Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4612
Peer-review started: January 10, 2023
First decision: January 21, 2023
Revised: January 26, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: July 6, 2023
Processing time: 171 Days and 10.5 Hours
Metabolic syndrome is a multifactorial disease, and the gut microbiota may play a role in its pathogenesis. Obesity, especially abdominal obesity, is associated with insulin resistance, often increasing the risk of type two diabetes mellitus, vascular endothelial dysfunction, an abnormal lipid profile, hypertension, and vascular inflammation, all of which promote the development of atherosclerotic cardio
To evaluate the outcomes of fecal microbiota transplantation (FMT) in patients with metabolic syndrome.
This was a randomized, single-blind placebo-controlled trial comparing FMT and a sham procedure in patients with metabolic syndrome. We selected 32 female patients, who were divided into eight groups of four patients each. All of the patients were submitted to upper gastrointestinal endoscopy. In each group, two patients were randomly allocated to undergo FMT, and the other two patients received saline infusion. The patients were followed for one year after the procedures, during which time anthropometric, bioimpedance, and biochemical data were collected. The patients also had periodic consultations with a nutritionist and an endocrinologist. The primary end point was a change in the gut microbiota.
There was evidence of a postprocedural change in microbiota composition in the patients who underwent FMT in relation to that observed in those who underwent the sham procedure. However, we found no difference between the two groups in terms of the clinical parameters evaluated.
There were no significant differences in biochemical or anthropometric parameters, between the two groups evaluated. Nevertheless, there were significant postprocedural differences in the microbiota composition between the placebo group. To date, clinical outcomes related to FMT remain uncertain.
Core Tip: The prevalence of metabolic syndrome is a pandemic that goes hand in hand with obesity and diabetes, affecting almost half of the world's population. Therapeutic approaches targeting dysbiosis and manipulation of the gut microbiome have become options and are being tested. Such approaches include the use of prebiotics, probiotics, synbiotics, antibiotics and fecal microbiota transplantation (FMT). It is known that FMT can alter the intestinal microbiota and increase its diversity, resulting in a microbiome that can help decrease body fat and increase insulin sensitivity, as well as facilitate the treatment of metabolic syndrome and obesity. This was a randomized controlled trial comparing FMT and a sham procedure in patients with the metabolic syndrome.
- Citation: da Ponte Neto AM, Clemente ACO, Rosa PW, Ribeiro IB, Funari MP, Nunes GC, Moreira L, Sparvoli LG, Cortez R, Taddei CR, Mancini MC, de Moura EGH. Fecal microbiota transplantation in patients with metabolic syndrome and obesity: A randomized controlled trial. World J Clin Cases 2023; 11(19): 4612-4624
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4612.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4612
The prevalence of metabolic syndrome parallels that of obesity and diabetes—up to 45% of the population worldwide—and is expected to rise as a consequence of increasing longevity and unhealthy lifestyles[1,2]. Obesity has become one of the most important public health problems in the United States and in many other resource-rich countries, as well as in transitional economies. The increase in the prevalence of obesity has resulted in increases in the incidence of associated diseases such as diabetes and hypertension[3,4].
Obesity, especially abdominal obesity, is associated with insulin resistance, often increasing the risk of type two diabetes, vascular endothelial dysfunction, an abnormal lipid profile, hypertension, and vascular inflammation, all of which promote the development of atherosclerotic cardiovascular disease[5,6]. Individuals in whom the metabolic risk factors for type two diabetes coexist with those for cardiovascular disease are classified as having metabolic syndrome[7].
There are many treatment modalities for obesity and metabolic syndrome. However, optimal management is still a challenge because multiple factors are involved in its physiopathology, such as genetic predisposition, sedentary lifestyle, and a specific distribution of body fat[8,9]. Therapeutic approaches targeting dysbiosis and manipulation of the gut microbiome have recently been developed. Such approaches include the use of prebiotics, probiotics, synbiotics, antibiotics, and fecal microbiota transplantation (FMT). It is known that FMT can change the gut microbiota and increase its diversity, resulting in a microbiome that could help decrease body fat and increase insulin sensitivity, as well as facilitating the treatment of metabolic syndrome and obesity. The gut microbiota is composed of trillions of microorganisms that can influence the human organism by various mechanisms, having been associated with many diseases and conditions, including obesity and metabolic syndrome[10-14].
The aim of this study was to evaluate the outcomes of FMT in patients with metabolic syndrome. To that end, we performed a randomized placebo-controlled clinical trial.
This was a randomized, single-blind, placebo-controlled clinical trial comparing FMT and a sham procedure in patients with metabolic syndrome. We selected patients who had been diagnosed with metabolic syndrome according to the 2006 International Diabetes Federation criteria[2]. Additional inclusion criteria were being female, being between 18 and 70 years of age, and having a body mass index (BMI) of 30–40 kg/m2. Patients who had previously undergone gastrointestinal surgery were excluded, as were those with immunodeficiency, those who had previously undergone treatment for obesity, and those who had used any weight loss medication, antibiotics, or probiotics within the last three months. The primary end point was a change in the gut microbiota.
After an initial screening for the characteristics of metabolic syndrome, the patients were referred for consultations with a nutritionist and an endocrinologist. Anthropometric, bioimpedance, and bio
The study was approved by the Research Ethics Committee of the Hospital das Clínicas (CAAE: 62319916.9.0000.0068) operated by the University of São Paulo School of Medicine, in the city of São Paulo, Brazil. All participating patients provided written informed consent.
Female patients with class I or II obesity were recruited following an advertisement at the entrance of the Hospital das Clínicas. We included 32 female patients (age range, 20–69 years) with class II obesity (BMI 30–40 kg/m2) and metabolic syndrome. Metabolic syndrome was defined as a fasting glucose level > 100 mg/dL or use of antidiabetic medications or insulin, plus at least two of the following criteria: triglycerides ≥ 150 mg/dL; high-density lipoprotein cholesterol < 50 mg/dL (the standard for women); blood pressure ≥ 130/85 mmHg or use of antihypertensive medication; and abdominal obesity, defined as a waist circumference ≥ 80 cm (the standard for women).
We chose to use a single feces donor, in an attempt to maintain the same bacterial diversity for all recipients, despite the fact that not all of the donations were made on the same day. To screen the donor, we used the protocol devised by van Nood et al[15]. The donor (a 30-year-old female) was a volunteer and was initially screened with a questionnaire on communicable diseases. Stool and blood samples were collected. The stool sample was screened for parasites, Clostridium difficile, and enteropathogenic bacteria. The blood sample was screened for the following: antibodies to human immunodeficiency virus; human T-cell lymphotropic virus types I and II; hepatitis A, B, and C; cytomegalovirus; Epstein–Barr virus; Treponema pallidum; Strongyloides stercoralis; and Entamoeba histolytica. The screening was repeated every 4 mo during the 1-year donation period. Immediately prior to each donation, another questionnaire was used in order to identify any recent illness[15].
On the day of the procedure, a stool sample was collected from each patient. In most cases, the samples were collected from a spontaneous evacuation by the patient prior to the procedure or by digital rectal extraction after the patient had been sedated. In one patient, it was necessary to perform proctoscopy to obtain the stool sample, which was captured with a snare. After the stool samples had been labeled, they were stored at −80°C.
On the day of donation, the microbiota solution was prepared by diluting 200 g of donor feces in 500 mL of sterile saline. The solution was stirred, after which the supernatant was strained and transferred to a sterile bottle[15]. Immediately after preparation, the microbiota solution was transported from the laboratory to the endoscopy center.
The 32 patients were divided into eight groups of four patients each. All of the patients were submitted to upper gastrointestinal endoscopy. In each group, two patients were randomly allocated to undergo FMT, and the other two patients received saline infusion. All procedures were performed at the endoscopy center of the Hospital das Clínicas.
All of the patients underwent upper gastrointestinal endoscopy under sedation. Infusions were performed with an oscope, which was advanced past the ligament of Treitz and released 200 mL of the microbiota or saline solution. In the FMT patients, the solution was infused within 4 h after the feces had been collected from the donor.
The patients underwent follow-up for one year after the procedure, during which time they had additional consultations with a nutritionist and an endocrinologist: at six weeks, six months, and one year. At each visit, anthropometric parameters, medication use, antibiotic use, and patient complaints were evaluated. Stool samples collected at each time point (baseline, six weeks, six months, and one year) were analyzed.
After the procedures, the patients were required to adhere to a standardized diabetic diet (1000 calories/day) and were instructed to keep a food diary for a period of one year. They were also instructed to use no probiotics and to inform the research team if they needed to use antibiotics.
A 200-mg aliquot of feces from each patient was analyzed with the QiaAmp DNA Stool Mini Kit (QIAGEN, Hilden, Germany), in accordance with the manufacturer’s protocol. The V4 region of the 16 S rRNA gene was amplified using the primers V4 F (TCGTCGGCAG CCAGTGATGTGTATAAGAGACAGGTGCCAGCMGCC GCGGTAA) and V4 R (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT)[16]. Amplification was performed in two steps with a custom Illumina preparation protocol (Illumina, San Diego, CA, USA). The samples were pooled and loaded into an Illumina MiSeq reagent cartridge (Illumina, San Diego, CA) for paired-end, 500-cycle sequencing at a final concentration of 12 pM. The library was clustered at a density of approximately 820 K/mm2. Image analysis, base calling, and data quality assessment were performed on the MiSeq platform. A DNA-free negative control was used, and polymerase-chain-reaction steps were performed. On a gel, no visible amplification signal was observed for the no-template control, indicating that bacterial contamination was minimal.
Bioinformatic analysis: The raw reads were demultiplexed and analyzed using QIIME software, version 1.9[17]. The software was used in order to remove barcodes and primer sequences, as well as to extract chimeric artifacts, align sequences, construct distance matrices, define operational taxonomic units (for phylogenetic tree construction), calculate diversity indices, and test hypotheses. After removing the barcodes and primers, we filtered the sequences, discarding the reads that were smaller than approximately 400 bp. We then checked for chimeras, using USEARCH[17], and excluded the sequences identified as chimeric. The sequences of the remaining libraries were grouped into operational taxonomic units, based on 97% similarity to sequences in the SILVA database, version 128[18]. The relative abundance of the bacteria was determined in relation to the main phyla and genera that appeared in at least 1% of the total found in both groups.
The alpha and beta diversity indices were calculated for each library. To calculate the alpha diversity, we used the Chao1 richness estimate[19], together with the Shannon and Simpson diversity indices[20,21]. To calculate the beta diversity, we constructed a principal coordinate analysis plot based on the weighted and unweighted UniFrac distance matrices[22,23]. Nucleic acid sequences are available at the Sequence Read Archive (accession number, PRJNA766355).
Initially, all variables were analyzed descriptively. For quantitative variables, we observed the minimum and maximum values, as well as calculating means, standard deviations, and quartiles. For qualitative variables, we calculated absolute and relative frequencies.
To compare means between the two groups, we used Student’s t-tests[24]. When the assumption of normality of the data was rejected, we used the nonparametric Mann–Whitney test[24]. To compare the groups over time, we used the nonparametric Mann–Whitney, Wilcoxon, and Friedman tests with Bonferroni correction[24].
The generalized linear model was used in order to compare the two groups, in relation to the clinical data, through linear and ordinal logistic regression. This model was also used in order to evaluate the effect of the independent variable (FMT) on the dependent variables—alpha diversity indices (with gamma distribution) and relative abundance of bacterial phyla and genera (with linear distribution). To detect between-group differences in beta diversity, we used permutational multivariate analysis of variance, with the adonis function for Bray–Curtis distances. For each variable, 999 permutations were used.
To study the correlations between the preprocedural and postprocedural periods, we employed Spearman’s correlation coefficient[24]. All statistical analyses were performed with the SPSS Statistics software package, version 17.0 (SPSS Inc., Chicago, IL, USA). The significance level adopted for all tests was 5%.
We included 32 patients with metabolic syndrome and one feces donor. Of the 32 patients evaluated, four did not complete the study: two withdrew after randomization; one became pregnant during follow-up; and one withdrew during follow-up. Therefore, the final sample comprised 28 patients: 15 in the FMT group and 13 in the placebo group as shown in the CONSORT flow diagram in the Supple
When we evaluated all 32 patients at baseline, there were no statistical differences between the two groups in terms of the mean age (55.20 + 10.22 years vs 53.62 + 13.09 years, P = 0.722), body weight (94.12 + 8.27 kg vs 89.29 + 5.70 kg, P = 0.867), or BMI (36.69 + 2.94 kg/m2vs 35.74 + 2.22 kg/m2, P = 0.719). Table 1 shows the change in body weight over the course of the study, by group, among the 28 patients who completed the study. Overall, no significant differences were found between the two groups regarding the general and clinical characteristics at the time of sample collection.
| Group | N | Mean | SD | Range | Median | IQR |
| FMT (n = 15) | ||||||
| Baseline | 14 | 94.18 | 7.95 | 82.00–110.00 | 93.50 | 87.75–100.38 |
| 6 wk | 14 | 93.86 | 9.94 | 82.00–114.00 | 93.00 | 85.00–100.50 |
| 6 mo | 14 | 94.21 | 11.24 | 78.00–115.00 | 94.00 | 84.50–99.25 |
| 1 yr | 14 | 95.79 | 11.05 | 81.00–116.00 | 95.00 | 86.50–102.25 |
| Placebo (n = 13) | ||||||
| Baseline | 12 | 91.28 | 10.60 | 82.00–120.00 | 89.50 | 83.25–94.50 |
| 6 wk | 12 | 89.79 | 9.53 | 80.50–112.00 | 86.50 | 82.25–96.75 |
| 6 mo | 12 | 89.58 | 10.87 | 77.00–115.00 | 88.50 | 81.25–93.75 |
| 1 yr | 12 | 90.50 | 13.11 | 73.00–117.00 | 86.50 | 81.25–99.75 |
No serious adverse events were reported in either group. We also observed no statistical differences between the two groups in terms of biochemical parameters (e.g., hematology, glucose, renal function, and liver chemistry), lean mass, or the percentage of body fat.
At 6 wk, 6 mo, and one year after the procedures, there were no statistical differences between the FMT and placebo groups for any of the following: body weight; BMI; waist circumference; hip circumference; fasting glucose; insulin; glycated hemoglobin (Table 2); the insulin resistance profile; the respiratory compensation point; and the lipid profile.
| Group | N | Mean | SD | Range | Median | IQR |
| FMT (n = 15) | ||||||
| Baseline | 11 | 6.75 | 1.09 | 5.60–8.90 | 6.30 | 6.10–7.60 |
| 6 wk | 11 | 6.65 | 1.09 | 5.60–9.10 | 6.10 | 6.00–7.60 |
| 6 mo | 11 | 6.95 | 1.19 | 5.40–9.50 | 6.50 | 6.20–7.70 |
| 1 yr | 11 | 7.34 | 1.85 | 5.60–11.50 | 6.70 | 5.80–8.30 |
| Placebo (n = 13) | ||||||
| Baseline | 11 | 6.99 | 1.99 | 4.80–12.50 | 6.60 | 6.00–7.50 |
| 6 wk | 11 | 6.75 | 1.38 | 5.00–10.20 | 6.30 | 6.00–7.40 |
| 6 mo | 11 | 6.97 | 1.98 | 4.90–12.20 | 6.40 | 5.90–7.20 |
| 1 yr | 11 | 7.29 | 2.56 | 4.90–13.80 | 6.30 | 5.90–8.10 |
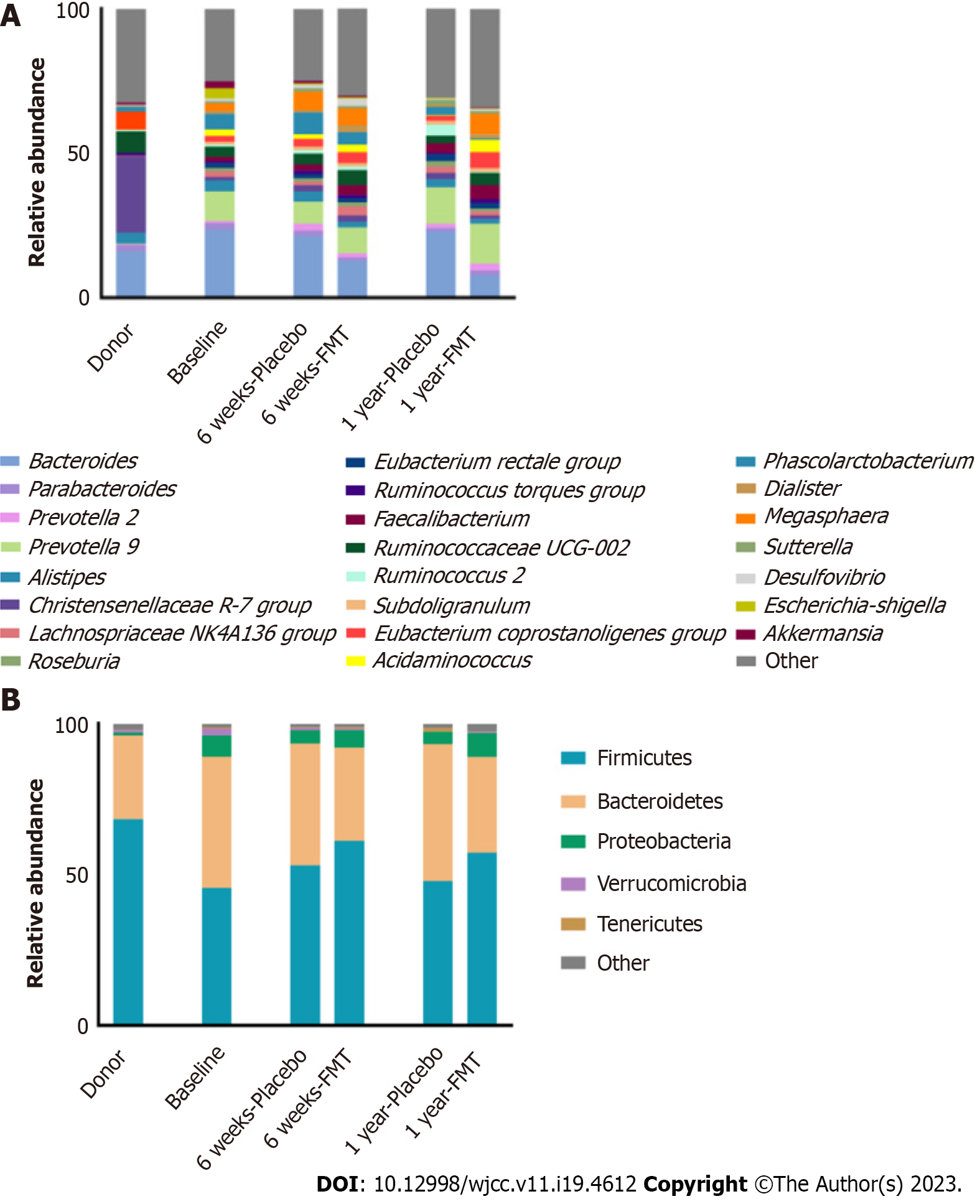
In both groups, the predominant phyla were Firmicutes and Bacteroidetes (Figure 1). At 6 wk after the procedures, the relative abundance of Firmicutes was higher in the placebo group than in the FMT group (Figure 1A and Supplementary Table 1). Over the course of the study period, the abundance of Firmicutes in the FMT group increased, whereas that of Bacteroidetes decreased in that group, resulting in the Firmicutes/Bacteroidetes ratio being higher in the FMT group than in the placebo group. In both groups, the most abundant genera were Bacteroides, Prevotella, Ruminococcaceae UCG-002, and Megasphaera (Figure 1B and Supplementary Table 2). Although there were no significant differences in the abundances of genera and phyla between the groups and among the time points, there was a marked increase in Ruminococcus 2 and Phascolarctobacterium in the placebo group, as well as a decrease in Bacteroides in the FMT group. Over the course of the study, the abundances of Prevotella, Ruminococcaceae UCG-002, and Faecalibacterium were higher in the FMT group than in the placebo group.
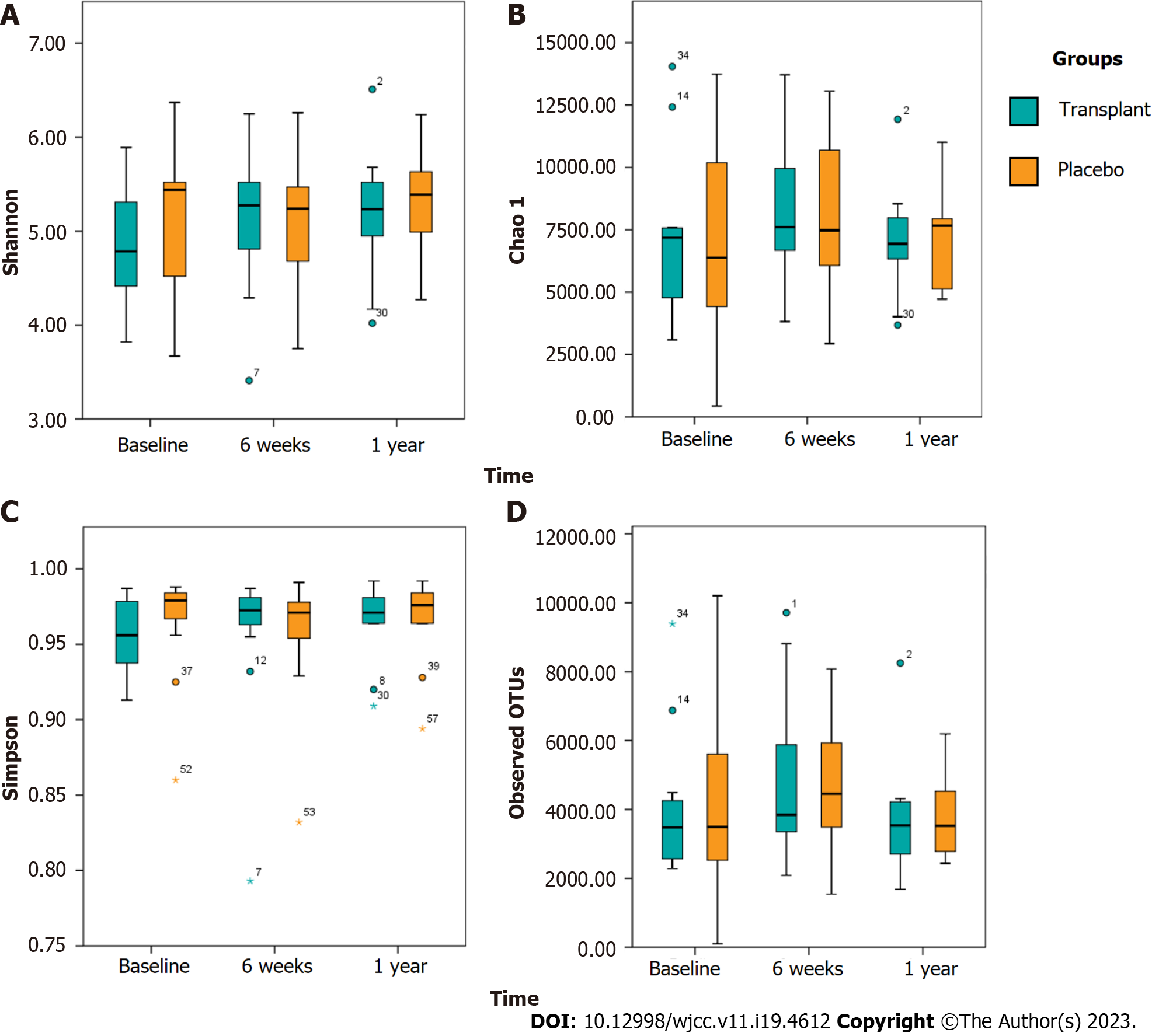
There were no significant differences between the FMT and placebo groups in terms of the alpha diversity (Figure 2). However, we observed a significant negative correlation between the Shannon diversity index and the percentage of body fat in both groups. We also found that the Chao1 richness estimate showed a significant negative correlation with fat mass, as did the Shannon and Simpson diversity indices.
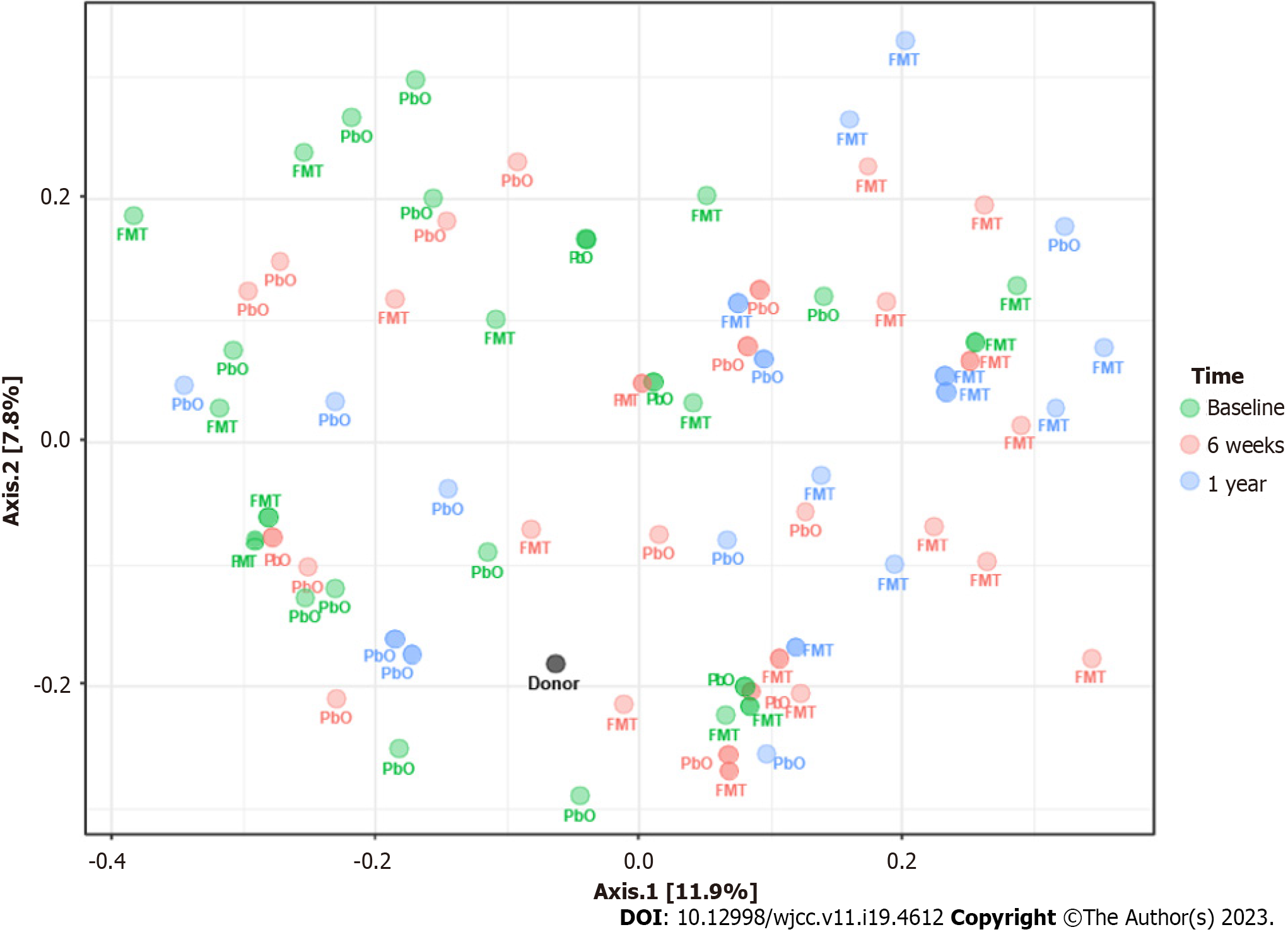
The beta diversity of the microbial community showed significant differences between the FMT and placebo groups over the course of the study (Figure 3): At baseline (F = 0.83294, R2 = 0.033542, P = 0.603); at 6 wk after the procedures (F = 1.9699, R2 = 0.070431, P = 0.039); and at 1 year after the procedures (F = 3.0656, R2 = 0.15278, P < 0.003).
The gut microbiota has been shown to play a role in a number of diseases, including inflammatory bowel disease[25-27], pseudomembranous colitis[28], primary sclerosing cholangitis[29], and cardiovascular disease. The gut microbiota also interacts with obesity, metabolic syndrome, and insulin resistance[30] which have common risk factors and are highly correlated.
The gut microbiota appears to be an important factor in the development of obesity and metabolic syndrome, FMT therefore being a possible treatment modality. Controlled studies have shown that FMT alters the gut microbiota of rats and reduces their weight, indicating that the microbiota is directly related to nutrient absorption capacity[12].
In one randomized controlled study of FMT, involving male patients with metabolic syndrome[31], the glycemic profile at six weeks after FMT was found to be better in the patients who received microbiota (from lean individuals) than in those who received autologous microbiota. In another randomized controlled trial[32], 38 obese male patients were randomized to receive autologous microbiota or allogeneic microbiota from lean donors. The authors found that, by six weeks after the procedures, there was a significant (11.5%) increase in insulin resistance among the patients who received allogeneic microbiota, although that increase was not maintained at 18 wk after the procedure, which suggests that FMT has only a short-term effect. In a double-blind randomized controlled study of FMT[33], 20 male patients with metabolic syndrome were allocated to receive autologous or allogeneic (vegan) microbiota. In that study, a slight improvement in insulin resistance was observed at two weeks after FMT in the patients receiving allogeneic microbiota.
In a systematic review of FMT in obese individuals with metabolic syndrome[34], the authors evaluated three randomized placebo-controlled studies involving a collective total of 63 patients. They identified no statistical differences between FMT and placebo in terms of the glycemic profile or weight loss. However, the studies evaluated differed in various aspects, including the number of donors, follow-up time, and means of preparation of the microbiota solution. Another systematic review of FMT in patients with obesity and metabolic syndrome[35], published in 2020, evaluated six studies: The three randomized placebo-controlled studies mentioned above[32,33] two randomized double-blind placebo-controlled studies[36] and the prepublication data of the present study. The authors of that review, which involved a collective total of 154 patients, detected no statistical difference between FMT and placebo in terms of the clinical parameters evaluated.
In the present study, there were no significant differences in biochemical or anthropometric parameters, between the two groups or at any of the time points evaluated. Nevertheless, there were significant postprocedural differences in the microbiota composition between the placebo group and the FMT group, as evidenced by the beta diversity results, which indicate that FMT is effective in changing the gut microbiome and that such changes can persist for at least one year after the procedure. However, other studies have shown that there is no difference in alpha diversity in the gut microbiome, as assessed with the Shannon diversity index, between patients receiving FMT and those receiving a placebo[33,34]. In one such study[33], the FMT group subjects were divided into two subgroups, based on the insulin sensitivity response observed: Metabolic responders and metabolic nonresponders. Among the metabolic responders, there was a significant increase (in relation to the baseline value) in the relative abundance of Akkermansia muciniphila. Human and animal studies have demonstrated that A. muciniphila is closely associated with improvements in insulin sensitivity[37]. Its beneficial effects may be due to a microbe-induced increase in the intestinal level of endocannabinoids and epithelial expression of Toll-like receptor two, which regulates gut barrier function and inflammation[37]. However, we observed no difference between our FMT group and our placebo group, in terms of the abundance of A. muciniphila, at six weeks or one year. The lack of a statistical difference regarding the abundance of A. muciniphila cannot be evaluated in isolation, as the health of the gut microbiome is defined in terms of diversity, stability, resistance, and resilience[38].
This trial has some limitations. As gut dysbiosis can be influenced by inflammation, diet, and environmental exposures[39], there was great variability among the individuals evaluated. This variability could be attributed, in part, to the fact that the recommended standardized diet was not followed. All of the patients evaluated were insulin resistant or had diabetes, and it is known that the use of medications such as metformin can change the diversity of the intestinal microbiota[40]. In addition, there is as yet no well-established protocol for preparing the donor intestinal microbiota. Although we followed the protocol devised by van Nood et al[2], it was not possible to ensure that the anaerobic bacteria remained viable until the time of transplantation. The two species most strongly associated with metabolic health—Faecalibacterium prausnitzii and A. muciniphila—are both anaerobic[41,42]. An anaerobic microbiota preparation protocol may be necessary to increase bacterial viability and engraftment success of strict anaerobes in FMT[43]. It would thus be possible to transplant a greater diversity of microbiota and keep the composition of the microbiota solution as close as possible to that of the donor sample. Furthermore, we opted for infusion by colonoscopy, which is just one of the many possible routes of delivery of FMT, and success rates have been shown to vary depending on the route of delivery[44]. Further studies are needed in order to understand the dose and duration of therapy required to maximize the therapeutic effect of FMT, while optimizing patient tolerance and compliance[45]. Moreover, we included only one donor (a female) and our sample comprised only female patients, whereas most comparable studies in the literature have evaluated male patients. Sex is recognized as an important factor in a variety of common conditions, including autoimmune, metabolic, cardiovascular, and psychiatric disorders[46]. Finally, our analysis was also limited not only by the small size of our sample but also by the small number of studies of the topic in the literature and their small sample sizes. As this was a single-center study, our findings may not reflect the outcomes that can be expected across global populations.
In the present study, there were no significant differences in biochemical or anthropometric parameters, between the two groups or at any of the time points evaluated. Nevertheless, there were significant postprocedural differences in the microbiota composition between the placebo group and the FMT group, as evidenced by the beta diversity results, which indicate that FMT is effective in changing the gut microbiome and that such changes can persist for at least 1 year after the procedure.
The prevalence of metabolic syndrome parallels that of obesity and diabetes—up to 45% of the population worldwide. New therapeutic methods have emerged to join efforts in the fight against obesity and metabolic syndrome.
To investigate new methods for the treatment of metabolic syndrome.
Evaluate the outcomes of fecal microbiota transplantation (FMT) in patients with metabolic syndrome.
This was a randomized, single-blind, placebo-controlled clinical trial comparing FMT and a sham procedure in patients with metabolic syndrome. The trial was registered at ensaiosclinicos.gov.br (identifier: U1111-1223-6951).
There was evidence of a postprocedural change in microbiota composition in the patients who underwent FMT in relation to that observed in those who underwent the sham procedure. However, we found no difference between the two groups in terms of the clinical parameters evaluated.
There were no significant differences in biochemical or anthropometric parameters, between the two groups evaluated. Nevertheless, there were significant postprocedural differences in the microbiota composition between the placebo group. To date, clinical outcomes related to FMT remain uncertain.
Studies with larger patient samples should be carried out in order to assess the potential effects of fecal microbiota transplantation on clinical parameters.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farhat H, Qatar; Sun SY, China S-Editor: Liu JH L-Editor: Webster JR P-Editor: Ju JL
| 1. | Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1964] [Cited by in RCA: 2375] [Article Influence: 339.3] [Reference Citation Analysis (0)] |
| 2. | Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3852] [Cited by in RCA: 4233] [Article Influence: 222.8] [Reference Citation Analysis (0)] |
| 3. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5030] [Article Influence: 628.8] [Reference Citation Analysis (2)] |
| 4. | Strauss RS, Pollack HA. Epidemic increase in childhood overweight, 1986-1998. JAMA. 2001;286:2845-2848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 676] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 5. | Lindsay RS, Howard BV. Cardiovascular risk associated with the metabolic syndrome. Curr Diab Rep. 2004;4:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Reaven GM. Banting Lecture 1988. Role of insulin resistance in human disease. 1988. Nutrition. 1997;13:65; discussion 64, 66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4273] [Cited by in RCA: 4495] [Article Influence: 224.8] [Reference Citation Analysis (0)] |
| 8. | Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 422] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 10. | Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1307] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 11. | Druart C, Dewulf EM, Cani PD, Neyrinck AM, Thissen JP, Delzenne NM. Gut microbial metabolites of polyunsaturated fatty acids correlate with specific fecal bacteria and serum markers of metabolic syndrome in obese women. Lipids. 2014;49:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4401] [Article Influence: 209.6] [Reference Citation Analysis (4)] |
| 13. | Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T; MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2727] [Cited by in RCA: 3196] [Article Influence: 266.3] [Reference Citation Analysis (2)] |
| 14. | Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070-11075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4639] [Cited by in RCA: 4457] [Article Influence: 222.9] [Reference Citation Analysis (1)] |
| 15. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2672] [Article Influence: 222.7] [Reference Citation Analysis (0)] |
| 16. | Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112-5120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4352] [Cited by in RCA: 4564] [Article Influence: 380.3] [Reference Citation Analysis (0)] |
| 17. | Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194-2200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9953] [Cited by in RCA: 9722] [Article Influence: 694.4] [Reference Citation Analysis (0)] |
| 18. | Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590-D596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15104] [Cited by in RCA: 17993] [Article Influence: 1499.4] [Reference Citation Analysis (0)] |
| 19. | Chao, A. (1984). Nonparametric Estimation of the Number of Classes in a Population. Scand J Stat. 11:265-270. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Pylro VS, Roesch LF, Morais DK, Clark IM, Hirsch PR, Tótola MR. Data analysis for 16S microbial profiling from different benchtop sequencing platforms. J Microbiol Methods. 2014;107:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Shannon CE. "A mathematical theory of communication". The Bell System Technical Journal. 1948;27:379-423. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34127] [Cited by in RCA: 14312] [Article Influence: 2385.3] [Reference Citation Analysis (0)] |
| 22. | Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228-8235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5374] [Cited by in RCA: 5540] [Article Influence: 291.6] [Reference Citation Analysis (0)] |
| 23. | Navas-Molina JA, Peralta-Sánchez JM, González A, McMurdie PJ, Vázquez-Baeza Y, Xu Z, Ursell LK, Lauber C, Zhou H, Song SJ, Huntley J, Ackermann GL, Berg-Lyons D, Holmes S, Caporaso JG, Knight R. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013;531:371-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 411] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 24. | Kittelson JM. A Review of: “Fundamentals of Biostatistics, 7th ed., by B. Rosner”. J Biopharm Stat. 2011;859. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Sasson AN, Ingram RJM, Zhang Z, Taylor LM, Ananthakrishnan AN, Kaplan GG, Ng SC, Ghosh S, Raman M. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2021;6:754-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 26. | Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 900] [Article Influence: 112.5] [Reference Citation Analysis (3)] |
| 27. | Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 28. | Shen NT, Maw A, Tmanova LL, Pino A, Ancy K, Crawford CV, Simon MS, Evans AT. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review With Meta-Regression Analysis. Gastroenterology. 2017;152:1889-1900.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 29. | Shah A, Macdonald GA, Morrison M, Holtmann G. Targeting the Gut Microbiome as a Treatment for Primary Sclerosing Cholangitis: A Conceptional Framework. Am J Gastroenterol. 2020;115:814-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Zhang Q, Hu N. Effects of Metformin on the Gut Microbiota in Obesity and Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2020;13:5003-5014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 31. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2005] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 32. | Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611-619.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 674] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 33. | Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, Wang Z, Levison BS, Cleophas MCP, Kemper EM, Dallinga-Thie GM, Groen AK, Joosten LAB, Netea MG, Stroes ESG, de Vos WM, Hazen SL, Nieuwdorp M. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 34. | Zhang Z, Mocanu V, Cai C, Dang J, Slater L, Deehan EC, Walter J, Madsen KL. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 35. | Proença IM, Allegretti JR, Bernardo WM, de Moura DTH, Ponte Neto AM, Matsubayashi CO, Flor MM, Kotinda APST, de Moura EGH. Fecal microbiota transplantation improves metabolic syndrome parameters: systematic review with meta-analysis based on randomized clinical trials. Nutr Res. 2020;83:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 36. | Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, Marchesi JR, McDonald JAK, Pechlivanis A, Barker GF, Miguéns Blanco J, Garcia-Perez I, Wong WF, Gerardin Y, Silverstein M, Kennedy K, Thompson C. Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin Gastroenterol Hepatol. 2020;18:855-863.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 37. | Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1031] [Cited by in RCA: 1183] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 38. | Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 1452] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 39. | Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3630] [Article Influence: 279.2] [Reference Citation Analysis (0)] |
| 40. | Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J; MetaHIT consortium, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1208] [Cited by in RCA: 1507] [Article Influence: 150.7] [Reference Citation Analysis (0)] |
| 41. | Vallianou NG, Stratigou T, Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Athens). 2019;18:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 42. | Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl Environ Microbiol. 2015;81:3655-3662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 408] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 43. | Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther. 2017;46:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 44. | Chu ND, Smith MB, Perrotta AR, Kassam Z, Alm EJ. Profiling Living Bacteria Informs Preparation of Fecal Microbiota Transplantations. PLoS One. 2017;12:e0170922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 46. | Davidovics ZH, Michail S, Nicholson MR, Kociolek LK, Pai N, Hansen R, Schwerd T, Maspons A, Shamir R, Szajewska H, Thapar N, de Meij T, Mosca A, Vandenplas Y, Kahn SA, Kellermayer R; FMT Special Interest Group of the North American Society of Pediatric Gastroenterology Hepatology, Nutrition, the European Society for Pediatric Gastroenterology Hepatology, Nutrition. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection and Other Conditions in Children: A Joint Position Paper From the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2019;68:130-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |