Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4579
Peer-review started: May 4, 2023
First decision: May 12, 2023
Revised: May 15, 2023
Accepted: May 25, 2023
Article in press: May 25, 2023
Published online: July 6, 2023
Processing time: 57 Days and 4 Hours
Polygoni Cuspidati Rhizoma et Radix (PCRR), a well-known traditional Chinese medicine (TCM), inhibits inflammation associated with various human diseases. However, the anti-inflammatory effects of PCRR in acute lung injury (ALI) and the underlying mechanisms of action remain unclear.
To determine the ingredients related to PCRR for treatment of ALI using multiple databases to obtain potential targets for fishing.
Recognized and candidate active compounds for PCRR were obtained from Traditional Chinese Medicine Systems Pharmacology, STITCH, and PubMed databases. Target ALI databases were built using the Therapeutic Target, DrugBank, DisGeNET, Online Mendelian Inheritance in Man, and Genetic Association databases. Network pharmacology includes network construction, target prediction, topological feature analysis, and enrichment analysis. Bioin
Thirteen bioactive compounds corresponding to the 433 PCRR targets were identified. In addition, 128 genes were closely associated with ALI, 60 of which overlapped with PCRR targets and were considered therapeutically relevant. Functional enrichment analysis suggested that PCRR exerted its pharmacological effects in ALI by modulating multiple pathways, including the cell cycle, cell apoptosis, drug metabolism, inflammation, and immune modulation. Molecular docking results revealed a strong associative relationship between the active ingredient and core target.
PCRR alleviates ALI symptoms via molecular mechanisms predicted by network pharmacology. This study proposes a strategy to elucidate the mechanisms of TCM at the network pharmacology level.
Core Tip: This study used network pharmacology and molecular docking to explore the treatment of acute lung injury (ALI) using Polygoni Cuspidati Rhizoma et Radix (PCRR), a traditional Chinese medicine (TCM). Functional enrichment analysis suggested that PCRR alleviated ALI symptoms by modulating multiple pathways, including inflammation, immune modulation, and drug metabolism. We identified 13 bioactive compounds and 60 therapeutically relevant genes. Molecular docking further confirmed the strong association between the active ingredients and core targets. This study provides insights into the mechanisms of TCM at the network pharmacology level for the treatment of ALI.
- Citation: Zheng JL, Wang X, Song Z, Zhou P, Zhang GJ, Diao JJ, Han CE, Jia GY, Zhou X, Zhang BQ. Network pharmacology and molecular docking to explore Polygoni Cuspidati Rhizoma et Radix treatment for acute lung injury. World J Clin Cases 2023; 11(19): 4579-4600
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4579.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4579
Acute lung injury (ALI) is a severe and potentially fatal condition that poses a significant threat to health, with high rates of morbidity and mortality[1,2], which is characterized by an excessive lung inflammatory response. Research indicates that the prolonged quality of life is negatively impacted, even in individuals who recover from ALI[3,4]. ALI can be caused by various factors including severe lung infections caused by viruses, Mycoplasma spp., and bacteria. ALI is characterized by pulmonary edema, tissue infiltration by inflammatory cells, and arterial hypoxemia. These clinical features result in damage to the alveolar epithelium and vascular endothelium, ultimately leading to a decrease in lung function[5-7].
Polygoni Cuspidati Rhizoma et Radix (PCRR) has been used in traditional Chinese medicine (TCM) to treat infections and inflammation[8]; its extracts and bioactive constituents exhibit these pharmacological properties[9,10]. However, the effect of PCRR on the pathogenesis of ALI remains unclear, and currently, no effective treatment has been identified.
Network pharmacology is a novel and powerful method in which bioinformatics integrates chemoinformatics, network biology, network analysis, and traditional pharmacology[11]. It is an interactive network that operates on the basis of the 'disease-gene-target-drug' concept. It offers the ability to predict the mechanism of action of drugs to treat diseases from a macro perspective[12]. The advan
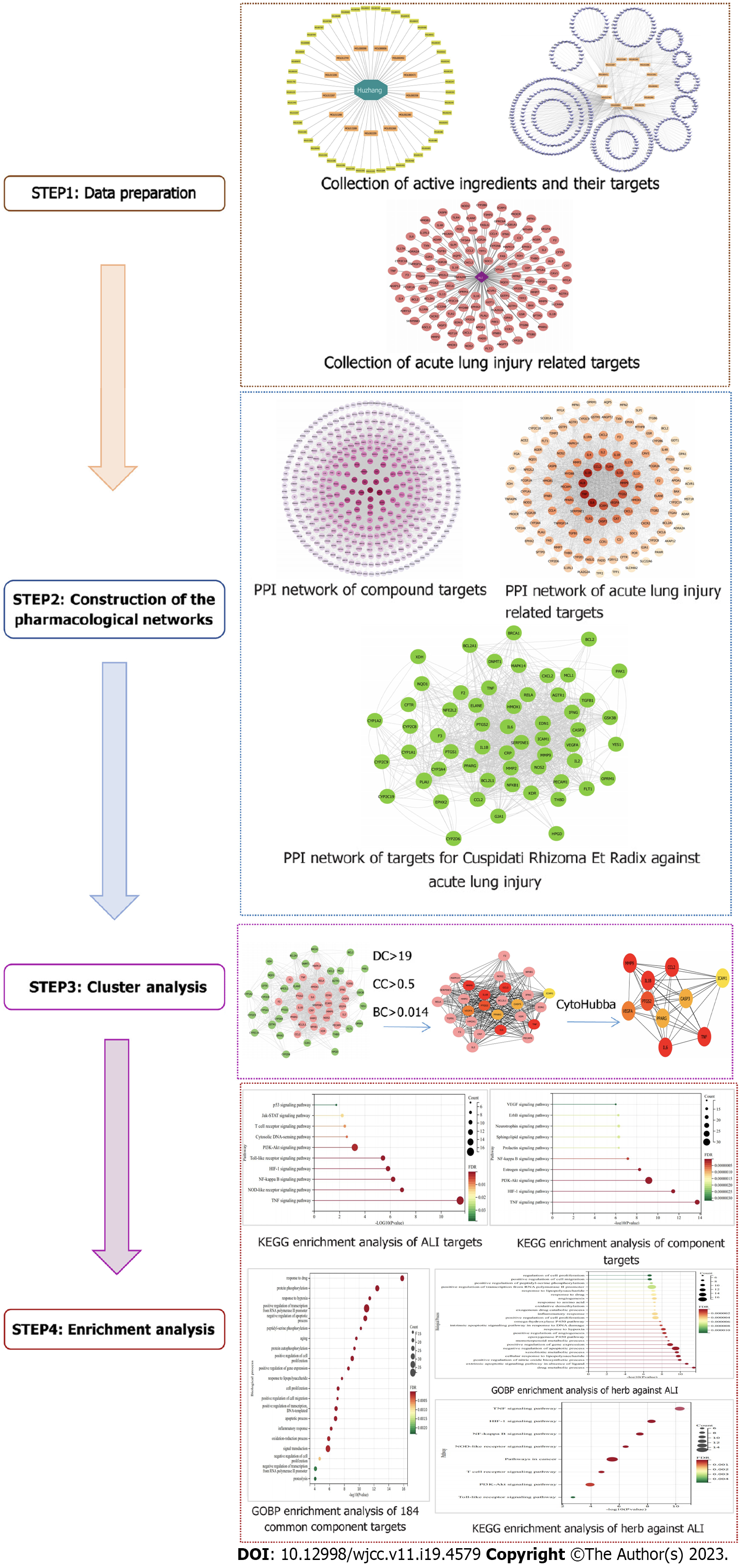
Figure 1 presents a schematic representation of the PCRR network pharmacology and molecular docking study for the treatment of ALI, which was established through four main steps: Data preparation, construction of pharmacological networks, cluster analysis, and enrichment analysis.
Traditional Chinese medicine database and analysis platform version 2.3 (TCMSP, https://tcmsp-e.com/), PubChem (https://pubchem.ncbi.nlm.nih.gov), STITCH version 5.0 (http://stitch.embl.de/), Therapeutic Target Database (TDD, http://db.idrblab.net/ttd/ last updated September 29, 2021), DrugBank (https://go.drugbank.com/); DisGeNET version 7.0 (https://www.disgenet.org/), Online Mendelian Inheritance in Man (OMIM, https://omim.org/, updated November 19, 2021), Genetic Association Database (https://geneticassociationdb.nih.gov/); STRING version 11.5 (https://www.string-db.org/), UniProt (http://ctdbase.org/, updated December 18, 2019), DAVID version 6.8 (https://david.ncifcrf.gov/home.jsp), Protein Data Bank (https://www.rcsb.org/) Cytoscape 3.7.2, AutoDock Tools 1.5.6, and Pymol2.1 software were used in this study.
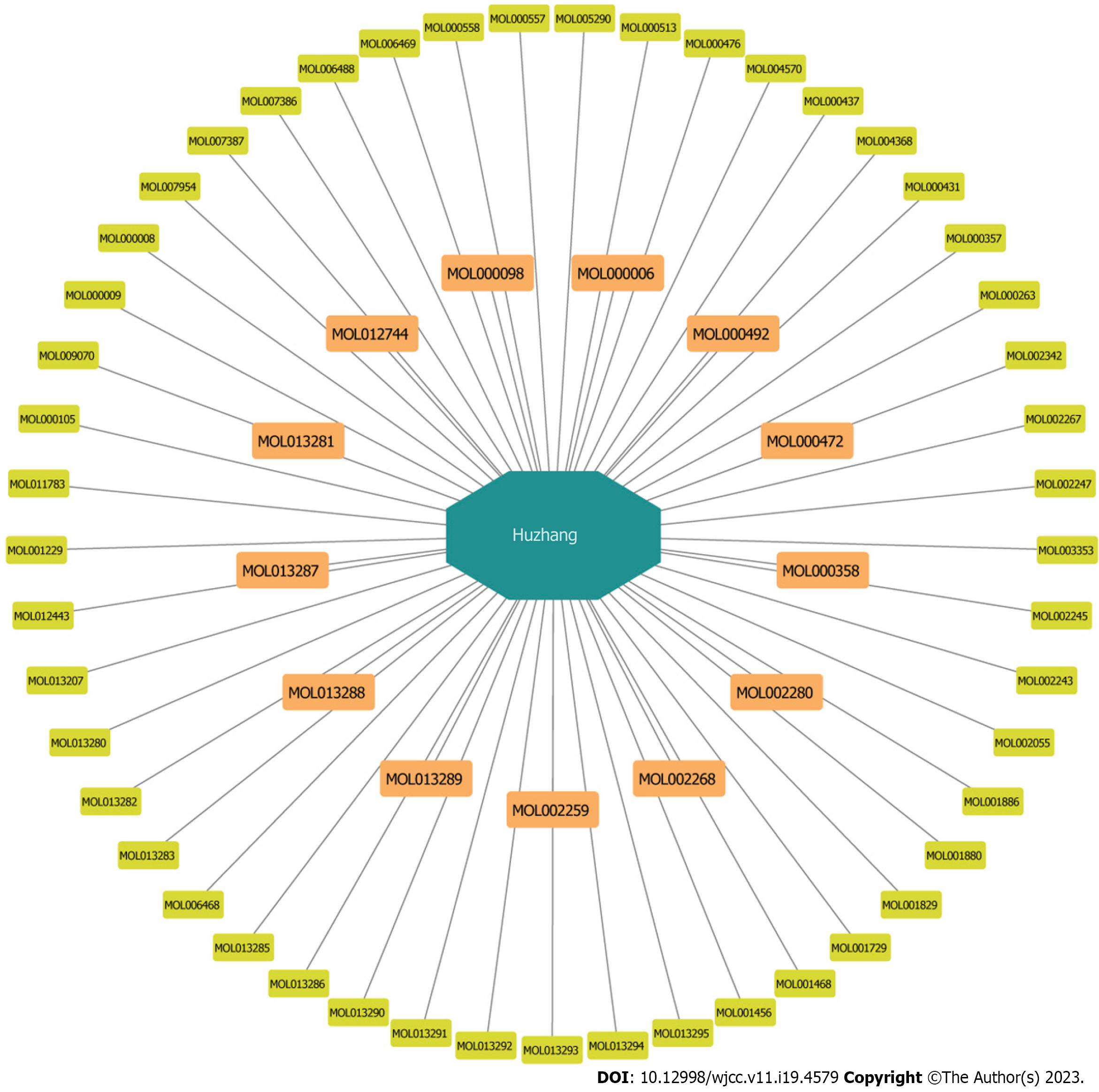
Construction of a database of PCRR components: Data related to the chemical compounds were derived from TCMSP, a unique systems pharmacology platform for Chinese herbal medicines that captures the relationships between drugs, targets, and diseases[13]. In total, 62 PCRR compounds were identified after removing duplicate data.
Screening for active ingredients of PCRR: The active ingredients of PCRR were collected from TCMSP based on the following absorption, distribution, metabolism, and excretion (ADME) parameters: Oral bioavailability (OB) > 30% and drug likeness (DL) > 0.18. The OB value was calculated on a silicon system using the OBioavail 1.1 model to determine the relationship between the compound, cytochrome P450 3A4 enzyme, and transporter (p-glycoprotein). The DL value is used to determine the ability of a prospective compound to become a drug by calculating the similarity between a potential compound and a known drug. Although drug-like compounds are not drugs, they have the potential to become drugs. For example, a bioactive molecule with high OB displays good DL, which is a qualitative concept utilized in drug design to optimize the pharmaceutical and pharmacokinetic properties of molecules, such as chemical stability and solubility. We selected 22 compounds with OB values of > 30% as screening targets. Next, we used TCMs with DL > 0.18 as the selection criteria and obtained 43 potential compounds.
Predicting PCRR targets: We searched for active chemical targets using the TCMSP2.3 (https://tcmsp-e.com/), PubChem (https://pubchem.ncbi.nlm.nih.gov), and STITCH5.0 (http://stitch.embl.de/) databases.
Targets associated with ALI: We collected therapeutic target data for ALI treatment from five resources: (1) Therapeutic Target Database (TTD, http://db.idrblab.net/ttd/); (2) DrugBank (https://go.drugbank.com/); (3) DisGeNET (https://www.disgenet.org/); (4) Online Mendelian Inheritance in Man (OMIM, https://omim.org/); and (5) Genetic Association Database (GAD, https://geneticassociationdb.nih.gov/). In these databases, we used the keyword “acute lung injury” to search for known therapeutic targets of diseases, and all target names were entered into the UniPort website (http://www.uniprot.org/) to search for human target gene names. Then, the ALI-related targets were imported into the DAVID database (http://david.abcc.ncifcrf.gov, version 6.7) for GO enrichment and KEGG network pathway analysis.
PPIs between human proteins were acquired from the STRING11.5 database (https://www.string-db.rg/). STRING consists of physical and functional interactions integrated from experimental repositories, public text collections, and computational prediction methods[14]. The action target was imported into the STRING database, defined as a human protein, and an interaction relationship was obtained. The result was saved in TSV format, which retained the node (nodes 1 and 2) and combined rate score information.
Network construction was performed in three steps. First, a PPI network of compound targets was developed by linking the compound targets. Second, a PPI network of ALI targets was constructed by linking known PCRR-related targets. Third, a PPI network of the ALI and PCRR targets was developed by intersecting these two networks. Graphical and diagrammatic networks were constructed using Cytoscape (version 3.7.2), a software package used to visualize network analysis.
The network analyzer function in Cytoscape 3.7.2 was used to further analyze mutual relations in the network model. Three topological properties, “degree,” “betweenness centrality,” and “closeness centrality” were adopted to estimate target topological importance. In addition, we selected significant cluster modules from the constructed PPI network using CytoHubba in Cytoscape 3.7.2.
We used DAVID 6.8[15,16] (https://david.ncifcrf.gov/) for GO and KEGG (http://www.kegg.jp/) pathway enrichment analyses. Smaller P values indicated greater enrichment, and only functional annotations with enrichment P values corrected by both Bonferroni and Benjamini (P < 0.05) were selected for further analysis.
Six potential action targets were selected from the top 10 median values in the Cytoscape 3.7.2 software analysis results. We performed molecular docking of six potential targets and key active ingredients. Crystal structures of the target proteins were obtained from the Protein Data Bank (https://www.rcsb.org/). After removing extraneous small molecules from the protein molecules using Pymol 2.1 software, the protein molecules were imported into the AutoDock Tools 1.5.6 software to remove water molecules, add hydrogen atoms, set the atom types, and finally saved as pdbqt files. Compound structures were imported from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and energy minimization of the downloaded compounds was performed using Chem3D, followed by conversion to the mol2 format. The small-molecule compounds were imported into the AutoDock Tools 1.5.6. Atomic charges were added, atomic types were assigned, all flexible bonds were made rotatable by default, and finally saved as pdbqt files. Finally, bulk molecular docking was performed using AutoDock Vina. The molecular docking results were analyzed, and the binding interactions of the compounds with the proteins were visualized using the Pymol 2.1 software.
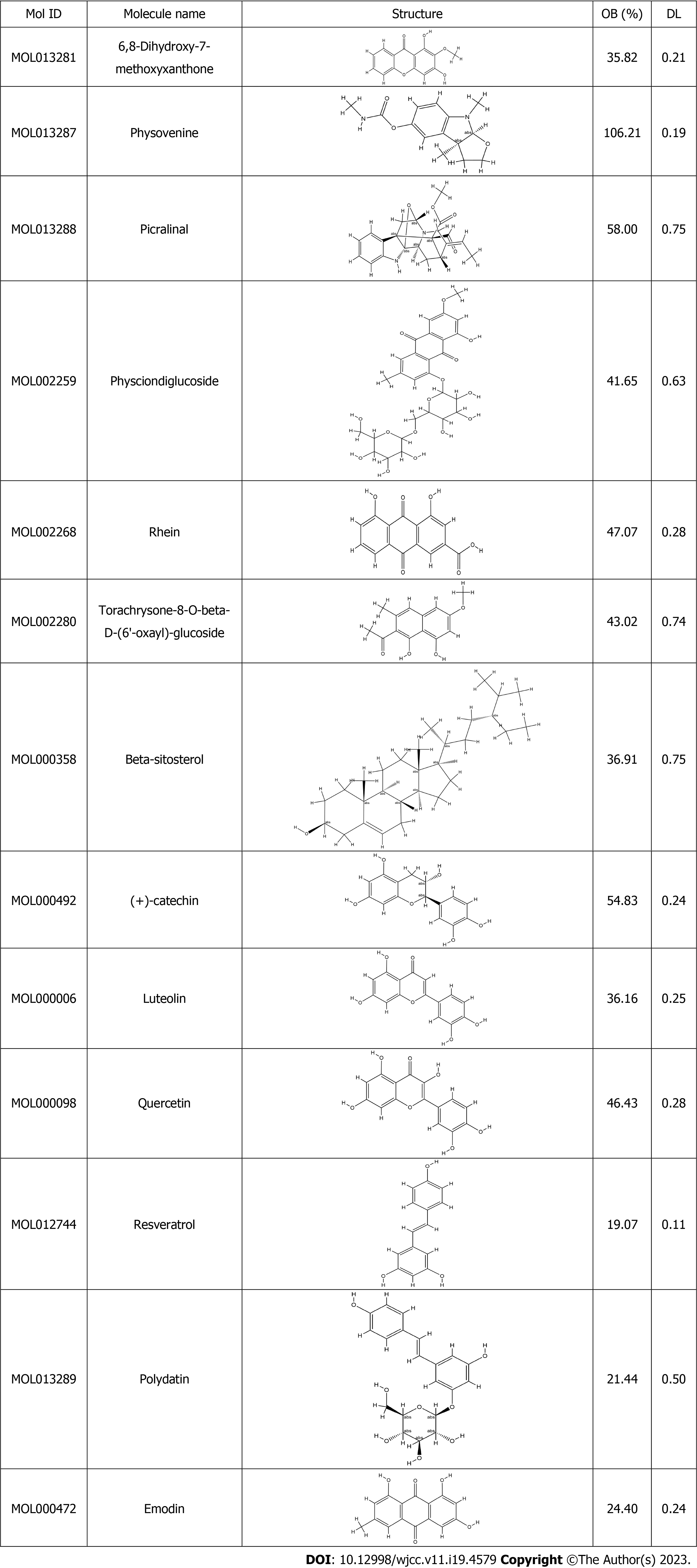
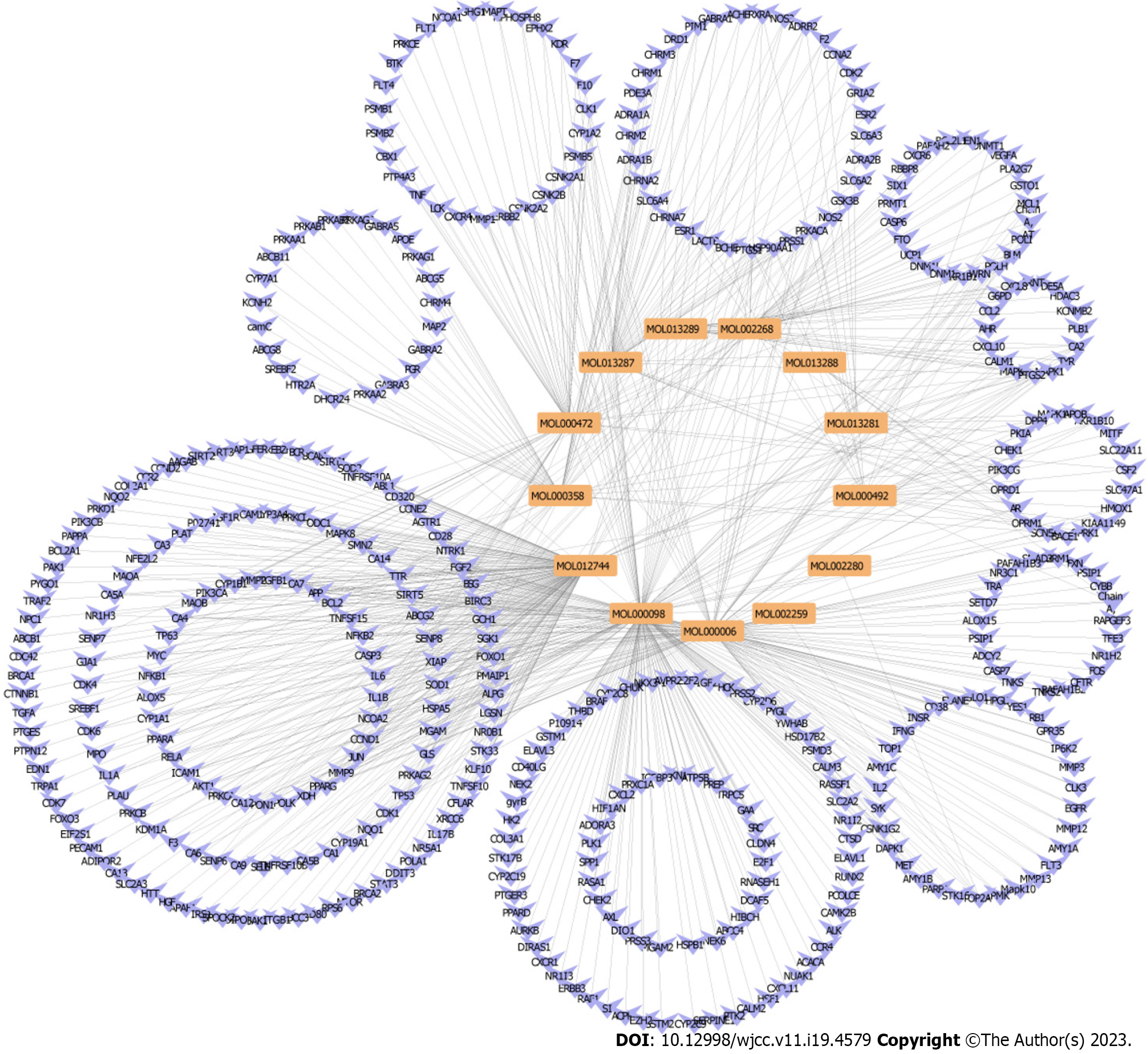
A search of the TCMSP database yielded 62 active ingredients for PCRR. Of these, 10 were initially screened based on their ADME parameters, specifically their oral bioavailability (OB) and drug-likeness (DL) (Figure 2). In addition, through comparison with literature data, polydatin[17,18], resveratrol[19,20], and emodin were identified as active ingredients[21,22] with anti-inflammatory effects. After identifying the relevant structure and target data from PubChem, 13 active ingredients and their corresponding targets were identified after the final screening (Figure 3).
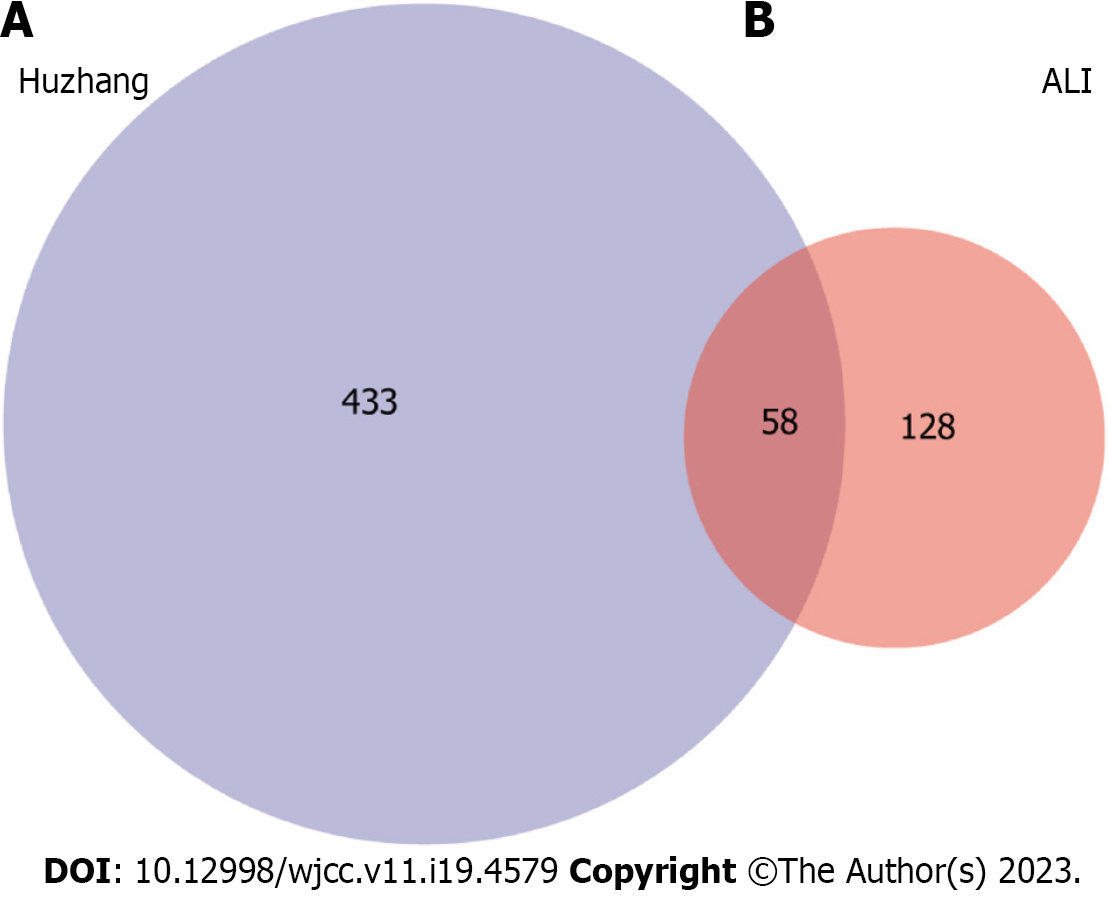
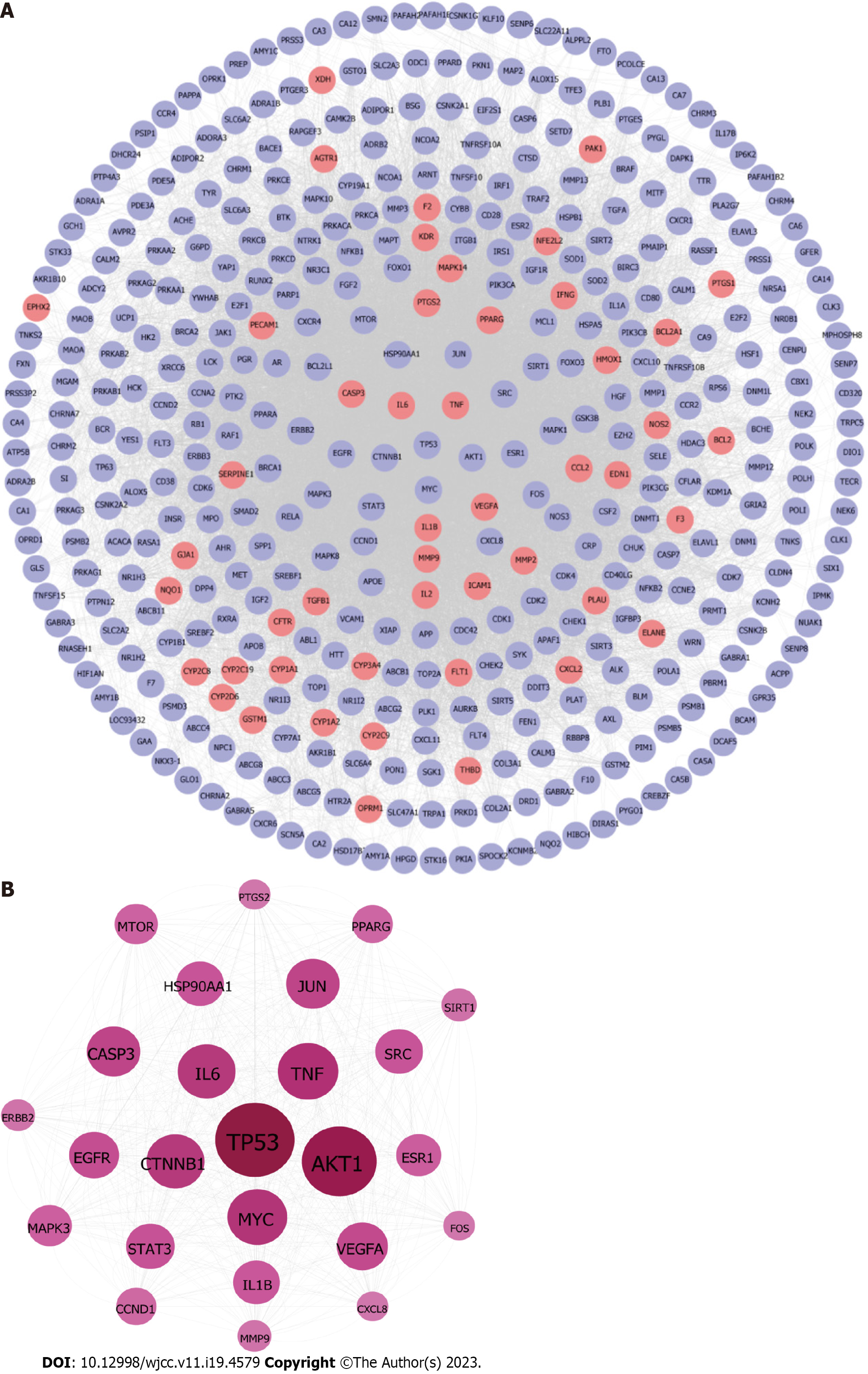
PCRR protein targets were searched using the TCMSP, STITCH, and PubChem databases for each of the 13 active chemical components. A total of 433 targets of the active ingredients of PCRR were identified after high-possibility screening and deduplication. In our study, we found that 184 out of the 433 putative targets were shared by two or more of these components (Figure 4). This indicates that these components are likely to be involved in similar biological processes (Figure 5A). This reflects the synergistic effects of multiple components on inflammation and the importance of these targets in the pathogenesis of ALI. These target genes include vascular endothelial growth factor A (VEGFA)[23], transforming growth factor beta-1 (TGFβ1)[24], and acetylcholinesterase[25], which are involved in the response to hypoxia, inflammation, and cell proliferation. Additionally, 184 targets were imported into the DAVID database to conduct KEGG network pathway analysis. The top 10 signal transduction pathways were selected by excluding a wide range of pathways from KEGG enrichment results: Tumor necrosis factor (TNF) signaling pathway, hypoxia-inducible factor-1 (HIF-1) signaling pathway, hosphoinositide 3-Kinase (PI3K)–Akt signaling pathway, estrogen signaling pathway, NF-kappa B (NF-kB) signaling pathway, prolactin signaling pathway, neurotrophin signaling pathway, sphingolipid signaling pathway, ErbB signaling pathway, and VEGF signaling pathway (Figure 5B).
ALI-related targets were identified using various databases, including TTD, DrugBank, DisGeNET, OMIM, and GAD. After compensating for redundant data, 128 ALI-related targets were identified (Figure 6A). Because the database names were irregular, official symbols were converted into UniProt IDs for subsequent analyses. The targets related to ALI were imported into the DAVID database to perform KEGG network pathway analysis. To narrow down the KEGG enrichment results, a selection was made to exclude certain pathways. As a result, the top 10 signal transduction pathways were identified: TNF signaling pathway, NOD-like receptor signaling pathway, NF-kB signaling pathway, HIF-1 signaling pathway, toll-like receptor (TLR) signaling pathway, PI3K–Akt signaling pathway, cytosolic DNA-sensing pathway, T cell receptor signaling pathway, JAK–signal transducer and activator of transcription (STAT) signaling pathway, and p53 signaling pathway (Figure 6B).
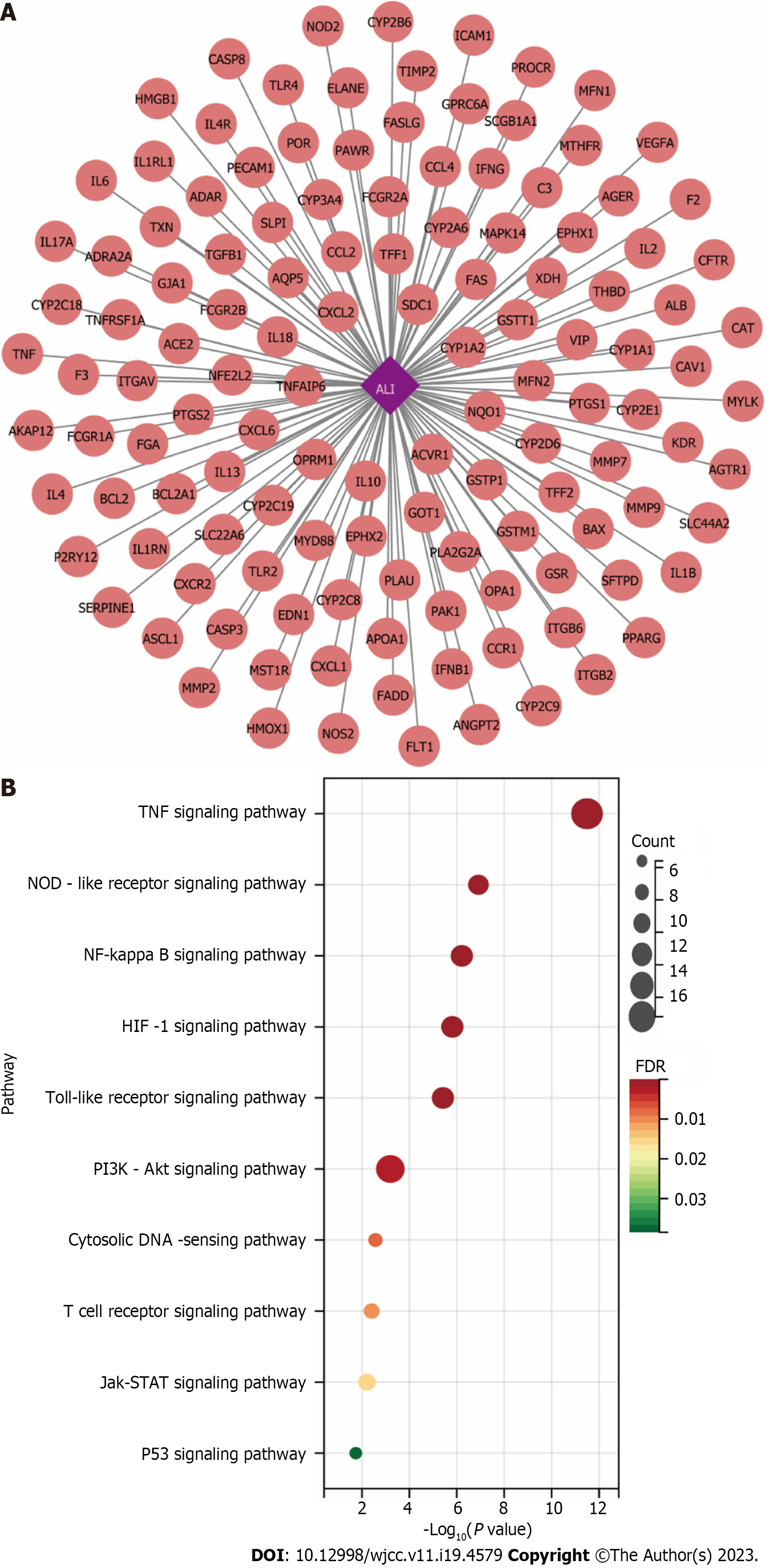
We identified 60 common targets between PCRR and ALI based on the intersection of protein targets (Figure 7). To construct and analyze the PPI network, we imported fifty eight target genes associated with the active ingredients and ALI into the STRING database.
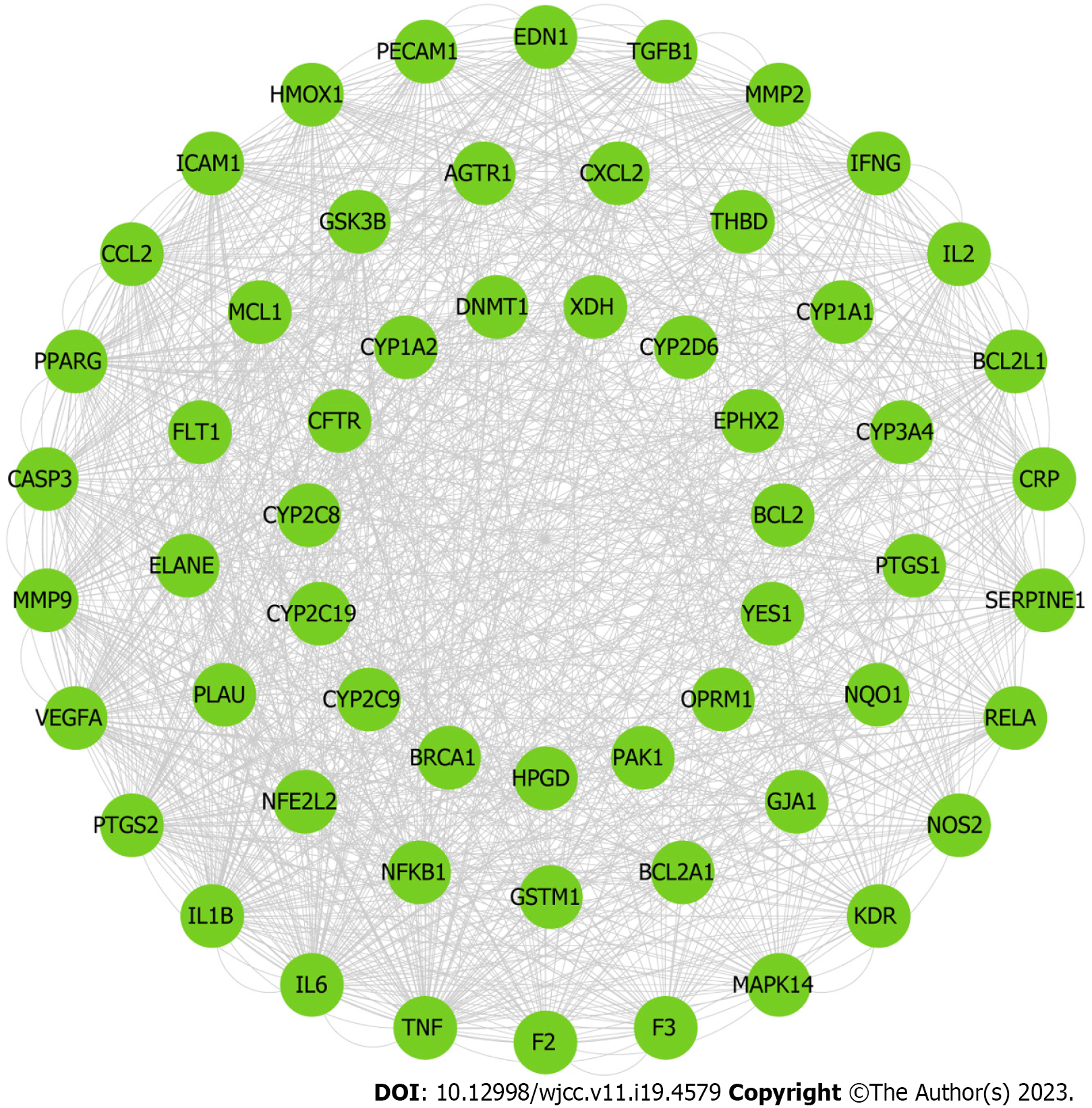
In order to determine the interactive effects of compounds modulated by PCRR, PPI networks of compound targets were developed. These networks consisted of 422 nodes (364 compound targets and 58 compound/ALI targets) and 16702 edges (Figure 8A) and were used to identify the interactions of PCRR-related proteins. To identify significant PCRR-related targets, we considered the median values for degree (39), betweenness centrality (0.003), and closeness centrality (0.45). As a result, we found 25 highly connected nodes with degree greater than 117, betweenness centrality higher than 0.003, and closeness centrality greater than 0.45. (Figure 8B). These targets included STAT3, cellular tumor antigen p53, receptor tyrosine-protein kinase erbB-2, epidermal growth factor receptor, interleukin (IL)-1β, caspase-3 (CASP3), peroxisome proliferator-activated receptor gamma (PPARG), heat shock protein HSP 90-alpha (HSP90AA1), NAD-dependent protein deacetylase sirtuin-1, catenin beta-1, serine/threonine-protein kinase mTOR (MTOR), prostaglandin G/H synthase 2 (PTGS2), transcription factor AP-1, proto-oncogene tyrosine–protein kinase Src, IL6, TNF, nuclear receptor subfamily 3 group A member 1 (ESR1), proto-oncogene c-Fos, RAC-alpha serine/threonine-protein kinase (AKT1), Myc proto-oncogene protein, VEGFA, C-X-C motif chemokine 8, G1/S-specific cyclin-D1, matrix metalloproteinase-9 (MMP9), and mitogen-activated protein kinase 3.
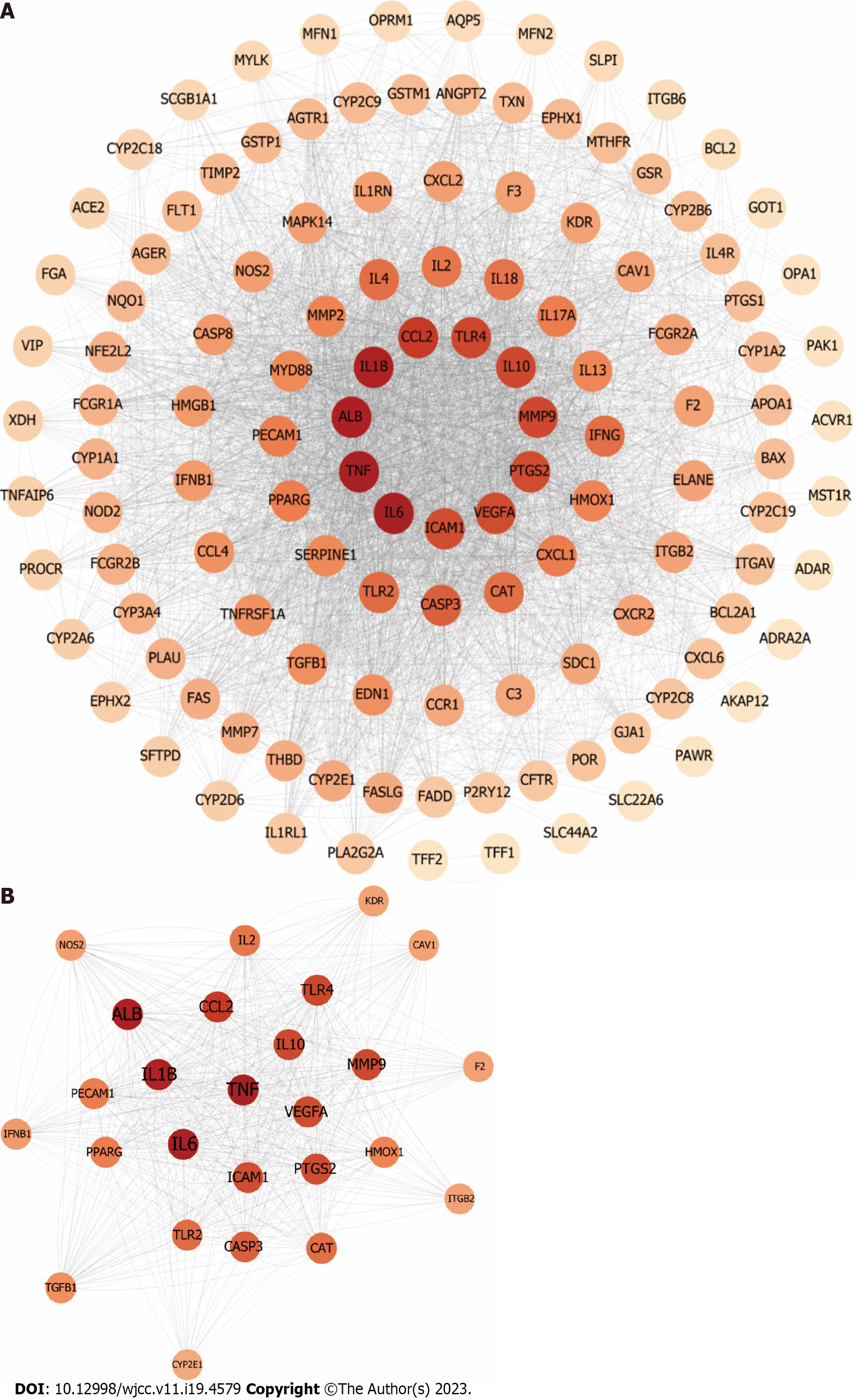
PPI networks of ALI-related targets were developed to identify the interactions of ALI-related targets with 125 nodes (ASCL1, TFF1, and TFF2 were not involved in protein interactions) and 3498 edges (Figure 9A). Significant ALI-related targets were identified as highly connected nodes with degree > 27, betweenness centrality > 0.49, and closeness centrality > 0.5, based on the median values for these measures (Figure 9B). These targets included PTGS2, cytochrome P450 2E1, PPARG, CASP3, IL1β, IL-2, heme oxygenase 1, platelet endothelial cell (EC) adhesion molecule, kinase insert domain receptor, MMP9, intercellular adhesion molecule 1 (ICAM1), TGFβ1, caveolin-1, integrin beta-2, nitric oxide synthase, IL-10, prothrombin, TLR4, TLR2, interferon beta, VEGFA, C-C motif chemokine 2 (CCL2), serum albumin, catalase, TNF, and IL-6.
A network of interacting targets was identified using Cytoscape, comprising of 58 targets. PIM1 and DRD1 were not involved in any protein interactions. The network consisted of a total of 1144 edges, which represented the interactions between the proteins (Figure 10).
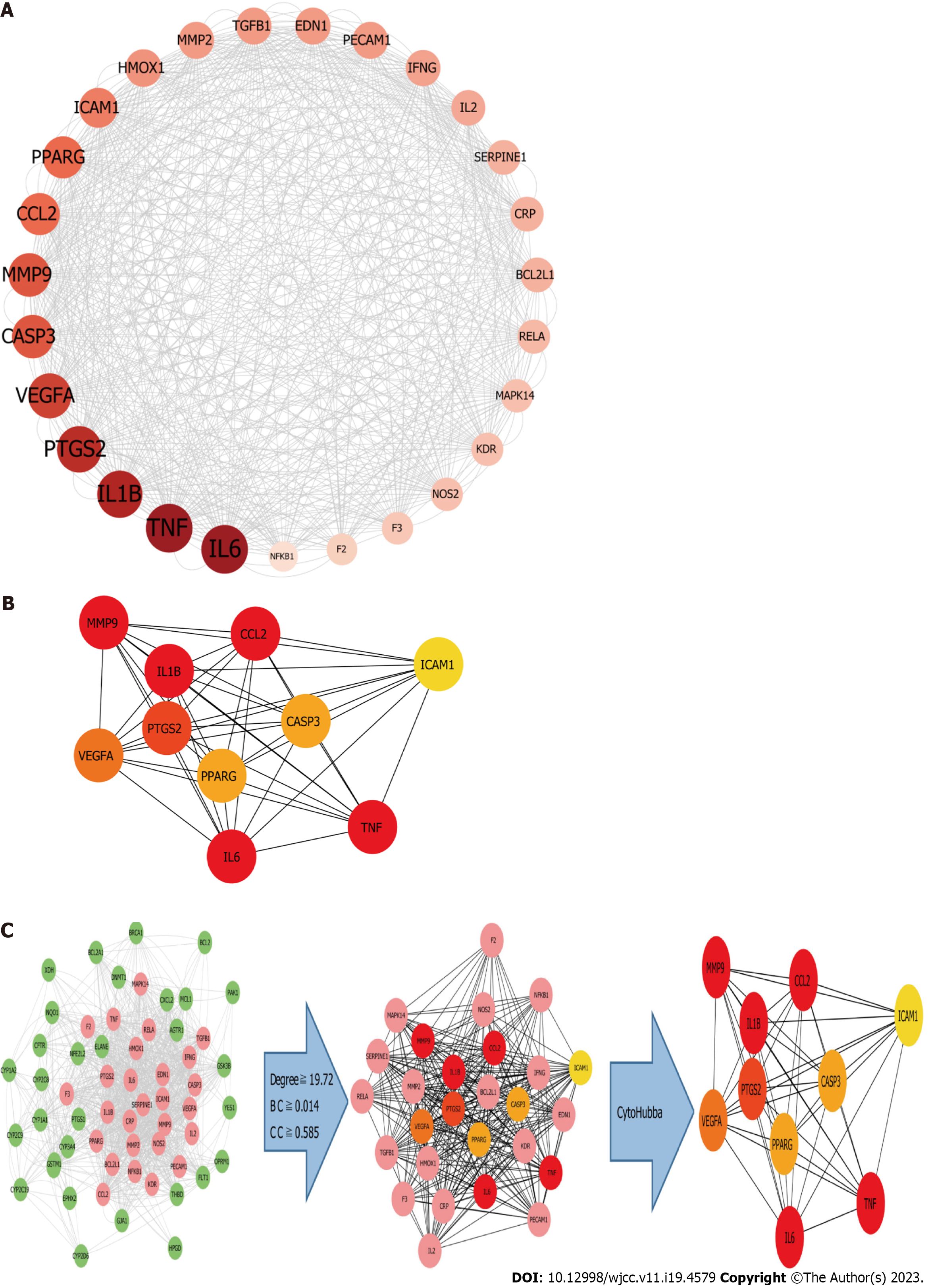
The analysis of the active ingredient-disease-target PPI network was conducted using three key parameters: degree, betweenness centrality, and closeness centrality, to identify topological features.In order to identify important targets, we considered those with median values above certain thresholds (“degree” ≥ 19.72, “betweenness centrality” ≥ 0.014, and “closeness centrality” ≥ 0.585). The results yielded 27 hub nodes and 296 edges (Figure 11A). The 27 key targets were analyzed using CytoHubba and ranked by MCC for the top 10 nodes (Figure 11C). The top ten targets were MMP9, CCL2, IL6, IL1B, ICAM1, TNF, VEGFA, PPARG, CASP3, and PTGS2 (Figure 11B).
Enrichment analysis was conducted using DAVID 6.8 on 58 targets, with a screening threshold of FDR < 0.01. Our analysis identified 83 GO items and eight KEGG pathways that were deemed relevant for further investigation.
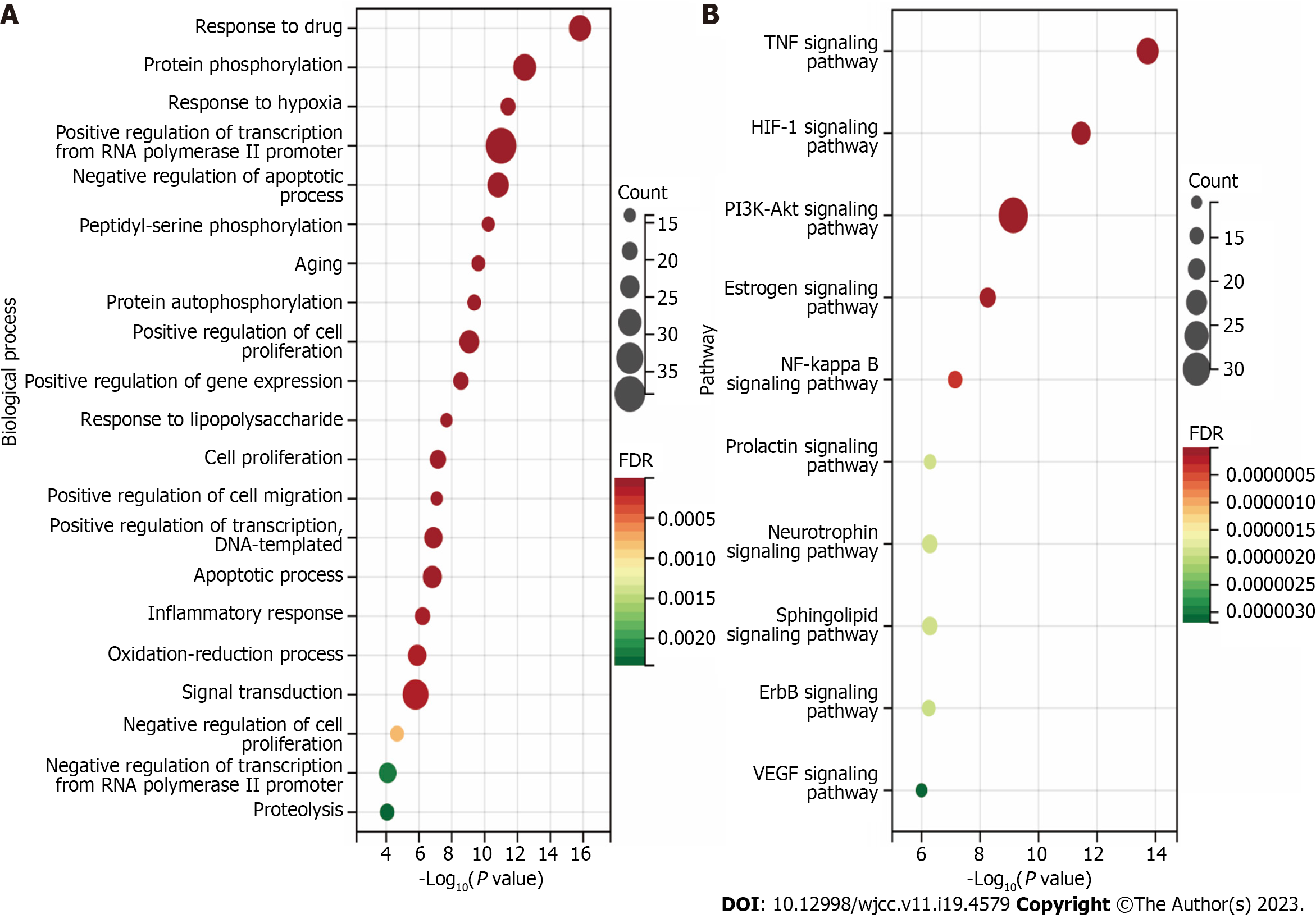
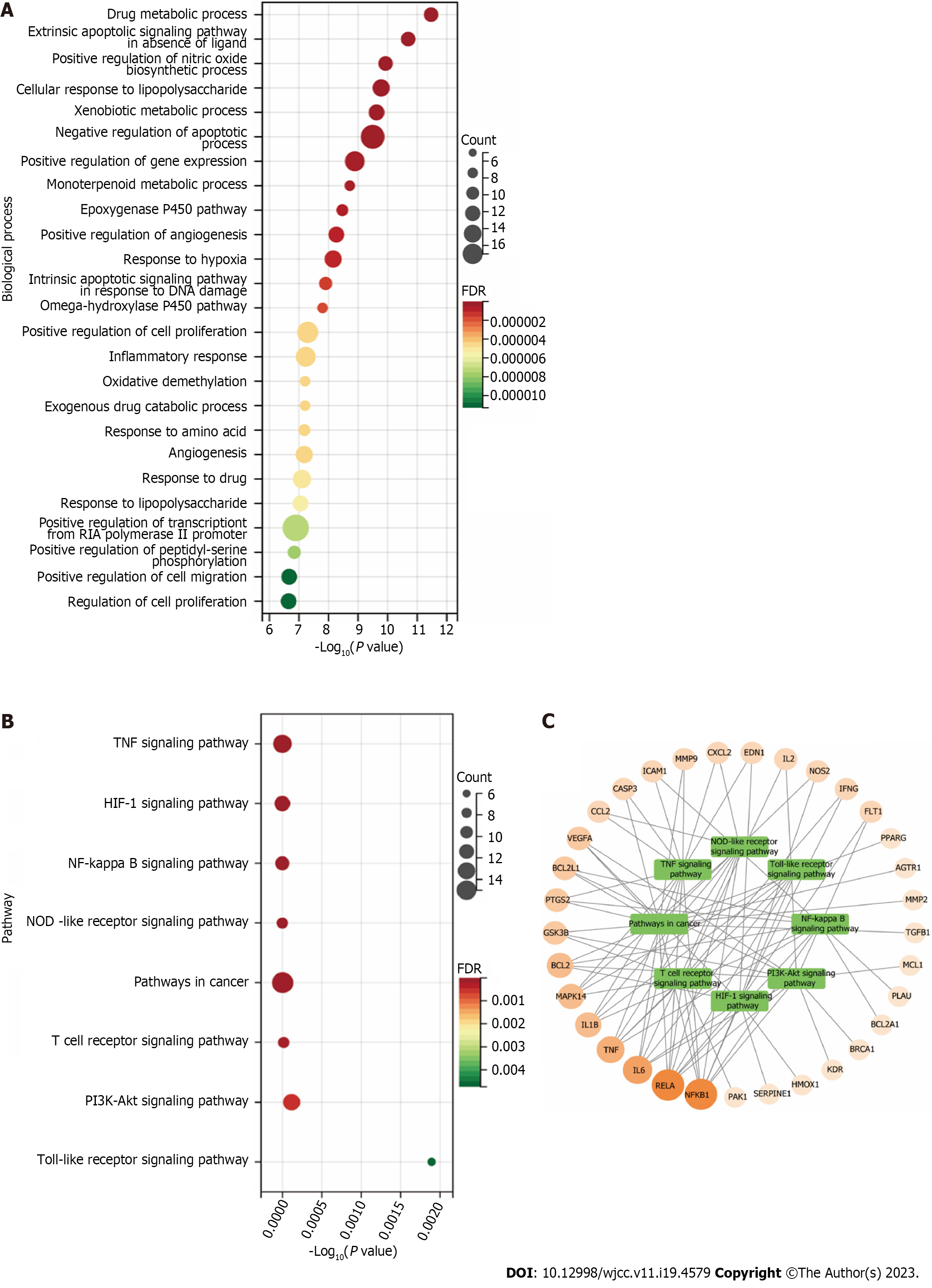
GO BP enrichment analysis: We ranked the 83 projects based on their FDR values and identified the top 25 biological processes (Figure 12A). They are primarily involved in apoptosis, proliferation, inflammatory responses, angiogenesis, and drug response. We identified the extrinsic apoptotic signaling pathway in the absence of a ligand (GO:0097192), negative regulation of the apoptotic process (GO:0043066), and the intrinsic apoptotic signaling pathway in response to DNA damage (GO:0008630). Regarding the regulation of proliferation, we identified positive regulation of cell proliferation (GO:0008284), regulation of cell proliferation (GO:0042127), positive regulation of cell migration (GO:0030335), positive regulation of gene expression (GO:0010628), and positive regulation of transcription from RNA polymerase II promoter (GO:0045944). To regulate the inflammatory response, we identified the cellular responses to lipopolysaccharide (GO:0071222), inflammatory response (GO:0006954), response to lipopolysaccharide (GO:0032496), and positive regulation of the nitric oxide biosynthetic process (GO:0045429). Finally, in drug response regulation, we identified drug metabolic process (GO:0017144), exogenous drug catabolic process (GO:0042738), and response to drugs (GO:0042493). Based on these five aspects, the therapeutic effect of PCRR on ALI may result from a complex multi-pathway synergetic effect.
KEGG pathway enrichment analysis: To investigate the possible mechanism behind the therapeutic effect of PCRR on ALI, we performed a KEGG pathway enrichment analysis on 60 targets. We screened eight pathways based on a threshold of FDR < 0.01 after excluding a wide range of pathways, including the TNF signaling pathway (hsa04668), HIF-1 signaling pathway (hsa04066), NF-kB signaling pathway (hsa04064), NOD-like receptor signaling pathway (hsa04621), pathways in cancer (hsa05200), T cell receptor signaling pathway (hsa04660), PI3K–Akt signaling pathway (hsa04151), and TLR signaling pathway (hsa04620) (Figure 12B). We also established a target pathway network based on the PCRR targets in each pathway (Figure 12C). The study's inclusion of mainstream pathways for ALI clinical research and drug treatment lends credibility to its findings. PCRR has multiple anti-inflammatory targets that are abundant and have a highly reliable mechanism of action.
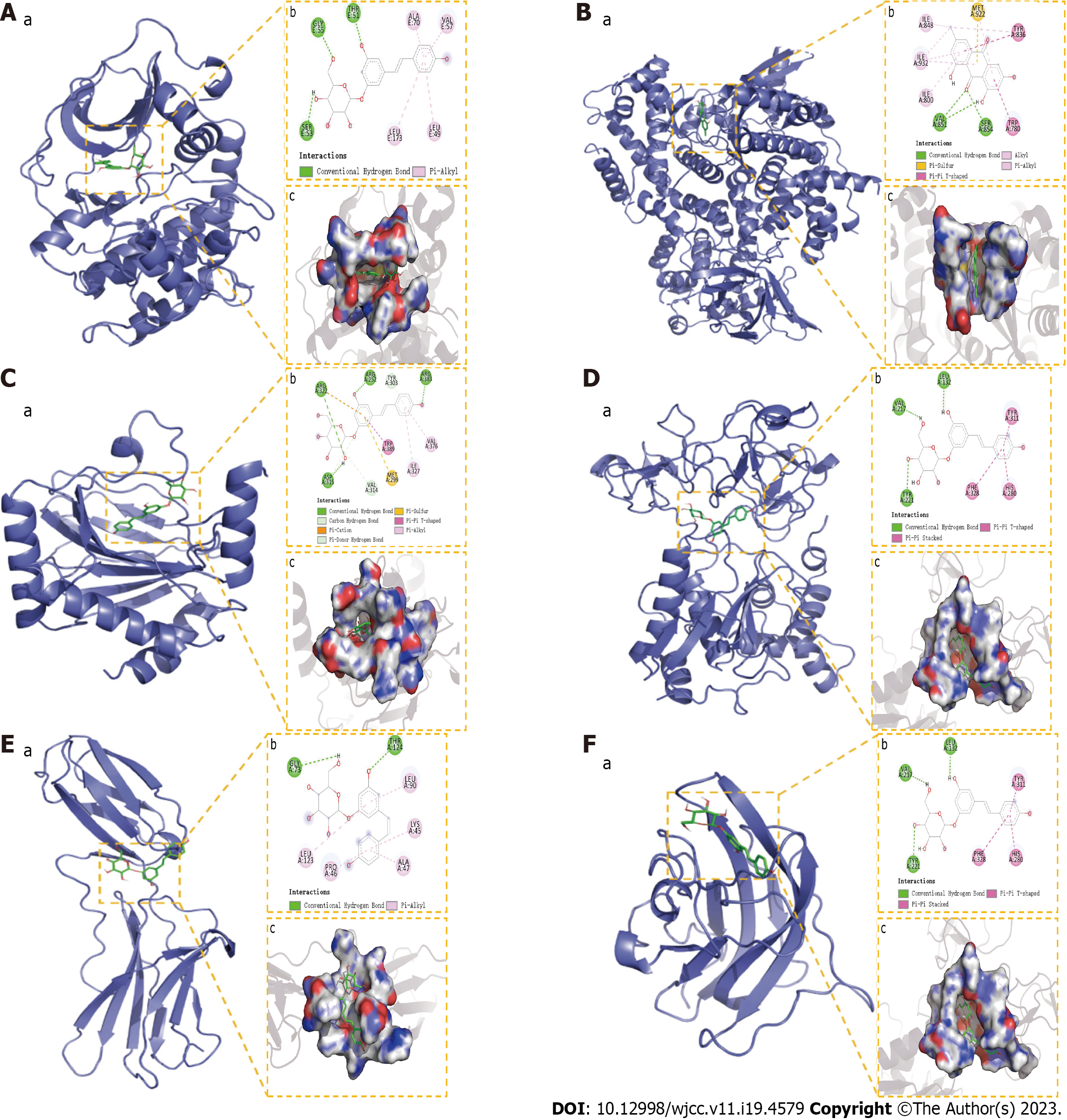
In this study, four compounds (emodin, polydatin, quercetin, and resveratrol) were molecularly docked to IL-6 (PDB ID:4O9H), AKT1 (PDB ID:2UZW), VEGFA (PDB ID:5DN2), PI3K (PDB ID:6PYS), MMP9 (PDB ID: AF-P41245-F1), and HIF-1α (PDB ID:5L9B). According to our findings, the docking score between components and proteins is considered meaningful when it is below -5 kcal/moL. The molecular docking results revealed that most compounds bonded well to the target proteins, with a good match (binding energy of < -5 kcal/moL). These six targets exhibited different binding abilities for the active components of PCRR. Among them, the lowest binding energy was that of polydatin to AKT, with a binding energy of -9.97 kJ/moL, followed by emodin to PI3K with a binding energy of -9.18 kJ/moL, polydatin to HIF-1α at -8.74 kJ/moL, polydatin to MMP9 at -8.61 kJ/moL, polydatin to IL-6 at -7.49 kJ/moL, and polydatin to VEGFA at -7.17kJ/moL. The molecular docking of the ingredients with key targets is demonstrated in Figures 13.
Network pharmacology has attracted increasing attention as a tool for analyzing the potential mechanisms of TCM in the treatment of diseases. TCM is a systematic theoretical approach. Herbal medicines contain various components that have coordinated and integrated roles. Thus, fully elucidating the mechanisms of action of TCMs by analyzing individual ingredients or targets is difficult. To establish the molecular mechanism of TCM in treating diseases, it is crucial to utilize network pharmacology based on big data bioinformatics. The chemical components isolated from PCRR include polydatin, resveratrol, and emodin, which are used to treat inflammatory diseases[26,27]. The main mechanism of ALI treatment using PCRR should be characterized by multiple components, targets, and pathways. Therefore, we explored this potential mechanism using a network pharmacology approach.
Through data screening, 13 active ingredients were identified: Quercetin, resveratrol, luteolin, emodin, beta-sitosterol, rhein, physovenine, (+)-catechin, polydatin, 6, 8-dihydroxy-7-methoxyxanthone, picralinal, physcion diglucoside, and torachrysone-8-O-beta-D-(6´-oxayl)-glucoside. Among them, quercetin has been proven to reduce inflammation and enhance immune function by inhibiting the levels of TNF-α and IL-1α via the TNF, PI3K-Akt, or TLR signaling pathways[28,29]. Resveratrol is well known for its anti-inflammatory and anti-apoptotic properties and has been demonstrated to significantly improve sepsis-induced acute respiratory distress syndrome by decreasing the release of proinflammatory factors and the ratio of alveolar macrophage apoptosis[30]. The attenuation of lung injury by luteolin may be explained by the attenuation of the inflammatory response via the ICAM-1, NF-kB, and partial iNOS signaling pathways[31]; emodin can inhibit the mTOR/HIF-1/VEGF pathway to alleviate ALI[32]. In conclusion, these active ingredients form the basis for the potential effects of PCRR on ALI. Based on the analysis of the target-pathway network and GO-BP analysis results, the potential mechanism of treating ALI using PCRR is probably related to its participation in cell apoptosis, proliferation, inflammatory response, and angiogenesis.
Excessive or inadequate apoptosis contributes to the pathogenesis of various diseases[33]. ECs express abundant VEGF, which promotes EC survival and maintains normal pH. When ECs are affected by adverse external factors, various factors (TNF-α and angiotensin II) are produced, resulting in cell apoptosis. Transcription factors such as HIF[34] regulate VEGFA through direct promoter binding. Experimental studies have indicated that the components that act on VEGF-A and include luteolin, quercetin, resveratrol, and emodin.
Lung epithelial injury results in the release of various soluble factors from fibroblasts, ECs, macrophages, injured and adjacent epithelial cells, underlying stroma, and plasma exudates. The PI3K-Akt signaling pathway plays a crucial role in regulating cell proliferation and is also involved in the development of inflammatory diseases[35]. The MMP family of enzymes is not normally expressed in healthy tissues[36]. After epithelial cell damage, MPP9 expression is elevated, and its inhibition enhances wound repair. Components such as luteolin, quercetin, and resveratrol in PCRR may affect cell proliferation by inhibiting MMP9 expression via the PI3K-Akt signaling pathway.
Inflammatory responses play important roles in its initiation and maintenance[37]. Proinflammatory cytokines, such as TNF-α and IL-1β, increase lung epithelium permeability, further inducing lung tissue injury and neutrophil accumulation, leading to pulmonary edema[38]. Increased IL-6 levels in ALI are associated with increased mortality and have been identified as biomarkers for monitoring ALI[39]. Pulmonary infections caused by polybiliary lipid compounds are among the most common causes of ALI. Lipid-polybiliary compounds bind to cell-surface receptors, which may increase inflammation and activate oxidative stress and an inflammatory cascade via the TLR4/NF-kB signaling pathway[40]. Quercetin suppresses TNF-induced apoptosis and inflammation by blocking the NF-kB signaling pathway[41]. Therefore, PCRR can inhibit inflammation by reducing TNF-α, IL-1β, and IL-6 levels by regulating the NF-kB signaling pathway.
Hypoxia, which is a low oxygen tension, results in the activation of genes that are responsible for various functions such as angiogenesis, iron metabolism, glucose metabolism, and cell proliferation/survival. The primary factor mediating this response is HIF-1[42]. VEGF plays a crucial role in promoting angiogenesis by exerting mitogenic and anti-apoptotic effects on endothelial cells (ECs), enhancing vascular permeability, and facilitating cell migration. The possible mechanism of PCRR in ALI treatment is related to upregulated antioxidant activity, angiogenesis promotion, and endothelial barrier repair through the HIF-1α/VEGF signaling pathway.
Using network pharmacology and molecular docking, we predicted the targets of the PCRR ingredients and explored the underlying mechanism of their potential therapeutic effects on ALI, focusing on four aspects: Inhibition of abnormal cell apoptosis, promotion of cell proliferation, reduction of systemic inflammatory response, and promotion of angiogenesis. After conducting a thorough analysis of signaling pathways, it was determined that the PI3K-Akt, HIF-1, and NF-kB pathways were the primary pathways responsible for the therapeutic effects of PCRR in ALI. PCRR may act on relevant targets, such as IL-6, AKT1, VEGFA, PI3K, MMP9, and HIF-1α, to inhibit abnormal cell apoptosis, promote cell proliferation, reduce systemic inflammatory response, and inhibition angiogenesis. TCMs consist of a combination of intricate ingredients that target various pathways and receptors, posing a challenge when attempting to analyze the mechanisms of action of their active components. The biological processes, molecular functions, and signaling pathways enriched by these targets were used to clarify the integral regulation of PCRR in ALI treatment, especially in the induction of infectious diseases, providing an important theoretical basis for subsequent experimental studies.
This study had some limitations. In this study, we employed network pharmacology and molecular docking techniques to forecast the mechanism of action of PCRR in the treatment of ALI. However, we did not conduct experimental verification to support our findings. Therefore, future studies should include cellular or animal experiments to confirm the conclusions of this study.
The background of this study is focused on Polygoni Cuspidati Rhizoma et Radix (PCRR), a traditional Chinese medicine (TCM) known for its anti-inflammatory effects in various human diseases. However, the anti-inflammatory effects and mechanisms of action of PCRR on acute lung injury (ALI) remain unclear. This study aimed to identify the active ingredients of PCRR for the treatment of ALI using multiple databases to obtain potential targets. In this study, a combination of network pharmacology, bioinformatics, and molecular docking techniques were used to investigate the mechanism of action of PCRR in ALI. The identification of these active ingredients and potential targets for treatment could lead to further research to evaluate the efficacy of PCRR as a therapeutic option for ALI.
The research motivation for this study is to explore the anti-inflammatory effects and underlying mechanisms of PCRR on ALI. Despite the known benefits of PCRR in the treatment of various inflammatory diseases, its effectiveness and mechanism of action in ALI are not fully understood. The objective of this study was to use network pharmacology and molecular docking techniques to identify active compounds and potential targets of PCRR for the treatment of ALI. The ultimate goal of this study was to propose a strategy to elucidate the mechanisms of TCM at the network pharmacology level and to provide insights into the development of novel therapeutic options for ALI.
The active compounds in PCRR are involved in the treatment of ALI. Multiple databases were used to identify potential targets for fishing and target genes closely associated with ALI. Network pharmacology and bioinformatics resources were utilized to predict the molecular mechanisms of PCRR in ALI. Verification of the combination of major active ingredients and core targets using molecular docking techniques. In this study, we present a proposed strategy to better understand the mechanisms of TCM through network pharmacology. Additionally, we emphasize the potential therapeutic benefits of PCRR in treating ALI.
The active compounds of PCRR were identified through data collected from various databases including Traditional Chinese Medicine Systems Pharmacology, STITCH, and PubMed. Target ALI databases were built using the Therapeutic Target, DrugBank, DisGeNET, Online Mendelian Inheritance in Man, and Genetic Association databases. Network pharmacology analysis Network construction, target prediction, topological feature analysis, and enrichment analysis were conducted to determine the potential targets and molecular mechanisms of PCRR in ALI. Bioinformatics analysis: Gene Ontology biological processes and Kyoto Encyclopedia of Genes and Genomes network pathway enrichment analyses were performed using bioinformatics resources from the Database for Annotation, Visualization, and Integrated Discovery. The combination of major active ingredients and core targets was verified using molecular docking techniques.
Thirteen bioactive compounds corresponding to the 433 PCRR targets were identified. A total of 128 genes closely associated with ALI were identified, out of which 60 genes overlapped with potential therapeutic targets identified by PCRR. These 60 genes were considered to be relevant for developing therapeutic interventions for ALI. According to functional enrichment analysis, PCRR was found to have pharmacological effects on ALI by regulating various pathways such as the cell cycle, apoptosis, drug metabolism, inflammation, and immune modulation. Molecular docking results revealed a strong associative relationship between the active ingredient and core target. This study suggests a method for understanding how TCM works at the network pharmacology level. Additionally, it emphasizes the potential of PCRR as a treatment for ALI. Overall, these results provide important insights into the potential therapeutic effects and mechanisms of action of PCRR in ALI, which could lead to further research evaluating the efficacy of PCRR as a therapeutic option for ALI.
This study identified 13 bioactive compounds in PCRR that are related to the treatment of ALI. Using network pharmacology and molecular docking techniques, we identified potential targets of PCRR in ALI and predicted the molecular mechanisms underlying its therapeutic effects. The findings of this study indicate that PCRR may have a beneficial impact on reducing inflammation in patients with ALI by regulating various pathways such as the cell cycle, cell apoptosis, drug metabolism, inflammation, and immune modulation. Overall, This study presents a network pharmacology-based approach to understand the mechanisms of TCM and emphasizes the therapeutic benefits of PCRR in treating ALI.
However, further experimental validation is required to confirm the predicted molecular mechanisms and therapeutic effects of PCRR in ALI. Future studies should explore the optimal dosage and administration methods for PCRR in treating ALI, as well as the potential adverse effects. Novel active ingredients and targets related to PCRR for the treatment of ALI can be identified through database mining and experimental verification. The network pharmacology approach used in this study can be applied to investigate the mechanisms of action of other TCMs and their potential therapeutic effects in various diseases. The integration of multiple omics data and machine learning techniques could enhance the accuracy and efficiency of predicting drug-target interactions and uncover the molecular mechanisms of TCM.
In the process of researching and writing this manuscript, I would like to express my gratitude to all those who have helped me.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mitsakakis K, Germany; Rombach M, Germany S-Editor: Ma YJ L-Editor: A P-Editor: Zhang XD
| 1. | Ng CS, Wan S, Arifi AA, Yim AP. Inflammatory response to pulmonary ischemia-reperfusion injury. Surg Today. 2006;36:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 692] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 3. | Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2636] [Cited by in RCA: 2793] [Article Influence: 139.7] [Reference Citation Analysis (0)] |
| 4. | Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32:1115-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Sweeney RM, Griffiths M, McAuley D. Treatment of acute lung injury: current and emerging pharmacological therapies. Semin Respir Crit Care Med. 2013;34:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 603] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 7. | Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev. 2017;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 8. | Hu WH, Chan GK, Lou JS, Wu QY, Wang HY, Duan R, Cheng MY, Dong TT, Tsim KW. The extract of Polygoni Cuspidati Rhizoma et Radix suppresses the vascular endothelial growth factor-induced angiogenesis. Phytomedicine. 2018;42:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Wang YG, Cao Y, Xu XX, Yang JC, Chen F. [Influence of Huzhang (Polygonum cuspidatum) on AQP - 1 and AQP - 5 mRNA expressions and immunohistochemical integral in lung tissue of acute lung injury rats]. Zhonghua Zhongyiyao Xuekan. 2015;33:2396–2399+12-13. [DOI] [Full Text] |
| 10. | Xu XH, Zheng N. [Polydatin protects diabetic nephropathy rats from renal inflammation by regulating the TLR4/NF-kB signal pathway]. Zhongguo Yiyuan Yaoxue Zazhi. 2018;38:1677-1680. [DOI] [Full Text] |
| 11. | Berger SI, Iyengar R. Network analyses in systems pharmacology. Bioinformatics. 2009;25:2466-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 12. | Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2254] [Cited by in RCA: 2793] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 13. | Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1623] [Cited by in RCA: 3117] [Article Influence: 283.4] [Reference Citation Analysis (0)] |
| 14. | Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362-D368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4345] [Cited by in RCA: 5062] [Article Influence: 562.4] [Reference Citation Analysis (0)] |
| 15. | Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24735] [Cited by in RCA: 27890] [Article Influence: 1743.1] [Reference Citation Analysis (0)] |
| 16. | Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10350] [Cited by in RCA: 11152] [Article Influence: 656.0] [Reference Citation Analysis (0)] |
| 17. | Zhao XJ, Yu HW, Yang YZ, Wu WY, Chen TY, Jia KK, Kang LL, Jiao RQ, Kong LD. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 18. | Huang B, Liu J, Meng T, Li Y, He D, Ran X, Chen G, Guo W, Kan X, Fu S, Wang W, Liu D. Polydatin prevents lipopolysaccharide (LPS)-induced Parkinson's disease via regulation of the AKT/GSK3β-Nrf2/NF-κB signaling axis. Front Immunol. 2018;9:2527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 19. | Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 20. | Jardim FR, de Rossi FT, Nascimento MX, da Silva Barros RG, Borges PA, Prescilio IC, de Oliveira MR. Resveratrol and brain mitochondria: a review. Mol Neurobiol. 2018;55:2085-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Cui Y, Chen LJ, Huang T, Ying JQ, Li J. The pharmacology, toxicology and therapeutic potential of anthraquinone derivative emodin. Chin J Nat Med. 2020;18:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Zhu T, Zhang W, Feng SJ, Yu HP. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway. Int Immunopharmacol. 2016;34:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Strauss E, Waliszewski K, Oszkinis G, Staniszewski R. Polymorphisms of genes involved in the hypoxia signaling pathway and the development of abdominal aortic aneurysms or large-artery atherosclerosis. J Vasc Surg. 2015;61:1105-13.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Booth HDE, Wessely F, Connor-Robson N, Rinaldi F, Vowles J, Browne C, Evetts SG, Hu MT, Cowley SA, Webber C, Wade-Martins R. RNA sequencing reveals MMP2 and TGFB1 downregulation in LRRK2 G2019S Parkinson's iPSC-derived astrocytes. Neurobiol Dis. 2019;129:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Prugh AM, Cole SD, Glaros T, Angelini DJ. Effects of organophosphates on the regulation of mesenchymal stem cell proliferation and differentiation. Chem Biol Interact. 2017;266:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Park B, Jo K, Lee TG, Lee IS, Kim JS, Kim CS. Polygonum cuspidatum stem extract (PSE) ameliorates dry eye disease by inhibiting inflammation and apoptosis. J Exerc Nutrition Biochem. 2019;23:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Yong Z, Yang Y, Cheng WT, Fan X. [Effects of polydatin on airway inflammation and TLR4/NF-κB signaling pathway in rats with chronic obstructive pulmonary disease]. Zhongyao Yaoli Yu Linchuang. 2019;35:35-40. [DOI] [Full Text] |
| 28. | Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, Rhee MH. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013;218:1452-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 29. | Milenković M, Arsenović-Ranin N, Stojić-Vukanić Z, Bufan B, Vučićević D, Jančić I. Quercetin ameliorates experimental autoimmune myocarditis in rats. J Pharm Pharm Sci. 2010;13:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Yang L, Zhang Z, Zhuo Y, Cui L, Li C, Li D, Zhang S, Cui N, Wang X, Gao H. Resveratrol alleviates sepsis-induced acute lung injury by suppressing inflammation and apoptosis of alveolar macrophage cells. Am J Transl Res. 2018;10:1961-1975. [PubMed] |
| 31. | Rungsung S, Singh TU, Rabha DJ, Kumar T, Cholenahalli Lingaraju M, Parida S, et al. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine. 2018;110:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Li X, Shan C, Wu Z, Yu H, Yang A, Tan B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1α/VEGF signaling pathway. Inflamm Res. 2020;69:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 33. | Chambers E, Rounds S, Lu Q. Pulmonary endothelial cell apoptosis in emphysema and acute lung injury. Adv Anat Embryol Cell Biol. 2018;228: 63-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Damert A, Ikeda E, Risau W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 158] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Wu MP, Zhang YS, Zhou QM, Xiong J, Dong YR, Yan C. Higenamine protects ischemia/reperfusion induced cardiac injury and myocyte apoptosis through activation of β2-AR/PI3K/AKT signaling pathway. Pharmacol Res. 2016;104:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715-L731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 37. | Brandenberger C, Kling KM, Vital M, Christian M. The role of pulmonary and systemic immunosenescence in acute lung injury. Aging Dis. 2018;9:553-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Zhao G, Zhang T, Ma X, Jiang K, Wu H, Qiu C, Guo M, Deng G. Oridonin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-induced RAW264.7 cells and acute lung injury. Oncotarget. 2017;8:68153-68164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Chen C, Shi L, Li Y, Wang X, Yang S. Disease-specific dynamic biomarkers selected by integrating inflammatory mediators with clinical informatics in ARDS patients with severe pneumonia. Cell Biol Toxicol. 2016;32:169-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Tang J, Xu L, Zeng Y, Gong F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int Immunopharmacol. 2021;91:107272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 41. | Chen T, Zhang X, Zhu G, Liu H, Chen J, Wang Y, He X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine (Baltimore). 2020;99:e22241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 42. | Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70:1469-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1249] [Article Influence: 65.7] [Reference Citation Analysis (0)] |