Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4664
Peer-review started: February 18, 2023
First decision: May 16, 2023
Revised: May 25, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: July 6, 2023
Processing time: 131 Days and 20.4 Hours
Transarterial chemoembolization (TACE) is widely performed for intermediate-stage or unresectable hepatocellular carcinoma (HCC), but approximately half of patients do not respond to TACE treatment. We describe a case of rapidly progressing of HCC after TACE and provide a possible hypothesis for this condition. The finding may contribute to identifying patients who obtain less benefit from TACE, thus avoiding the unnecessary waste of medical resources and treatment during the golden hour window.
A 61-year-old woman had been diagnosed with chronic hepatitis B infection and HCC at Barcelona Clinic Liver Cancer stage B, which had been treated by segmental hepatectomy 14 mo ago. The tumor recurred in the two months after surgery. She received an initial TACE and then underwent systemic therapy with lenvatinib 8 mg daily due to an increased level of alpha-fetoprotein (AFP) after the first TACE. However, the tumor continued to progress with an increased level of AFP, and she underwent a second TACE, after which the tumor volume did not obviously decrease on the contrast-enhanced computed tomography image. One month later, she had a third TACE to control the residual HCC tumors. Two weeks after that, the HCC had increased dramatically with tea-colored urine and yellowish skin turgor. Eventually, the patient refused further treatment and went into hospice care.
Intense hypoxia induced by TACE can trigger rapid disease progression in infiltrative HCC patients with a large tumor burden
Core Tip: We report an hepatocellular carcinoma (HCC) case with a large tumor burden and infiltrative tumor pattern who exhibited rapidly increased tumor volume within two weeks after undergoing a third trans-arterial chemoembolization (TACE). Although the Barcelona Clinic Liver Cancer staging system classifies multinodular HCC without portal invasion or extrahepatic spread in stage B, it appears that TACE is not suitable for those with a large tumor burden or infiltrative tumor pattern. In addition, hypoxia is an important factor for tumor development, metastasis, and drug resistance. Our case suggests that intense hypoxia induced by TACE may lead to the rapid progression of HCC.
- Citation: Yeo KF, Ker A, Kao PE, Wang CC. Hypothetical hypoxia-driven rapid disease progression in hepatocellular carcinoma post transarterial chemoembolization: A case report. World J Clin Cases 2023; 11(19): 4664-4669
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4664.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4664
Primary liver cancer was the third-leading cause of cancer death globally in 2020, with an age-standardized incidence rate of 19.3 cases per 100000 people and an age-standardized mortality rate of 17.7 per 100000 people[1]. Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for approximately 75%–85% of cases. Clinically, trans-arterial chemoembolization (TACE) is used as the first-line treatment for patients with Barcelona Clinic Liver Cancer (BCLC) stage B and some unresectable HCC[2,3]. TACE injects cytotoxic agents into the arteries, followed by the embolization of tumor blood vessels, which induces strong cytotoxic and ischemic effects to destroy tumor cells. However, the response rate of intermediate-stage HCC patients receiving TACE is low for unclear reasons, with a pooled objective response rate of about 52%[4]. To our best knowledge, only a few studies have investigated the baseline characteristics of those not benefitting from TACE, such as large tumor volume, high tumor number, and poor performance status[5,6]. In our case, an intermediate-stage HCC patient underwent a third TACE and experienced rapid disease progression within two weeks. This case may provide information on the failure of and resistance to TACE in HCC treatment.
Progressive yellowish skin turgor and firm sensation over the epigastric area.
A 61-year-old woman had been diagnosed with HCC at BCLC stage B as well as chronic hepatitis B infection 14 mo earlier. Because the typical image of contrast-enhanced abdominal computed tomography (CT) showed three heterogeneous arterial enhancing lesions with delayed phase wash out in segments S4 and S8, with the largest measuring 7.3 cm, she received segmental hepatectomy and cholecystectomy on October 5, 2021 based on the extensive criteria of the University of California San Francisco[7]. Tumor recurrence emerged two months later, and we performed a TACE followed by systemic therapy of lenvatinib 8 mg daily due to an elevated alpha-fetoprotein (AFP) level after the TACE. The subsequent CT confirmed the progression of HCC volume, so we arranged a second course of TACE after the lenvatinib treatment.
The patient had a history of major depression with good medication control.
The patient denied smoking, alcohol use, and betel nut use. She also denied any family history of malignancy. There was no family history of hepatitis B or hepatitis C.
The patient’s height and weight were 162 cm and 60 kg. Her vital signs were stable, with a body temperature of 37.5°C, pulse rate of 90 bpm, respiration rate of 17 breaths per minute, and blood pressure of 136/76 mmHg. A physical examination revealed icteric sclera on the day of admission. A palpable firm mass lesion was observed at the epigastric area.
The laboratory data revealed mild anemia, including a low red blood cell count of 392 × 106 cells/μL and a high mean corpuscular hemoglobin of 32.7 pg. The hemoglobin, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin concentrations were within normal values. Coagulation tests showed a low platelet count of 126000 platelets/μL and a prothrombin time/international normalized ratio within the normal value. Alanine aminotransferase and aspartate aminotransferase were elevated at 273 U/L and 272 U/L, respectively, while total bilirubin was 4.7 mg/dL. The renal function tests and electrolyte tests were within normal ranges.
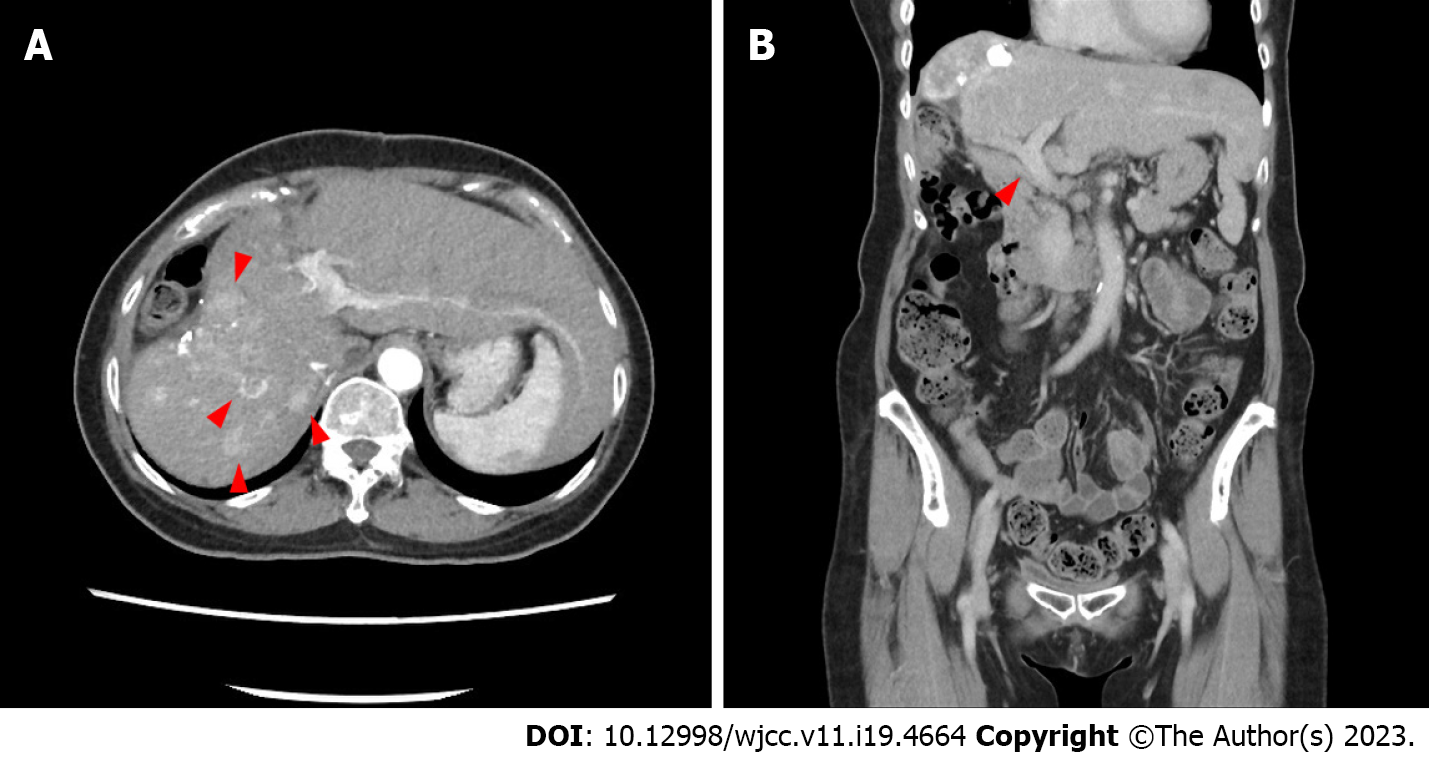
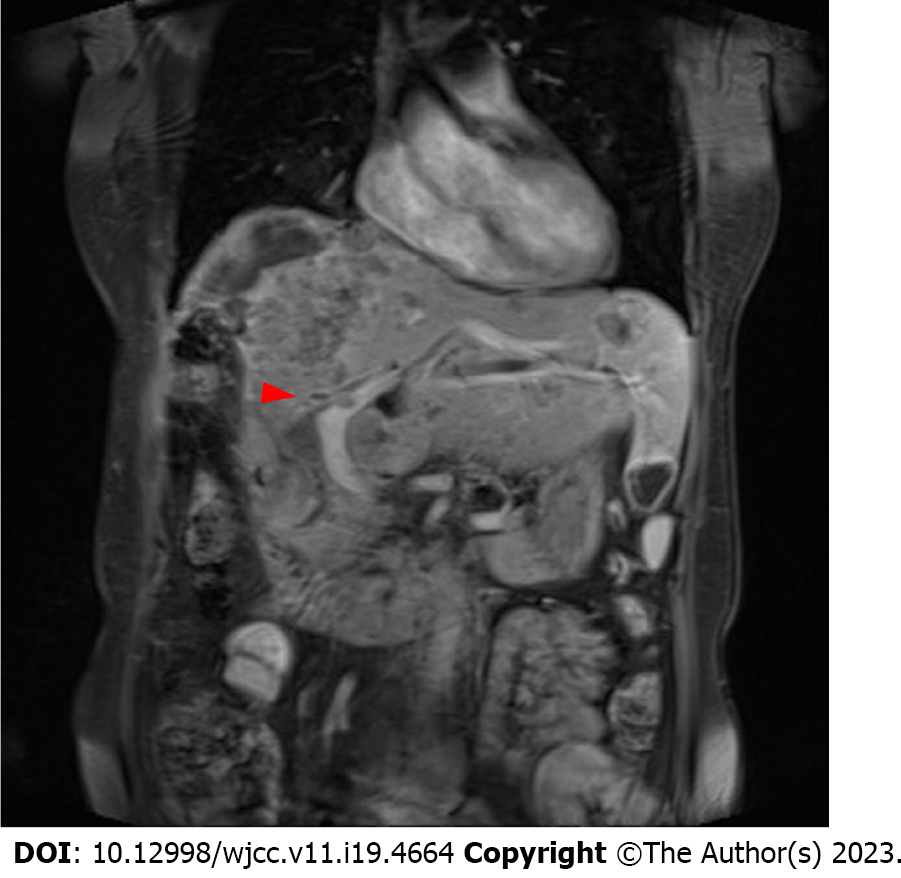
An abdominal contrast CT showed increased residual HCC volume (Figure 1A) after the second TACE course. The coronal view showed a patent portal vein and normal bile ducts (Figure 1B). Contrast-enhanced magnetic resonance imaging (MRI) was arranged taken two weeks after a third TACE course because of an episode of jaundice. It revealed rapidly progressing of HCC volume in a short time, with left intra-hepatic duct (IHD) and portal vein tumor invasions (Figure 2).
HCC with rapid, extensive progression and left IHD invasion after TACE.
The abdominal enhanced CT still showed increased residual HCC volume and a high AFP level after the second TACE. Therefore, a third TACE was done one month later, after which a firm abdomen over the epigastric area, tea-colored urine, and yellowish skin turgor appeared within two weeks. Abdominal sonography showed dilated left IHDs and a greatly increased liver tumor burden, which were confirmed by a contrast-enhanced MRI. Although we hypothesized that mutations in hypoxia-related genes may contribute to the disease progression after TACE, we did not perform liver biopsies to confirm these mutations due to laboratory limitation in our hospital. We described an endoscopic retrograde cholangiopancreatography intervention for bile duct drainage and immunotherapy to the patient and her husband, which had previously been described in the outpatient clinic before the series of TACE, but she refused and opted for hospice care.
For personal and religious reasons, the patient decided to pursue hospice care and was referred for home hospice care.
TACE is considered a first-line treatment for unresectable, multinodular, or intermediate-stage HCC, according to the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases[2,3]. A systematic review of TACE therapy’s efficacy for HCC found an objective response rate of 52.5% (95% confidence interval: 43.6-61.5), and overall survival was 70.3% at one year and 32.4% at five years[4]. However, approximately half of intermediate-stage HCC patients had a poor response to TACE, and the best window for other anticancer therapies was missed due to TACE. In this report, our patient with extensive multinodular HCC had a poor response to the second TACE and rapidly progressed to BCLC stage C HCC within two weeks after the third. Despite the probability of the natural course of the disease, it appeared that TACE stimulated the tumors and rapidly led to this upward stage of transition. In the past decade, several studies have revealed that hypoxia can cause tumor development, tumor angiogenesis, and drug resistance and even promote metastasis, which are mediated by hypoxia-markers including hypoxia-inducible factors (HIF), COX-2, AMP-activated protein kinase and glucose transporter[8-14]. It was reasonably hypothesized that the strong tumor hypoxia induced by TACE may cause drug resistance, rapid growth, invasion, angio
Due to the nature of the case report, causality cannot be established and external validity is limited. However, it was unusual for tumors to grow so rapidly in two weeks, indicating that they were likely caused by the third TACE. Large cohort studies and clinical trials are required to explore this relationship. A strength of this study is that our case identified a potential causal relationship in which TACE stimulates tumors to grow rapidly with portal invasion, bile duct invasion, or extrahepatic spread—that is, the upward stage of transition—among specific intermediate-stage patients with a large tumor burden. This case may raise global awareness of the current limitations of the BCLC staging system and contribute to reducing the incidence of ineffective and even harmful TACE treatment in specific patients.
Several guidelines recommend TACE as the first-line treatment for intermediate-stage HCC patients. However, even without portal invasion or extrahepatic spread, cases with a large tumor burden and multi-foci tumor infiltration tends not to respond to TACE, and HCC may even increase dramatically.
We extend our gratitude to the reviewers and editor for their valuable feedback.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: koganti SB, United States; Radhakrishnan K, South Korea S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64132] [Article Influence: 16033.0] [Reference Citation Analysis (174)] |
| 2. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3008] [Article Influence: 429.7] [Reference Citation Analysis (3)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6013] [Article Influence: 859.0] [Reference Citation Analysis (3)] |
| 4. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 518] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 5. | Tsai YJ, Hsu CY, Huang YH, Su CW, Lin HC, Lee RC, Chiang JH, Huo TI, Lee SD. Early identification of poor responders to transarterial chemoembolization for hepatocellular carcinoma. Hepatol Int. 2011;5:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Kim HY, Park JW, Joo J, Jung SJ, An S, Woo SM, Kim HB, Koh YH, Lee WJ, Kim CM. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Decaens T, Roudot-Thoraval F, Hadni-Bresson S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Sulpice L, Calmus Y, Hardwigsen J, Ducerf C, Pageaux GP, Dharancy S, Chazouilleres O, Cherqui D, Duvoux C. Impact of UCSF criteria according to pre- and post-OLT tumor features: analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl. 2006;12:1761-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Godet I, Shin YJ, Ju JA, Ye IC, Wang G, Gilkes DM. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat Commun. 2019;10:4862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 9. | Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1094] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 10. | Erin N, Grahovac J, Brozovic A, Efferth T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist Updat. 2020;53:100715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 341] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 11. | Lai JP, Conley A, Knudsen BS, Guindi M. Hypoxia after transarterial chemoembolization may trigger a progenitor cell phenotype in hepatocellular carcinoma. Histopathology. 2015;67:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Semenza GL. Intratumoral Hypoxia and Mechanisms of Immune Evasion Mediated by Hypoxia-Inducible Factors. Physiology (Bethesda). 2021;36:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Huang M, Wang L, Chen J, Bai M, Zhou C, Liu S, Lin Q. Regulation of COX-2 expression and epithelial-to-mesenchymal transition by hypoxia-inducible factor-1α is associated with poor prognosis in hepatocellular carcinoma patients post TACE surgery. Int J Oncol. 2016;48:2144-2154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Qu K, Yan Z, Wu Y, Chen Y, Qu P, Xu X, Yuan P, Huang X, Xing J, Zhang H, Liu C, Zhang J. Transarterial chemoembolization aggravated peritumoral fibrosis via hypoxia-inducible factor-1α dependent pathway in hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |