 1C). This result was similar to a previous report[17], indicating that our centrifugation and detection protocol was feasible.
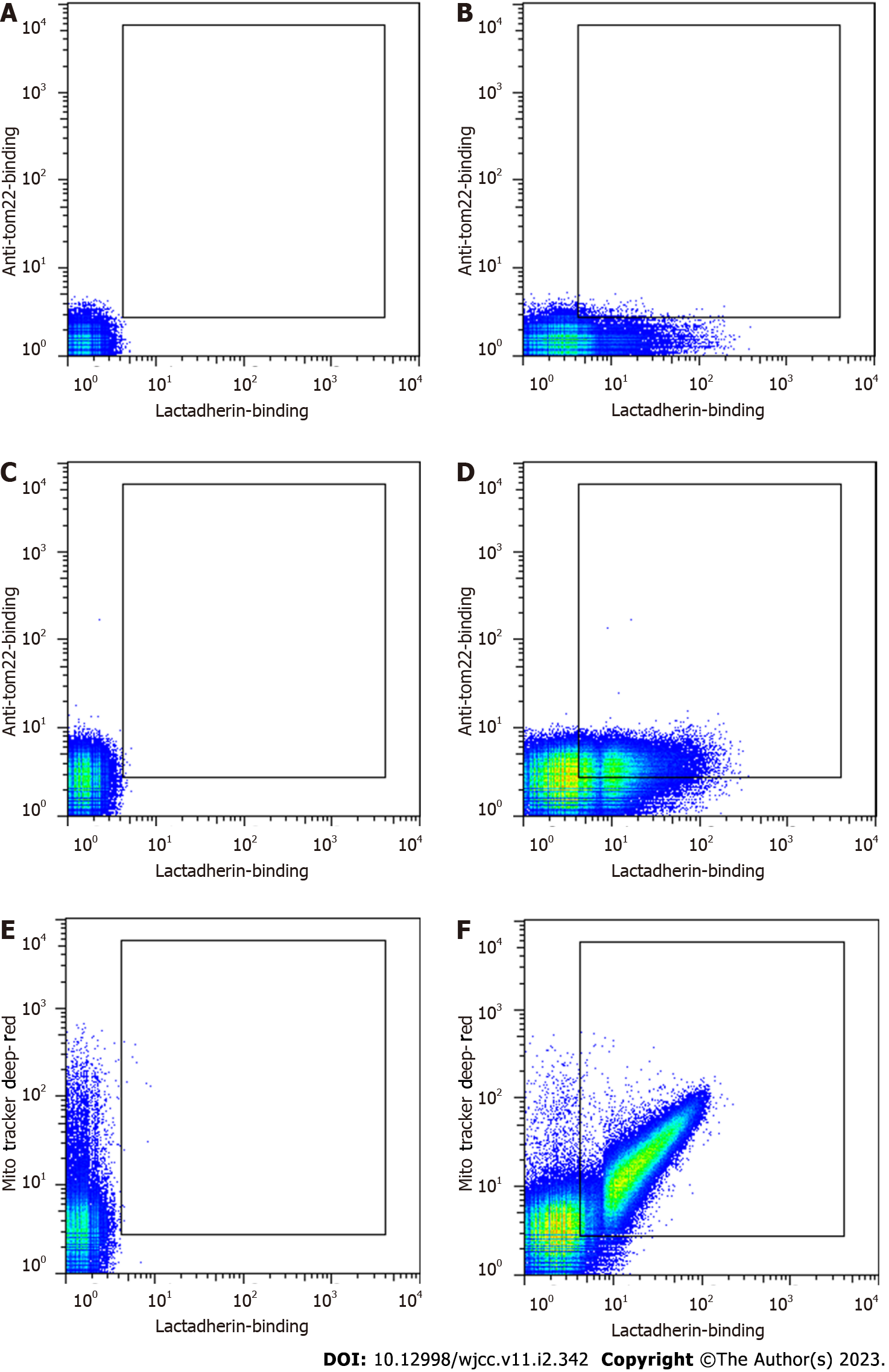
Confocal scanning microscopy and flow cytometry were used to examine whether the MVs isolated from the plasma of patients with sepsis were rich in mitochondrial content. The binding of lactadherin to PS was used to distinguish MVs from events caused by noise or exosomes. MitoTracker Deep Red and APC-anti-tom22 were used to indicate whether the MVs contained mitochondrial components.
Bright field microscopy revealed that the MVs were approximately elliptical in shape and of different sizes (Supplementary Figure 1). Upon laser excitation at wavelengths suitable for FITC and Mito
To correctly identify mitoMVs, we determined a flow cytometry threshold with a blank MV sample to correct for intrinsic autofluorescence (Figure 2A). To correct for spectral overlap, we also incubated the MV samples with lactadherin-FITC, anti-tom22-APC or MitoTracker Deep Red to establish thresholds. These thresholds are shown in Figure 2B, C and E. The MVs from septic patients stained positive for both lactadherin and tom22 (Figure 2D) and for both lactadherin and MitoTracker (Figure 2F), confirming the presence of mitochondrial components.
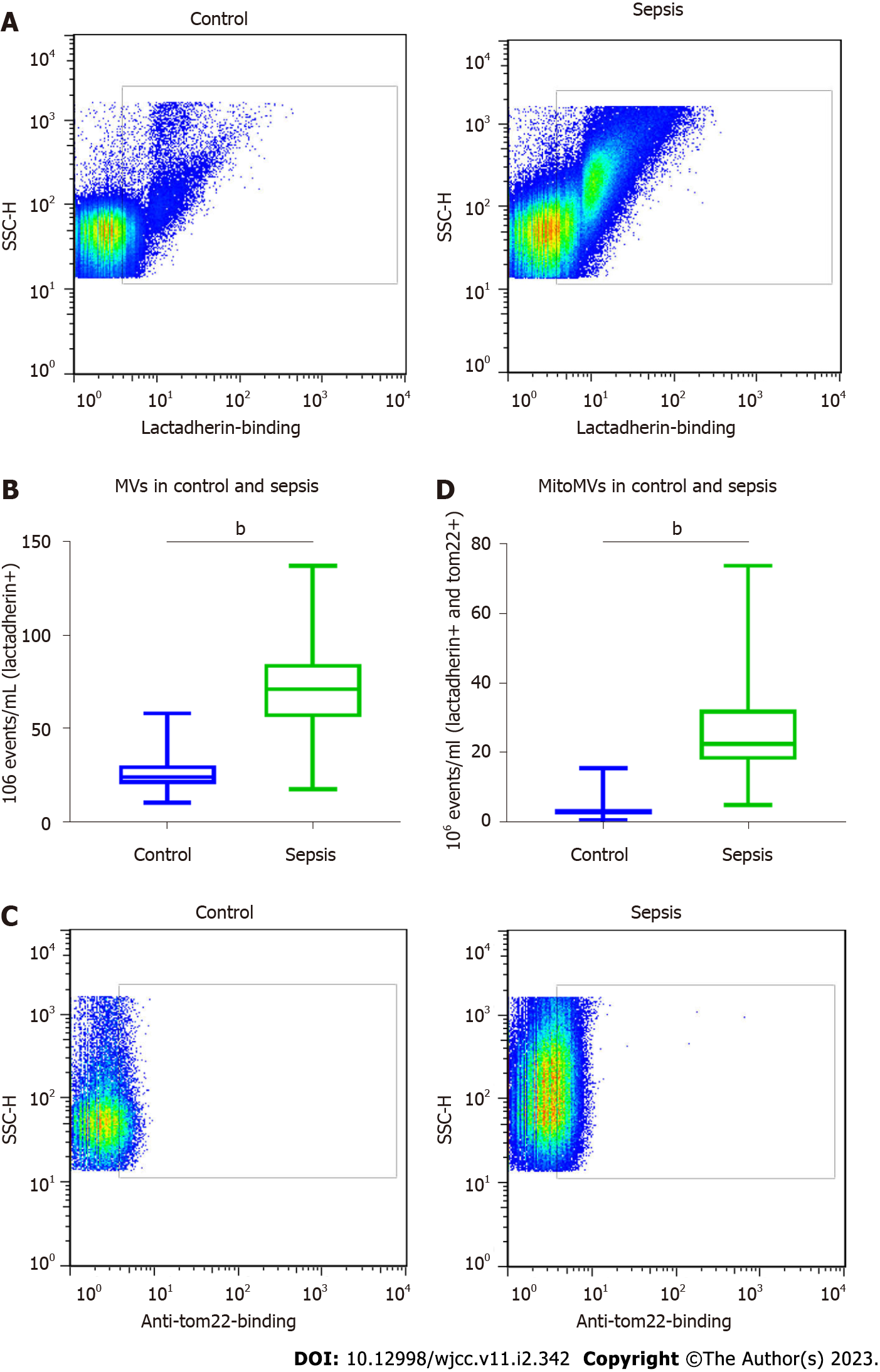
We determined the numbers of MVs and mitoMVs in plasma samples from the patients with sepsis and controls. The number of MVs in the healthy controls was 23.72 (20.10–30.21) × 106 events/μL, whereas that in the sepsis group was 73.27 (64.08–84.49) × 106 events/μL. These numbers were significantly different between the two groups (P < 0.001; Figure 3A and B). We analysed the number of mitoMVs in the healthy control and sepsis groups. The number of mitoMVs in the healthy control group was 3.12 (2.16–3.82) × 106 events/μL, and that in the sepsis group was 22.53 (17.78–32.29) × 106 events/μL. These values also suggested significant differences between the two groups (P < 0.001; Figure 3C and D, respectively). Regarding cytokines, the plasma levels of the endothelial activation marker sVCAM-1 and the inflammatory markers IL-8, IL-6, TNF-α and CRP were dramatically increased in patients with sepsis after their admittance to intensive care units and diagnosis compared with the healthy control group, and the difference was significant (P < 0.001; Table 1). These data suggest that, in the plasma of patients with sepsis, the increase in the number of mitoMVs was more significant than the increase in the number of MVs and there were endothelial dysfunction and severe inflammatory response in patients with sepsis.
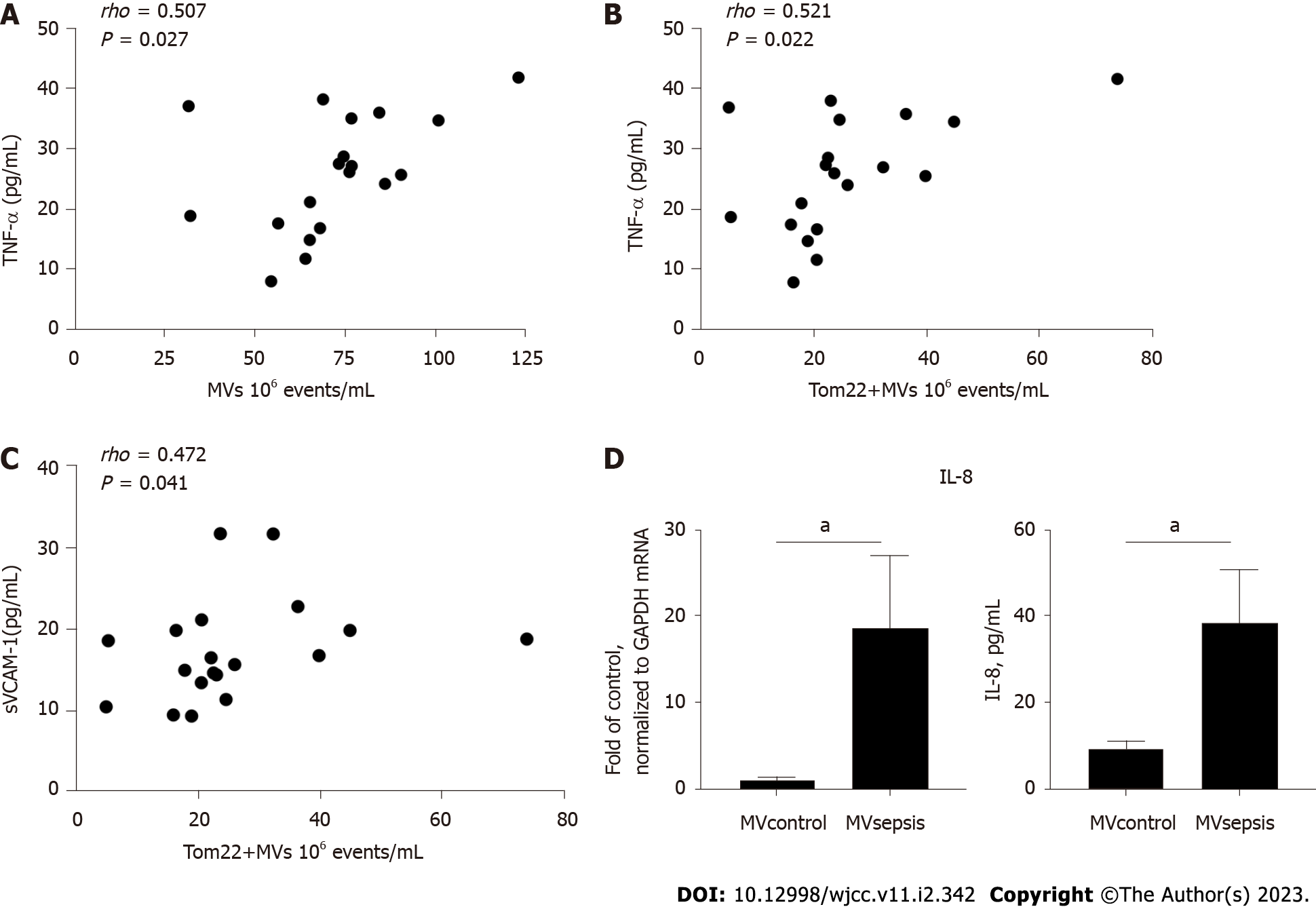
Because MVs and mitochondrial components are related to endothelial dysfunction[18] and inflammation[19], we analysed the relationships among MVs, mitoMVs and cytokines in sepsis. In the sepsis group, the number of mitoMVs was related to expression of TNF-α (P = 0.022, r = 0.521; Figure 4B, respectively) and to expression of sVCAM-1 (P = 0.041, r = 0.472; Figure 4C, respectively). Second, expression of MVs was related to expression of TNF-α (P = 0.027, r = 0.507; Figure 4A), but the correlation coefficient was lower than that of mitoMVs and TNF-α. Finally, we did not find IL-6, IL-8 or CRP to be correlated with MVs or mitoMVs despite performing the same correlation analysis.
Since TNF-α is a strong proinflammatory factor and sVCAM-1 is a marker of endothelial activation, the above results indicate that MVsepsis may mediate the sepsis-induced inflammatory response through the endothelium. Therefore, we isolated MVs from the plasma of patients with sepsis and healthy controls and applied them to HUVECs in vitro. Compared with MVcontrol, MVsepsis induced an increase in IL-8 mRNA expression in HUVECs. In the HUVEC supernatant, IL-8 expression in the MVsepsis treatment group was significantly higher than that in the MVcontrol treatment group (P < 0.05, Figure 4D). This indicates that MVsepsis are able to activate ECs.
Given that MVs exert their biological effects by transferring their cargo to recipient cells[20,21], confocal microscopy was used to explore whether the mitochondrial content in MVsepsis could be taken up by HUVECs. Incubation of MVsepsis with HUVECs resulted in accumulation of MitoTracker-positive MVs around the nucleus and in the significantly increased red fluorescence intensity of HUVECs, which indicated that mitochondrial content of MVsepsis was transferred to HUVECs (Figure 5).
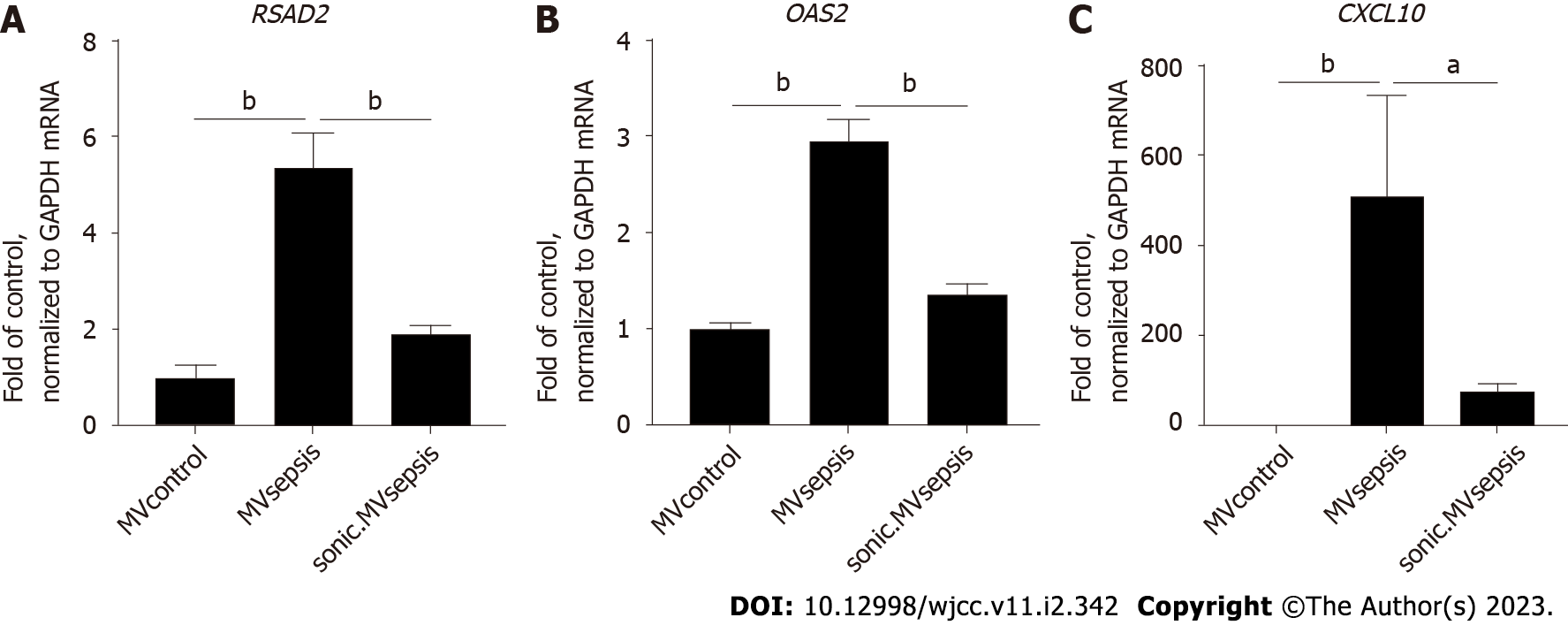
Since several components of mitochondrial DAMPs have the capacity to induce activation of type I IFN response[22], we determined the mRNA expression levels of CXCL10, RSAD2 and OAS2, all of which are representative of the type I IFN signalling pathway, in HUVECs stimulated by circulating MVs. qRT-PCR showed that, compared with MVcontrol, MVsepsis significantly promoted HUVEC expression of the above genes (P < 0.01, Figure 6). Among these genes, CXCL10 expression was increased to the greatest extent (approximately 500-fold). In addition, the treatment of MVsepsis by sonication and reincubation with HUVECs significantly reduced the entry of MitoTracker-labelled MVsepsis into HUVECs (Figure 5) and reduced expression of RSAD2, OAS2 and CXCL10 (Figure 6). This result indicates that the mitochondrial content of MVsepsis is an important factor driving type I IFN signal activation.
In this study, we demonstrated that MVsepsis were abundant in mitochondrial content and that the levels of MVs and mitoMVs were significantly higher in patients with sepsis than in the healthy controls. MitoMVs were significantly correlated with TNF-α and sVCAM-1. MVsepsis, in particular, have the capacity to increase expression of type I IFN pathway members in HUVECs. This capacity was significantly reduced after destruction of mitoMVs by sonication. Collectively, mitoMVs constitute a subset of MVsepsis and may contribute to endothelial activation.
Mitochondria play important roles in energy metabolism, cell signal transduction, and apoptosis regulation. Therefore, mitochondria are one of the most easily affected cellular organelles in disease-induced dysfunction[23]. Therefore, characterizing mitochondria has increasingly become an important method to explore the pathogenesis of related diseases[24]. The most commonly used mediators are mitochondrial fluorescent probes, such as MitoTracker Deep Red, and antibodies against tom complexes, such as anti-tom22[25]. The former is chemically reactive and linked to thiol groups in the mitochondria[26]. Therefore, it is not affected by the mitochondrial membrane potential and is highly sensitive. The latter reacts with the tom complex via antigen–antibody interactions, which is specific and potentially indicates that mitochondrial proteins are present in an immunologically accessible form. In this study, the above two methods were used to label the mitochondrial content in MVs, and the results were positive. This provides reliable evidence that MVs isolated from human plasma carry mito
Mitochondrial dysfunction contributes to several inflammatory diseases, ranging from sepsis[27] to rheumatoid arthritis[28]. Mitochondrial DAMPs, which are released by damaged mitochondria, have the ability to directly activate inflammatory responses[29]. In particular, Irene et al[30] showed that the MVs isolated from the sera of children with autism spectrum disorder not only contained mtDNA, one type of mitochondrial DAMP, but could also stimulate human microglia to secrete IL-1β. Similarly, we found mitoMVs. The expression of mitoMVs in patients with sepsis was significantly higher than that of the control group. Compared with the increase in MVs in the control group, the increase in mitoMVs in the sepsis group was more marked. This demonstrates that mitoMVs indicate the presence of sepsis and may participate in the immune response induced by sepsis.
Previous studies have shown that the levels of various cytokines are elevated in sepsis[31,32] and can be used as markers of inflammation in sepsis[33]. Therefore, ELISA was used to detect expression of CRP, IL-6, IL-8 and TNF-α in the sera of patients with sepsis. Compared with those in the healthy control group, expression of the above inflammatory factors in the sepsis group were significantly higher. This confirms activation of the bodily inflammatory response in patients with sepsis. Correlation of MVs and mitoMVs with the above cytokines was analysed. MVs and mitoMVs were correlated with expression of TNF-α and mitoMVs were correlated with expression of sVCAM-1. This indicates that MVsepsis are potentially involved in sepsis-mediated immune activation and may mediate the immune response through endothelial activation. TNF-α is a strong proinflammatory cytokine[34] that participates in oedema formation, leukocyte adhesion to vascular ECs via the expression of adhesion molecules, and promotion of oxidative stress at sites of inflammation, and VCAM-1 regulates inflammation-associated vascular adhesion and serves as a marker of endothelial activation[35].
MVs are the medium of information transmission between cells, and many studies have shown that MVs produced under pathological conditions have the ability to induce activation of recipient cells[18,36]. Therefore, based on the above inference, we explored the effect of plasma MVs on ECs in vitro. ELISA and qRT-PCR showed that compared with MVcontrol, MVsepsis increased expression of IL-8 in HUVECs. This result is similar to the findings of Hosseinkhani et al[37] that indicated that MVsepsis induced activation of ECs.
The type I IFN signalling pathway is related to inflammation[38] and is a manifestation of endothelial activation[39]. Several components of mitochondrial DAMPs can induce activation of type I IFN signals. In particular, increasing evidence shows that delivering MV cargo to recipient cells is an important way for MVs to exert their biological effects[21,40]. Therefore, the uptake of MVsepsis mitochondrial content and its ability to induce activation of type I IFN signals in HUVECs were investigated. MitoMVs were taken up by HUVECs, which accounted for the mechanism by which MVsepsis increased expression of type-I-IFN-dependent genes in HUVECs. This is similar to the study of Puhm et al[41]. They stimulated monocytes to produce mitoMVs via lipopolysaccharides, which are characteristic components of the Gram-negative bacterial cell wall, and these mitoMVs induced activation of type I IFN signalling in ECs. This study, together with our results, suggests that mitoMVs may be an effective target in sepsis-induced endothelial activation and for the treatment of sepsis in future. ECs with activated type I IFN signals have been shown to secrete adhesion molecules[42] and chemokines[43], which can amplify the activation and damage of ECs by inducing the adherence of monocytes[44] and neutrophils[45] to ECs. Many studies have shown a role for type I IFN signalling in vascular abnormalities associated with impaired blood vessel dilation. In particular, the latest research shows that type I IFN signals also mediate abnormal blood clotting induced by septic bacterial infection[46]. Finally, our results may also indicate that mitoMVs are not the only factor driving the elevated expression of type-I-IFN-dependent genes. Compared with MVcontrol, MVsepsis subjected to sonication still slightly promoted the expression of type I IFN signalling genes. Caielli et al[47] showed that mtDNA in its free form[48], which may not be completely eliminated during the process of MV isolation, can induce the activation of type I IFN signalling.
This study had some limitations. First, expression of genes that are representative of type I IFN signalling in HUVECs should be further assessed at the protein level. Second, human microvascular endothelial cells, which are more relevant to the pathophysiology of sepsis than HUVECs, are not used to perform the ex vivo studies. Finally, since sonication does not only disrupt mitochondria, it is possible that other mitoMV components or nonmitochondrial contents induce type I IFN responses.
MitoMVs were increased in the plasma of patients with sepsis when compared with the healthy control group. The number of mitoMVs was correlated with inflammatory and endothelial activation markers. Moreover, mitoMVs from patients with sepsis induced expression of type-I-IFN-dependent genes in ECs. Therefore, circulating mitoMVs may have potential as a novel intervention target for sepsis-induced endothelial activation.
Sepsis is a life-threatening complication of infection that involves endothelial injury that contributes to multiple organ failure. Microvesicles (MVs), which can transfer nucleic acids, organelles and proteins to target cells, are recognized as important mediators in intercellular communication.
MVs carrying mitochondrial content (mitoMVs) have been implicated in several diseases. However, it is not clear whether mitoMVs are involved in sepsis.
To explore whether mitoMVs constitute a subset of MVs isolated from plasma of patients with sepsis and contribute to endothelial activation.
Confocal microscopy and flow cytometry were used to characterize the presence of mitoMVs and their expression in plasma. Human umbilical vein endothelial cells were stimulated with circulating MVs to evaluate their effect on endothelial activation.
MVs isolated from patients with sepsis were rich in mitochondrial content and the levels of MVs and mitoMVs were significantly higher in patients with sepsis than in healthy controls. The number of mitoMVs was positively associated with tumour necrosis factor-α and soluble vascular cell adhesion molecule 1. MitoMVs isolated from the plasma of patients with sepsis induced elevated expression of type-I-IFN-dependent genes in endothelial cells.
MitoMVs were increased in the plasma of patients with sepsis and may activate the type I IFN signalling pathway in endothelial cells.
MitoMVs may have potential as a novel interventional target for sepsis-induced endothelial injury.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal; Jovandaric M, Serbia; Rodrigues AT, Brazil S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17095] [Article Influence: 1899.4] [Reference Citation Analysis (2)] |
| 2. | Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, Jimenez E, Sakr Y; ICON investigators. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 836] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 3. | Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial Responses in Sepsis. Am J Respir Crit Care Med. 2020;202:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 4. | Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular Vesicles in Angiogenesis. Circ Res. 2017;120:1658-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 463] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 5. | Zacharia E, Antonopoulos AS, Oikonomou E, Papageorgiou N, Pallantza Z, Miliou A, Mystakidi VC, Simantiris S, Kriebardis A, Orologas N, Valasiadi E, Papaioannou S, Galiatsatos N, Antoniades C, Tousoulis D. Plasma signature of apoptotic microvesicles is associated with endothelial dysfunction and plaque rupture in acute coronary syndromes. J Mol Cell Cardiol. 2020;138:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Gkaliagkousi E, Nikolaidou B, Gavriilaki E, Lazaridis A, Yiannaki E, Anyfanti P, Zografou I, Markala D, Douma S. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diab Vasc Dis Res. 2019;16:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Chen HP, Wang XY, Pan XY, Hu WW, Cai ST, Joshi K, Deng LH, Ma D. Circulating Neutrophil-Derived Microparticles Associated with the Prognosis of Patients with Sepsis. J Inflamm Res. 2020;13:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Mobarrez F, Fuzzi E, Gunnarsson I, Larsson A, Eketjäll S, Pisetsky DS, Svenungsson E. Microparticles in the blood of patients with SLE: Size, content of mitochondria and role in circulating immune complexes. J Autoimmun. 2019;102:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Voukalis C, Shantsila E, Lip GYH. Microparticles and cardiovascular diseases. Ann Med. 2019;51:193-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, Mackman N. Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner. Blood. 2011;118:2366-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Mostefai HA, Meziani F, Mastronardi ML, Agouni A, Heymes C, Sargentini C, Asfar P, Martinez MC, Andriantsitohaina R. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am J Respir Crit Care Med. 2008;178:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Bernimoulin M, Waters EK, Foy M, Steele BM, Sullivan M, Falet H, Walsh MT, Barteneva N, Geng JG, Hartwig JH, Maguire PB, Wagner DD. Differential stimulation of monocytic cells results in distinct populations of microparticles. J Thromb Haemost. 2009;7:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 14. | Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O'Neill LA. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167:457-470.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1311] [Cited by in RCA: 1586] [Article Influence: 176.2] [Reference Citation Analysis (0)] |
| 15. | O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1864] [Cited by in RCA: 2092] [Article Influence: 232.4] [Reference Citation Analysis (0)] |
| 16. | Chiva-Blanch G, Crespo J, Suades R, Arderiu G, Padro T, Vilahur G, Cubedo J, Corella D, Salas-Salvadó J, Arós F, Martínez-González MA, Ros E, Fitó M, Estruch R, Badimon L. CD142+/CD61+, CD146+ and CD45+ microparticles predict cardiovascular events in high risk patients following a Mediterranean diet supplemented with nuts. Thromb Haemost. 2016;116:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Xu R, Greening DW, Rai A, Ji H, Simpson RJ. Highly-purified exosomes and shed microvesicles isolated from the human colon cancer cell line LIM1863 by sequential centrifugal ultrafiltration are biochemically and functionally distinct. Methods. 2015;87:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | Li B, Huang Q, Lin C, Lu R, Wang T, Chen X, Liu Z, Liu Y, Wu J, Wu Y, Liao S, Ding X. Increased circulating CD31+/CD42b-EMPs in Perthes disease and inhibit HUVECs angiogenesis via endothelial dysfunction. Life Sci. 2021;265:118749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 526] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 20. | Xu J, Feng Y, Jeyaram A, Jay SM, Zou L, Chao W. Circulating Plasma Extracellular Vesicles from Septic Mice Induce Inflammation via MicroRNA- and TLR7-Dependent Mechanisms. J Immunol. 2018;201:3392-3400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 21. | Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L, Hedin U, Maegdefessel L, Fish JE, Rayner KJ. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler Thromb Vasc Biol. 2018;38:49-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 22. | Li S, Hu Q, Huang J, Wu X, Ren J. Mitochondria-Derived Damage-Associated Molecular Patterns in Sepsis: From Bench to Bedside. Oxid Med Cell Longev. 2019;2019:6914849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Coppotelli G, Ross JM. Mitochondria in Ageing and Diseases: The Super Trouper of the Cell. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 2462] [Article Influence: 189.4] [Reference Citation Analysis (0)] |
| 25. | Taylor TH, Frost NW, Bowser MT, Arriaga EA. Analysis of individual mitochondria via fluorescent immunolabeling with Anti-TOM22 antibodies. Anal Bioanal Chem. 2014;406:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Chazotte B. Labeling mitochondria with MitoTracker dyes. Cold Spring Harb Protoc. 2011;2011:990-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Mantzarlis K, Tsolaki V, Zakynthinos E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid Med Cell Longev. 2017;2017:5985209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 28. | Fearon U, Canavan M, Biniecka M, Veale DJ. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:385-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 290] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 29. | Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2990] [Cited by in RCA: 2795] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 30. | Tsilioni I, Theoharides TC. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J Neuroinflammation. 2018;15:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Baghel K, Srivastava RN, Chandra A, Goel SK, Agrawal J, Kazmi HR, Raj S. TNF-α, IL-6, and IL-8 cytokines and their association with TNF-α-308 G/A polymorphism and postoperative sepsis. J Gastrointest Surg. 2014;18:1486-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Jekarl DW, Kim JY, Lee S, Kim M, Kim Y, Han K, Woo SH, Lee WJ. Diagnosis and evaluation of severity of sepsis via the use of biomarkers and profiles of 13 cytokines: a multiplex analysis. Clin Chem Lab Med. 2015;53:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 857] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 34. | Zelová H, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013;62:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 594] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 35. | Kong DH, Kim YK, Kim MR, Jang JH, Lee S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 452] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 36. | Qiu Q, Dan X, Yang C, Hardy P, Yang Z, Liu G, Xiong W. Increased airway T lymphocyte microparticles in chronic obstructive pulmonary disease induces airway epithelial injury. Life Sci. 2020;261:118357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Hosseinkhani B, Kuypers S, van den Akker NMS, Molin DGM, Michiels L. Extracellular Vesicles Work as a Functional Inflammatory Mediator Between Vascular Endothelial Cells and Immune Cells. Front Immunol. 2018;9:1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 38. | Chen K, Liu J, Cao X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J Autoimmun. 2017;83:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 39. | Jones Buie JN, Oates JC. Role of interferon alpha in endothelial dysfunction: insights into endothelial nitric oxide synthase-related mechanisms. Am J Med Sci. 2014;348:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, Krasnodembskaya AD. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017;196:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 562] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 41. | Puhm F, Afonyushkin T, Resch U, Obermayer G, Rohde M, Penz T, Schuster M, Wagner G, Rendeiro AF, Melki I, Kaun C, Wojta J, Bock C, Jilma B, Mackman N, Boilard E, Binder CJ. Mitochondria Are a Subset of Extracellular Vesicles Released by Activated Monocytes and Induce Type I IFN and TNF Responses in Endothelial Cells. Circ Res. 2019;125:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 42. | Kobayashi T, Takaku Y, Yokote A, Miyazawa H, Soma T, Hagiwara K, Kanazawa M, Nagata M. Interferon-beta augments eosinophil adhesion-inducing activity of endothelial cells. Eur Respir J. 2008;32:1540-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Nakano M, Fujii T, Hashimoto M, Yukawa N, Yoshifuji H, Ohmura K, Nakaizumi A, Mimori T. Type I interferon induces CX3CL1 (fractalkine) and CCL5 (RANTES) production in human pulmonary vascular endothelial cells. Clin Exp Immunol. 2012;170:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Venkatesh D, Ernandez T, Rosetti F, Batal I, Cullere X, Luscinskas FW, Zhang Y, Stavrakis G, García-Cardeña G, Horwitz BH, Mayadas TN. Endothelial TNF receptor 2 induces IRF1 transcription factor-dependent interferon-β autocrine signaling to promote monocyte recruitment. Immunity. 2013;38:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Buie JJ, Renaud LL, Muise-Helmericks R, Oates JC. IFN-α Negatively Regulates the Expression of Endothelial Nitric Oxide Synthase and Nitric Oxide Production: Implications for Systemic Lupus Erythematosus. J Immunol. 2017;199:1979-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Yang X, Cheng X, Tang Y, Qiu X, Wang Z, Fu G, Wu J, Kang H, Wang J, Wang H, Chen F, Xiao X, Billiar TR, Lu B. The role of type 1 interferons in coagulation induced by gram-negative bacteria. Blood. 2020;135:1087-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 47. | Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R, Baisch J, Phelps K, Clayton S, Gong M, Wright T, Punaro M, Palucka K, Guiducci C, Banchereau J, Pascual V. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med. 2016;213:697-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 48. | Faust HE, Reilly JP, Anderson BJ, Ittner CAG, Forker CM, Zhang P, Weaver BA, Holena DN, Lanken PN, Christie JD, Meyer NJ, Mangalmurti NS, Shashaty MGS. Plasma Mitochondrial DNA Levels Are Associated With ARDS in Trauma and Sepsis Patients. Chest. 2020;157:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |