Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4723
Peer-review started: April 13, 2023
First decision: May 8, 2023
Revised: May 10, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: July 6, 2023
Processing time: 78 Days and 3.4 Hours
Posterior reversible encephalopathy syndrome (PRES) is characterized mainly by occipital and parietal lobe involvement, which can be reversible within a few days. Herein, we report a rare case of PRES that developed after craniotomy for an unruptured intracranial aneurysm (UIA).
A 59-year-old man underwent clipping surgery for the treatment of UIA arising from the left middle cerebral artery. Clipping surgery was performed unevent
Our unique case highlights that, to our knowledge, this is the second report of PRES developing after craniotomy for the treatment of UIA. Surgeons must keep PRES in mind as one of the causes of perioperative neurological abnormality following clipping of an UIA.
Core Tip: Posterior reversible encephalopathy syndrome (PRES) is a clinicoradiological syndrome characterized by predominant parietal and occipital involvement, which can be reversible within a few days. We report a rare case of PRES that developed after clipping surgery for an unruptured intracranial aneurysm (UIA). In our case, the important causative factor of PRES was suspected to be a sudden increase in cerebral perfusion pressure associated with temporary M1 occlusion. Our unique case highlights that, to our knowledge, this is the second report of PRES developing after craniotomy for the treatment of UIA. Surgeons must keep PRES in mind as one of the causes of perioperative neurological abnormality following clipping of an UIA.
- Citation: Hwang J, Cho WH, Cha SH, Ko JK. Posterior reversible encephalopathy syndrome following uneventful clipping of an unruptured intracranial aneurysm: A case report. World J Clin Cases 2023; 11(19): 4723-4728
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4723.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4723
Posterior reversible encephalopathy syndrome (PRES) is a clinical and radiological entity in which reversible changes occur in the central nervous system (CNS), associated with typical features on magnetic resonance imaging (MRI)[1]. The main symptoms of PRES include insidious onset of headache, altered mentality, seizures, and cortical blindness, with edematous changes primarily in the occipital cortex and/or white matter bilaterally on radiological imaging[1,2]. The term ‘PRES’ was first introduced in 1996 by Hinchey and his colleagues[3]. It has been mainly described in relation to the hypertensive crisis—particularly in the setting of renal failure, sepsis, eclampsia, and the use of immunosuppressant drugs, such as calcineurin inhibitors[4]. Since then, many reports have focused on the imaging findings and pathophysiology of this condition. Although various underlying conditions of PRES have been reported, to the best of our knowledge, only one case of PRES that developed after clipping surgery for the treatment of an unruptured intracranial aneurysm (UIA) has been reported[5]. Here, we would like to report the second case of PRES.
Sudden deterioration of consciousness developed at the 4th hour after surgical clipping of an UIA.
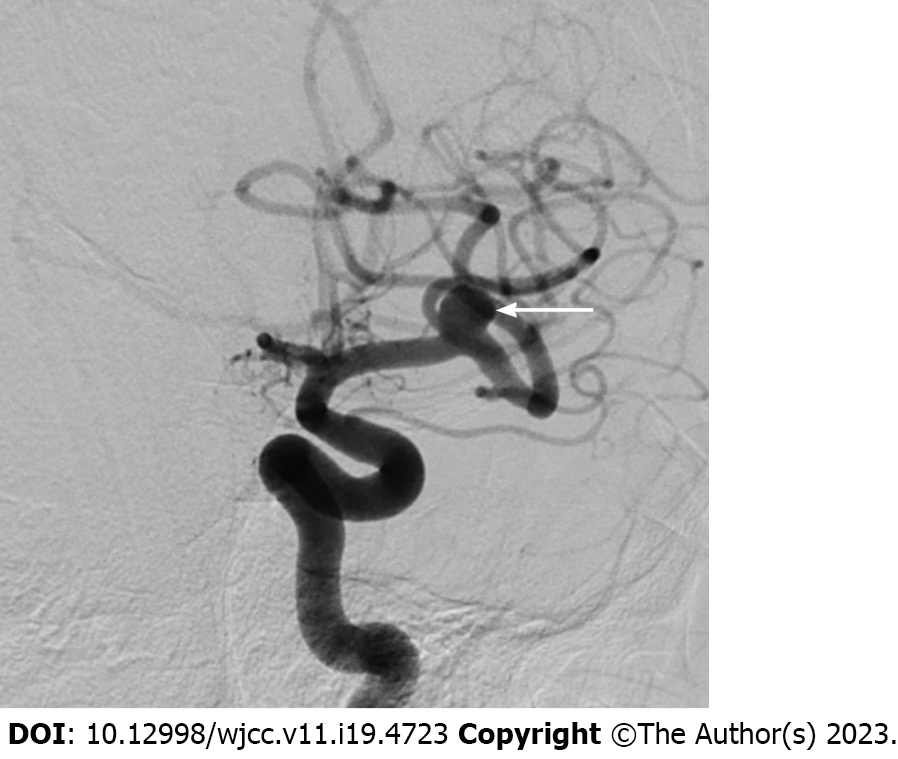
A 59-year-old man who had undergone uneventful coiling for a ruptured anterior communicating artery aneurysm one month ago was readmitted to our hospital for surgical clipping of the left middle cerebral artery (MCA) aneurysm unfavorable for coiling. He recovered uneventfully from the subarachnoid hemorrhage one month ago and had no neurological abnormalities. All other preoperative evaluations, including hematological tests, were unremarkable. Digital subtraction an
He had no specific medical history other than high blood pressure, which was well controlled by amlodipine (5 mg/d).
The patient admitted to smoking ten cigarettes daily for > 20 years. The remaining personal and family histories did not contribute.
The patient’s vital signs were: Blood pressure, 129/84 mmHg; heart rate, 67 beats per min; body temperature, 37.0°C; and respiratory rate, 21 breaths per min. Neurological examination demonstrated stuporous mentality (Glasgow Coma Scale score of 10) and profound global aphasia. The function of cranial nerves was intact. He had normal tone in all limbs and normal power in the left limbs, but slightly reduced power in (4/5) in the right limbs. Sensory examination was normal. The tendon reflexes of the extremities were normal.
There were no abnormalities in routine blood tests, blood biochemistry, blood coagulation, and routine urine tests.
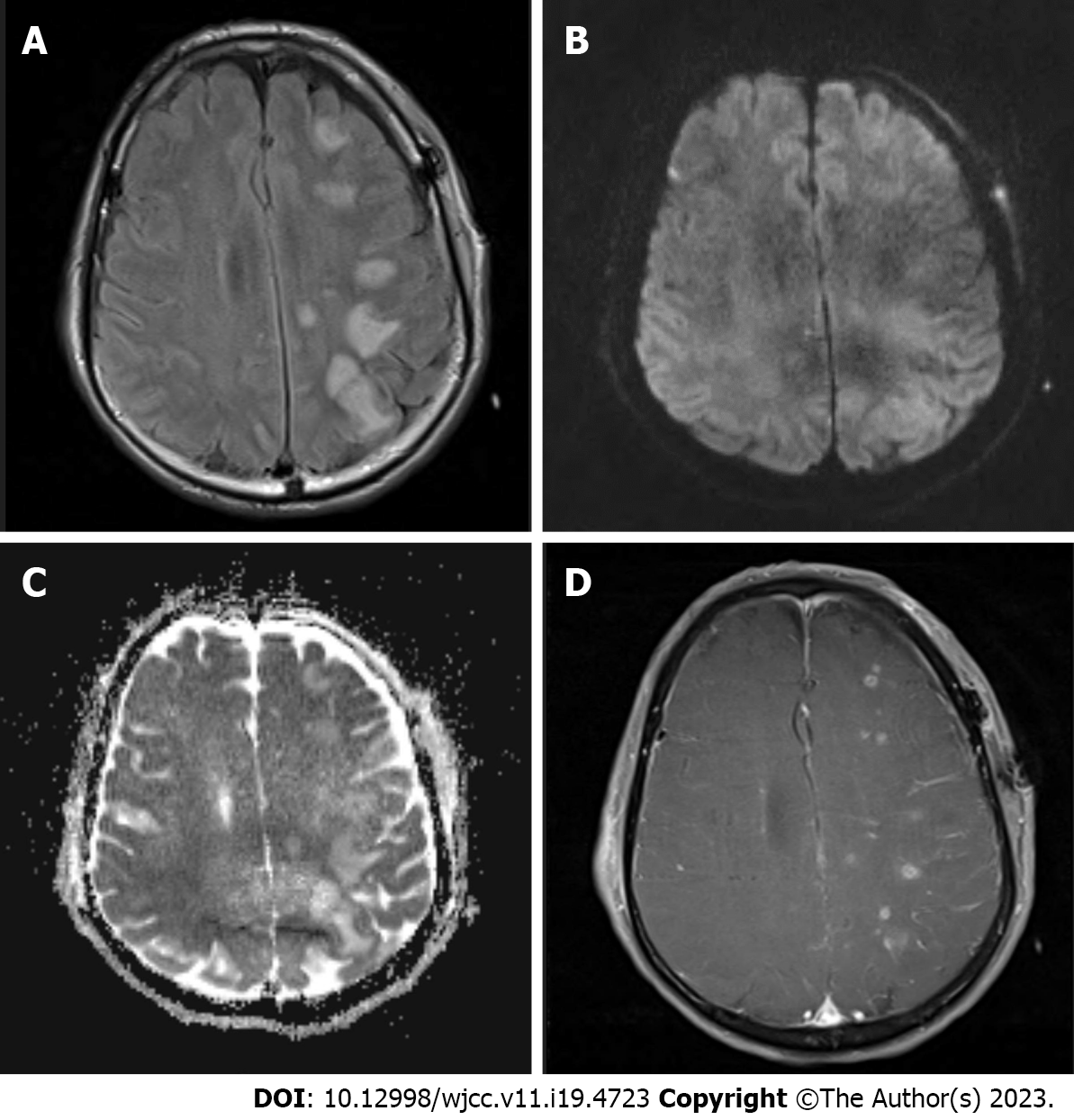
After confirming absence of an abnormal finding on non-contrast brain CT performed immediately, MRI was carried out in succession. It showed widespread high signal intensities especially in the cortex and subcortical region of the ipsilateral fronto-parieto-occipital lobes in fluid attenuation inversion recovery images (Figure 2A). The lesions were iso or high signal intensity in diffusion-weighted images (Figure 2B) and high signal intensity in apparent diffusion coefficient maps (Figure 2C) without any signs of diffusion restriction, consistent with vasogenic edema. In contrast-enhanced three-dimensional T1-weighted images, lesions showed patchy enhancement (Figure 2D). Susceptibility-weighted images did not demonstrate hemorrhage. Since major arteries were still clearly depicted on magnetic resonance angiography besides clip artifacts, vascular problems including reversible cerebral vasoconstriction syndrome could be excluded from the diagnosis. The CT perfusion study was negative.
The imaging findings were suggestive of PRES in the left hemisphere.
The patient gradually recovered with dexamethasone injections and supportive care, without additional neurologic signs.
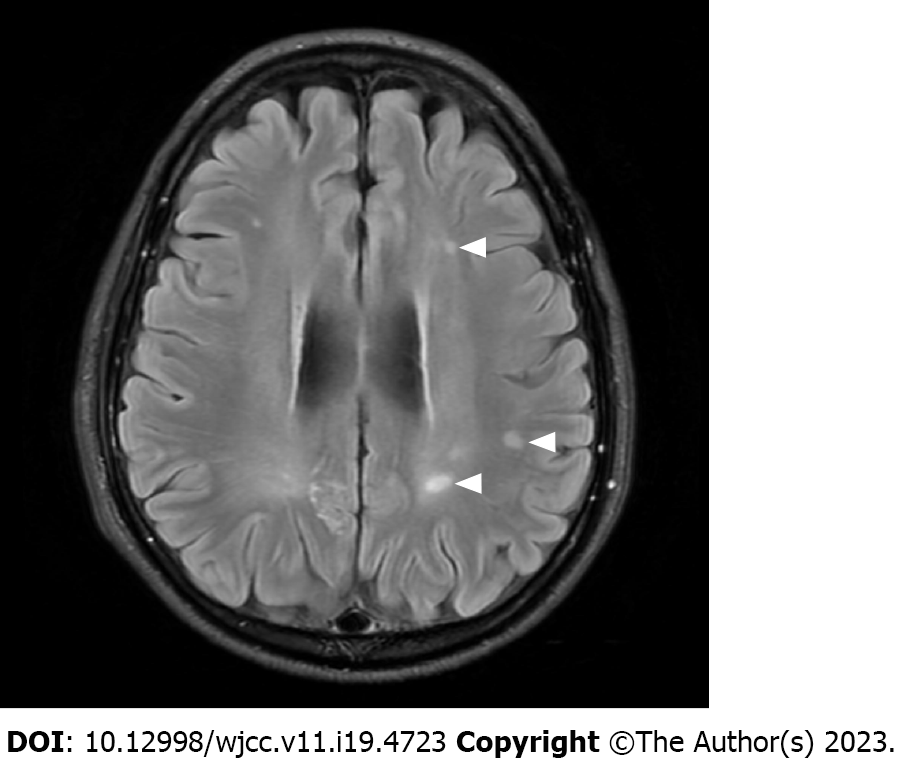
He was discharged with a modified Rankin scale score of 0 at 3 wk after surgery. On serial MRI scans, most of the lesions gradually disappeared within few weeks. However, several lesions in the subcortical white matter persisted even after 1 year, despite being reduced in size (Figure 3).
PRES is a clinicoradiological syndrome characterized mainly by occipital and parietal lobe involvement, which can be recovered within a few days or weeks[1]. In our patient, PRES was diagnosed based on the typical clinical presentation and MRI finding after excluding CNS infections, air embolus, and sinus thrombosis. As clinical symptoms resolve after several weeks in most cases of PRES, neurological abnormalities gradually improved from the 4th day after surgery and our patient fully recovered without any deficit 3 wk after surgery. MRI typically shows symmetrical high signal intensity in both parieto-occipital cortical-subcortical white matter in fluid attenuation inversion recovery images[2]. However, our case showed an atypical finding of unilateral involvement in PRES. The involvement in PRES could be asymmetrical or rarely unilateral. Reports of unilateral lesions include those related to subarachnoid hemorrhage-induced cerebral vasospasm and PRES associated with MCA occlusion[6]. Since PRES was first described in 1996, various MRI findings have been reported. In addition to the typical site of PRES involvement, almost any region of the brain can also be involved[1,6]. Involvement of the basal ganglia or brain stem while sparing the subcortical regions was named “central-variant” PRES accounting for about 4% of the cases, and it was distinctly reported that 10% of the patients were involved in the splenium of the corpus callosum[2,7]. Restricted diffusion is sometimes observed in PRES, and resulted in irreversible cell death[8]. It has been rarely reported that intracranial hemorrhage can also be accompanied by this condition in around 5%–17% of the cases, and it could present as minimal hemorrhages, intraparenchymal hematoma, and subarachnoid hemorrhage[1,9]. However, an intracranial hemorrhage-related finding was not found in our case, even in susceptibility-weighted images.
The pathophysiology of PRES remains a mystery nearly 25 years after its initial description. The most well-known and accepted theory is an increase in arterial pressure above the upper limit for cerebral autoregulation, causing vasogenic edema[4]. This theory is supported by hypertension, which is common in patients with PRES. However, this hypertension theory does not explain all situations. Although rare, PRES has been reported in patients with normal or slightly elevated blood pressure, and serious cerebral edema has been reported in PRES without severe hypertension[4,6]. In the present case, the overall intraoperative mean blood pressure was maintained at around 60 mmHg and no blood pressure surge was reported immediately after surgery. Therefore, we hypothesized that a sudden increase in cerebral perfusion pressure associated with temporary M1 occlusion and intracranial hypotension secondary to the cerebrospinal fluid (CSF) leak caused a degree of hyperperfusion that precipitated PRES. These assumptions support the reason for PRES spreading within the brain hemisphere ipsilateral to the treated UIA, and not bilateral involvement. It was also inferred that diffuse subarachnoid hemorrhage, which occurred one month before surgery, partially contributed to increased susceptibility to autoregulation breakdown. Another mechanism to explain the development of PRES is the activation of immune system through cascade as a result of inducing endothelial dysfunction[10]. Kur et al[11] reported three cases of systemic lupus erythematosus (SLE) with PRES, assuming that PRES may be a feature of disease activity with nephritis and hypertension or a result of immunosuppressive therapy in patients with SLE. Patients with autoimmune diseases are more susceptible to endothelial dysfunction and consequently to the occurrence of PRES[12].
To the best of our knowledge, only one case of PRES that developed after clipping surgery for the treatment of UIA has been reported[5]. The authors supposed that the cause of PRES in their case was rapid blood pressure fluctuations accompanying general anesthesia for clipping surgery, and PRES, which occurs after craniotomy, is unilateral and can become severe in the craniotomy area and leave sequelae. Recently, Fukushima et al[13] reported a case of delayed leucoencephalopathy after coil embolization of the UIA, which was supposed to be caused by delayed hypersensitivity to the bioactive polyglycolic-polylactic acid coil. The diagnosis of PRES was excluded as an etiology for the white matter lesion because of unilateral involvement of the lesions. However, their imaging findings are consistent with the current unilateral PRES. Since the causes of PRES are very diverse and difficult to define accurately, it could be assumed that several case reports with different diagnoses for phenomena similar to actual PRES have been reported. According to a report studying cerebral hyperperfusion syndrome and related conditions, such as hypertensive encephalopathy, PRES, and reversible cerebral vasoconstriction syndrome, these syndromes can share similar pathophysiological mechanism such as cerebral vasoconstriction, endothelial damage, blood-brain barrier dysfunction, brain edema, and, sometimes intracerebral hemorrhage, with fatalities described in all reports[14]. Despite knowledge of these syndromes, they still remain unknown. However, it is important to be aware of this condition as it can be cured through early diagnosis and treatment[12].
Our unique case highlights that, to our knowledge, this is the second report of PRES developing after craniotomy for the treatment of UIA. Although it may be very rare, prolonged temporary occlusion time and CSF leak may cause the development of PRES in the brain with impaired autoregulation. Surgeons must keep PRES in mind as one of the causes of neurological abnormality after clipping of an UIA.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Reis F, Brazil; Shao A, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 483] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Hugonnet E, Da Ines D, Boby H, Claise B, Petitcolin V, Lannareix V, Garcier JM. Posterior reversible encephalopathy syndrome (PRES): features on CT and MR imaging. Diagn Interv Imaging. 2013;94:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2250] [Cited by in RCA: 2148] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 4. | Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 716] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 5. | Chihara H, Hatano T, Ando M, Takita W, Tokunaga K, Hashikawa T, Funakoshi Y, Kamata T, Higashi E, Nagata I. A Case of Posterior Reversible Encephalopathy Syndrome After Surgical Clipping of Unruptured Cerebral Aneurysm. World Neurosurg. 2019;124:323-327. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Çamlıdağ İ, Cho YJ, Park M, Lee SK. Atypical Unilateral Posterior Reversible Encephalopathy Syndrome Mimicking a Middle Cerebral Artery Infarction. Korean J Radiol. 2015;16:1104-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | McKinney AM, Jagadeesan BD, Truwit CL. Central-variant posterior reversible encephalopathy syndrome: brainstem or basal ganglia involvement lacking cortical or subcortical cerebral edema. AJR Am J Roentgenol. 2013;201:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23:1038-1048. [PubMed] |
| 9. | Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol. 2009;30:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 713] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 11. | Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33:2178-2183. [PubMed] |
| 12. | Ferreira TS, Reis F, Appenzeller S. Posterior reversible encephalopathy syndrome and association with systemic lupus erythematosus. Lupus. 2016;25:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Fukushima Y, Nakahara I. Delayed leucoencephalopathy after coil embolisation of unruptured cerebral aneurysm. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Delgado MG, Bogousslavsky J. Cerebral Hyperperfusion Syndrome and Related Conditions. Eur Neurol. 2020;83:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |