Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4544
Peer-review started: April 14, 2023
First decision: April 26, 2023
Revised: May 3, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: July 6, 2023
Processing time: 77 Days and 2.4 Hours
Limb body wall complex (LBWC) is a fatal malformation characterized by major defects in the fetal abdominal or thoracic wall, visceral herniation, significant scoliosis or spina bifida, limb deformities, craniofacial deformities, and umbilical cord abnormalities (short or absent umbilical cord). Early diagnosis of this condition is of great clinical significance for clinical intervention and pregnancy decision-making. With the rapid development of fetal ultrasound medicine, early pregnancy (11-13+6 wk) standardized prenatal ultrasound examinations have been widely promoted and applied.
To explore the value of prenatal ultrasound in the diagnosis of fetal LBWC syndrome during early pregnancy.
The ultrasonographic data and follow-up results of 18 cases of fetal LBWC diagnosed by prenatal ultrasound during early pregnancy (11-13+6 wk) were retrospectively analyzed, and their ultrasonographic characteristics were analyzed.
Among the 18 fetuses with limb wall abnormalities, there were spinal dysplasia (18/18, 100%), varying degrees of thoracoschisis and gastroschisis (18/18, 100%), limb dysplasia in 6 cases (6/18, 33%), craniocerebral malformations in 4 cases (4/18, 22%), thickening of the transparent layer of the neck in 5 cases (5/18, 28%), and umbilical cord abnormalities in 18 cases (18/18, 100%), single umbilical artery in 5 cases.
Prenatal ultrasound in early pregnancy can detect LBWC as early as possible, and correct prenatal evaluation provides important guidance value for pregnancy decision-making and early intervention.
Core Tip: Limbs-body wall complex (LBWC) is a fatal malformation characterized by severe defects in the abdominal or chest wall of the fetus, resulting in protruding viscera, severe scoliosis or spina bifida, limb deformities, craniofacial malformations, and abnormal umbilical cord (short or absent). Our study focuses on the significance of using prenatal ultrasound in detecting LBWC during early pregnancy, where we also provide a retrospective analysis of ultrasound data and post-abortion consequences of 18 confirmed cases of LBWC. We aim to investigate further and identify the ultrasound characteristics of LBWC in early pregnancy to enhance our knowledge of this condition.
- Citation: Ye CH, Li S, Ling L. Analysis of characteristic features in ultrasound diagnosis of fetal limb body wall complex during 11-13+6 weeks. World J Clin Cases 2023; 11(19): 4544-4552
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4544.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4544
Limb body wall complex (LBWC) is a fatal malformation characterized by major defects in the fetal abdominal or thoracic wall, visceral herniation, significant scoliosis or spina bifida, limb deformities, craniofacial deformities, and umbilical cord abnormalities (short or absent umbilical cord)[1,2]. Early diagnosis of this condition is of great clinical significance for intervention and pregnancy decision-making. With the rapid development of fetal ultrasound medicine[3,4], early pregnancy (11-13+6 wk) standardized prenatal ultrasound examinations have been widely promoted and applied. However, there are relatively few reports on early pregnancy ultrasound screening for LBWC. In this study, we retrospectively analyzed the sonographic data and post-abortion results of 18 cases diagnosed with LBWC in early pregnancy to explore the ultrasound features of prenatal diagnosis of LBWC in early pregnancy and enhance the understanding of LBWC.
From March 2015 to October 2022, a total of 35486 pregnant women underwent early pregnancy (11-13+6 wk) fetal nuchal translucency (NT) ultrasound examination at our hospital. Among them, 18 cases of LBWC were detected and confirmed after induced abortion. The pregnant women's ages ranged from 21 years to 39 years, with an average age of 28 years. The gestational age of the fetuses was calculated based on the menstrual cycle, and all were singleton pregnancies.
GE Voluson E8 and Philips EPIQ7 color Doppler ultrasound diagnostic instruments were used in our study, with a 2-8 MHz two-dimensional convex array probe. In accordance with the requirements of the Chinese "Prenatal Ultrasound Screening Guidelines", pregnant women were informed of the appropriate gestational age, content, purpose, importance, and limitations of early pregnancy prenatal ultrasound screening. In this study, all patients were placed in a supine position, and during the examination, in addition to routine checks for the number of fetuses, fetal heartbeat, placenta, and amniotic fluid, the main growth parameters of the fetus and the spectrum of the venous duct were measured. In cases with multiple pregnancies, chorionicity and amnionicity were determined. The focus was on obtaining the fetal mid-sagittal plane and measuring the crown-rump length and NT thickness on this plane, taking the average of three measurements. The presence or absence of the fetal nasal bone was also observed.
In addition, the following ultrasound screening planes were included: starting from the fetal head and sequentially examining the skull and brain. The lateral ventricle transverse plane primarily observed the lateral ventricle level cranial halo, cerebral falx, and bilateral choroid plexus in the lateral ventricles. With the abdomen, the umbilical cord abdominal wall entrance transverse plane primarily observed the structure at the umbilical cord abdominal wall entrance. All planes were saved in the workstation. If a defect in the fetal abdominal wall or thoracoabdominal wall was found, it was necessary to carefully observe whether there were abnormal echo masses and the size, shape, and structural echoes of the masses, whether the physiological curvature of the spine had changed, the development of the limbs, and whether there were any abnormalities in the umbilical cord.
As shown in Table 1, the ages of pregnant women ranged from 21 years to 39 years old, with an average of 27-years-old. Gestational age ranged from 11-13+6 wk, with an average gestational age of 12+6 wk. Among the 18 cases, 11 cases of the 18 cases were screened for Down's syndrome in the first trimester and all were found to be low risk.
| No. | Age | Gestational age in wk | Pregnancy history, G/P/A/L | Embryo transplantation | Down syndrome screening status |
| 1 | 29 | 13 | G1P0 | No | Unchecked |
| 2 | 28 | 13+6 | G2P0A1 | Yes | Unchecked |
| 3 | 25 | 13+5 | G1P0 | No | Low-risk |
| 4 | 21 | 12+3 | G1P0 | No | Low-risk |
| 5 | 23 | 12+4 | G1P0 | No | Low-risk |
| 6 | 26 | 12 | G2P0A1 | No | Low-risk |
| 7 | 30 | 11+2 | G2P0A1 | No | Low-risk |
| 8 | 29 | 13 | G1P0 | No | Low-risk |
| 9 | 38 | 11+3 | G1P0 | Yes | Low-risk |
| 10 | 25 | 13+2 | G1P0 | No | Low-risk |
| 11 | 28 | 12+6 | G2P0A1 | No | Low-risk |
| 12 | 24 | 11+6 | G1P0 | No | Low-risk |
| 13 | 32 | 12+3 | G2P1L1 | No | Low-risk |
| 14 | 26 | 12+4 | G1P0 | No | Unchecked |
| 15 | 39 | 13+6 | G1P0 | Yes | Unchecked |
| 16 | 31 | 13+5 | G2P0A1 | Yes | Unchecked |
| 17 | 21 | 13+2 | G1P0 | No | Low-risk |
| 18 | 26 | 13 | G1P0 | No | Low-risk |
As shown in Table 2, all 18 cases of LBWC fetuses were single live fetuses. Among the 11 cases, 5 had increased NT thickness, 2 had cystic hygromas, and 7 were not measurable.
| No. | NT | Nasal bone visible | Ductus venosus |
| 1 | 2.0 | Yes | Visible, no reverse a-wave |
| 2 | Unmeasurable | No | Invisible |
| 3 | Unmeasurable | No | Visible, no reverse a-wave |
| 4 | Unmeasurable | Yes | Invisible |
| 5 | Unmeasurable | No | Invisible |
| 6 | 2.3 | Yes | Visible, no reverse a-wave |
| 7 | 3.8 | Yes | Invisible |
| 8 | Unmeasurable | No | Visible, no reverse a-wave |
| 9 | Unmeasurable | Yes | Invisible |
| 10 | 1.3 | Yes | Visible, no reverse a-wave |
| 11 | 1.8 | Yes | Invisible |
| 12 | 3.4 | Yes | Visible, no reverse a-wave |
| 13 | 1.1 | Yes | Visible, no reverse a-wave |
| 14 | 4.7 | Yes | Visible, no reverse a-wave |
| 15 | 2.8 | Yes | Visible, no reverse a-wave |
| 16 | Unmeasurable | No | Invisible |
| 17 | 3.6 | Yes | Visible, no reverse a-wave |
| 18 | 2.4 | Yes | Visible, no reverse a-wave |
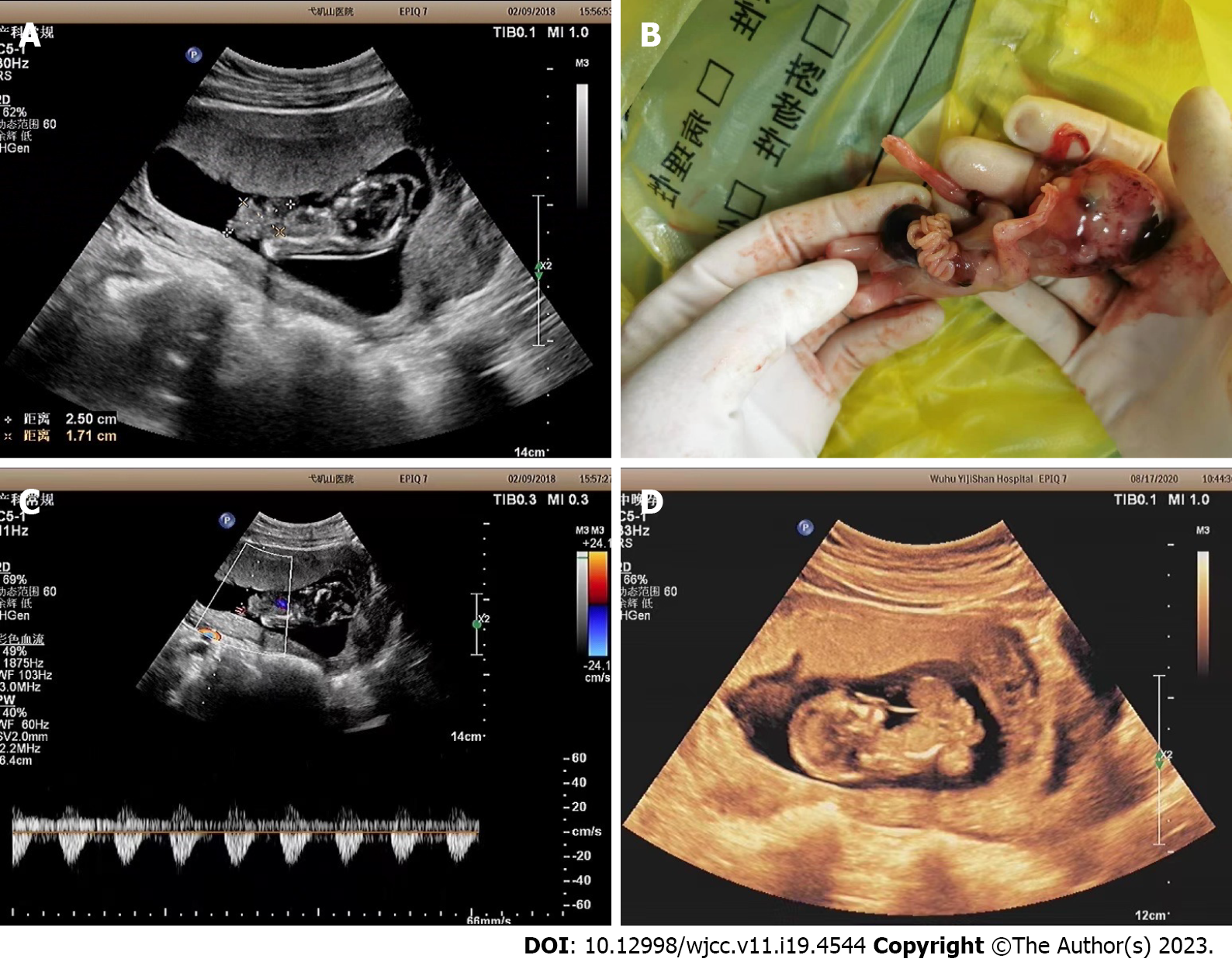
As shown in Table 3, all fetuses had severe thoracoschisis or gastroschisis (18/18, 100%), either spinal scoliosis and torsion (18/18, 100%). Among them, 6 cases (6/18, 33%) had limb developmental abnormalities, 4 cases (4/18, 22%) had cranial malformations, and 1 case had spina bifida. Umbilical cord abnormalities, such as being too short or absent, were observed in 11 cases (11/18, 61%). Color Doppler examination revealed only one umbilical artery in 5 fetuses. None of the fetuses had detectable facial abnormalities (Figure 1). All 18 pregnant women chose to terminate their pregnancies, which was confirmed after induced abortions.
| No. | Ultrasonic anomaly | |||||
| Body wall defect and bulge | Spine | Limbs | Brain | Umbilical cord | Others | |
| 1 | Gastroschisis (bulge is liver) | Torsion | Both feet varus | - | Short | |
| 2 | Gastroschisis (bulge is liver) | Torsion | - | - | Undetectable | Cloacal malformation |
| 3 | Thoracoschisis and Gastroschisis (bulge are heart, liver, intestinal tube) | Twist into angles | - | Brain herniation | Short | |
| 4 | Gastroschisis (bulge are liver, gastric vesicles and intestinal tubes) | Twisted arrangement irregularity | - | Encephalocele | Undetectable | Fetal cystic hygroma |
| 5 | Gastroschisis (bulge are liver, gastric vesicles and intestinal tubes) | Torsion | Miss left upper limb | Meningoencephalocele | Undetectable | |
| 6 | Gastroschisis (bulge are liver, gastric vesicles and intestinal tubes) | Curved, middle and lower segments not shown | - | Short | ||
| 7 | Gastroschisis (bulge are liver, gastric vesicles and intestinal tubes) | Twist into angles | Miss one upper limb | Undetectable | ||
| 8 | Gastroschisis (bulge are liver, gastric vesicles and intestinal tubes) | Abnormal bending, abnormal alignment | Short | Fetal cystic hygroma | ||
| 9 | Gastroschisis (bulge is liver) | Torsion | Meningoencephalocele | Undetectable | ||
| 10 | Gastroschisis (bulge are liver, gastric vesicle and intestinal tube) | Lordotic bending | - | Short | Single umbilical artery | |
| 11 | Thoracoschisis and gastroschisis (bulge are heart, liver, intestinal tube and gastric vesicle) | Lordotic bending | - | Undetectable | ||
| 12 | Gastroschisis (bulge are liver, intestinal tube) | Lordotic bending | - | Short | ||
| 13 | Gastroschisis (bulge are liver, intestinal tube) | Angle the side | - | Short | ||
| 14 | Gastroschisis and malformation (bulge are liver and intestine) | Lordotic bending | Miss left upper arm | - | Short | |
| 15 | Gastroschisis and malformation (bulge are liver, intestinal tube and gastric vesicle) | Side bending and twisting | - | Short | Single umbilical artery | |
| 16 | Gastroschisis (bulge are liver, gastric vesicles and intestinal tubes) | Lordotic bending | Lower limb fusion | - | Undetectable | |
| 17 | Gastroschisis (bulge are liver, gastric vesicles and intestinal tubes) | Torsion | Miss right lower limb | Short | ||
| 18 | Acromphalus (bulge is liver) | Torsion | - | Short | Megacystis | |
LBWC is an uncommon, intricate, and fatal multi-malformation syndrome characterized by encephalocele and/or craniofacial clefts, thoracoschisis or gastroschisis, and limb deformities. Other features may include spinal scoliosis, genitourinary abnormalities, and short umbilical cords. The initial diagnostic criteria for LBWC were established by Van Allen et al[5] in 1987, and a diagnosis can be confirmed by the presence of two of the following three malformations: any type of limb defect, encephalocele or anencephaly with facial cleft, thoracoschisis or gastroschisis with visceral herniation.
The etiology of LBWC remains uncertain, there are three pathogenic mechanisms that have been discussed by foreign scholars[6-10]. Firstly, abnormal folding of the trilaminar embryonic disc during the first 4 wk of embryonic development. Secondly, early amniotic rupture with amniotic band syndrome. Finally, early embryonic vascular rupture leading to widespread blood flow impairment.
(1) Abnormal folding of the trilaminar embryonic disc during the first 4 wk of embryonic development, which represents the complete failure of folding along the three axes (cranial, caudal, and lateral) of the body. Abnormal longitudinal folding results in thoracoschisis or gastroschisis, while abnormal transverse folding prompts abdominal contents to protrude into an expansive amniotic sac, which inserts peripherally into the placental chorionic plate, resulting in absent or short umbilical cords. As a result of the compression of abdominal viscera, the spine and thoracic cavity cannot develop symmetrically, leading to severe spinal scoliosis and trunk abnormalities. Poor spinal rotation and incomplete pelvic closure may lead to congenital limb deformities[7].
(2) Early amniotic rupture. Early amniotic rupture caused by mechanical pressure or amniotic bands can lead to severe deformities such as spinal malformations, gastroschisis, and extremely short umbilical cords if the rupture occurs at the caudal end, with the lower half of the embryo entering the extraembryonic cavity through the rupture[8]. This mechanism was questioned by Paul et al[11], who reported a case of intact LBWC at 10 wk of gestation without rupture of the amniotic cavity.
And (3) early embryonic vascular rupture or developmental abnormality. Some studies[10] suggest that vascular rupture during early pregnancy (at 4-6 wk of gestation) leads to the interruption of normal blood supply to the developing embryo, resulting in ischemia, hypoxia, hemorrhagic necrosis, and abnormal development of the embryonic disc, which in turn affects the closure of the abdominal wall and extraembryonic cavity.
Arshad believes that the pathogenesis of LBWC is more inclined towards the theory of early embryonic abnormal folding[12], with the malformations associated with LBWC depending on the degree of abnormal folding of the embryonic disc. On the other hand, Sahinoglu et al[13] and others believe that due to the complexity of LBWC malformations, all of the above pathogenic mechanisms are possible, leading to different subgroups.
There are many literature reports and controversies about the classification of LBWC, and most studies currently divide it into two types based on the characteristic abnormalities of LBWC[13-16]. Type I is based on craniofacial defects, while Type II is based on thoracoschisis or gastroschisis. However, Sahinoglu et al[13] proposed a new phenotypic classification, dividing it into three types, Type I still refers to craniofacial defects, while Type II refers to gastroschisis located above the umbilicus and large chest-abdominal wall defects. Type III pertains to defects located below the umbilicus, while the chest wall remains intact. This article primarily concentrates on thoracoschisis and gastroschisis, which aligns with the research conducted by Akinmoladun et al[17].
At present, early pregnancy ultrasound examination is considered the safest and most reliable imaging technique for screening severe fetal structural malformations. Owing to the relatively large extraembryonic coelom in early pregnancy, it becomes straightforward to distinctly display the extent of thoracoschisis or gastroschisis as well as the protrusion of organs in the thoracoabdominal cavity into the extraembryonic coelom, making the diagnosis relatively easy. With the widespread popularity of early pregnancy (11-13+6 wk) prenatal ultrasound examinations, many severe fetal structural malformations have been detected. Some scholars believe that the most characteristic ultrasonographic clue for LBWC in early pregnancy is spinal curvature[18]. The basis for ultrasound diagnosis of LBWC may be when the fetus has a large abdominal or chest-abdominal wall defect, herniation of the viscera, short or absent umbilical cord combined with spinal abnormalities[19,20].
The sonographic characteristics of the 18 LBWC fetuses in our investigation can be summarized as follows: (1) The most common and severe malformation in LBWC is the thoracoschisis or gastroschisis[21,22]. Due to the absence of abdominal wall development, the defect area is large and may be accompanied by chest wall defects, resulting in the opening of the thoracoabdominal viscera to the amniotic cavity. Prenatal ultrasound shows a large defect at the insertion of the fetal umbilical cord on the abdominal wall, with abnormal echo masses at the defect site, and the fetal liver, stomach bubble, and intestine protruding into the extraembryonic coelom. The umbilical cord is difficult to display or appears too short. Daskalakis et al[23] and others believe that abdominal wall defects can be roughly divided into three types, gastroschisis, umbilical protrusion, and body stalk-like malformation. LBWC can be considered when accompanied by spinal curvature or torsion, anencephaly or encephalocele, facial abnormalities, and limb development abnormalities; (2) All 18 fetuses had spinal developmental abnormalities, manifested as spinal kyphosis, torsion or scoliosis, causing the fetus to be in a flexed or hyperextended state, making it difficult to obtain the mid-sagittal section required for measuring crown-rump length in prenatal ultrasound screening guidelines, and thus unable to accurately determine the gestational age of the fetus by ultrasound. In this study, all cases were calculated by menstrual age. In this group, the earliest large abdominal wall defect was seen at 11+2 wk. In this group, all cases had varying degrees of thoracoschisis or gastroschisis and abnormal spinal curvature; (3) Increased fetal NT, similar to the findings of Daniilidis et al[24]. In our study, 4 cases out of 18 LBWC cases were normal, 5 cases had increased NT, and 9 cases could not be measured due to spinal torsion or concomitant cranial abnormalities. Although LBWC fetuses often have abnormal NT thickening or fetal edema, 8 cases in this group underwent early pregnancy Down syndrome screening and all were classified as low risk. Previous studies[13,25] have shown that fetuses with LBWC have a normal chromosomal karyotype and the recurrence rate in subsequent pregnancies is very low; (4) Cranial abnormalities. In our 18 cases, 3 had encephalocele, and 1 had exencephaly; (5) Limb abnormalities. In our study, limb abnormalities were manifested as clubfoot, limb agenesis, or fusion; and (6) Short or absent umbilical cord. Other associated malformations include megacystis, fetal cystic hygroma, and abnormal development of the cloacal cavity.
Owing to the numerous resemblances in ultrasound manifestations between LBWC and Cantrell's pentalogy[25], the latter primarily encompasses omphalocele, ectopia cordis, lower sternal defects, anterior diaphragmatic defects, and pericardial defects. Its hallmark features are omphalocele and ectopia cordis. Nevertheless, Cantrell's pentalogy does not involve spinal and limb developmental abnormalities, which serve as crucial distinguishing factors from LBWC[26].
As LBWC is a lethal disease, enhancing early detection is of utmost importance. Prenatal ultrasound should be well-acquainted with the sonographic features of LBWC, such as thoracoschisis and gastroschisis, severe spinal scoliosis, neural tube defects, limb abnormalities, and absence or shortening of the umbilical cord. Furthermore, when spinal scoliosis and omphalocele are observed, the likelihood of LBWC should be strongly considered. In conclusion, early pregnancy ultrasound can effectively diagnose LBWC, and precise prenatal evaluation holds significant value for informing parental decision-making.
There are relatively few reports on early pregnancy ultrasound screening for Limb body wall complex (LBWC). In this study, we retrospectively analyzed the sonographic data and post-abortion results of 18 cases diagnosed with LBWC in early pregnancy to explore the ultrasound features of prenatal diagnosis of LBWC in early pregnancy and enhance the understanding of LBWC.
In this study, we retrospectively analyzed the sonographic data and post-abortion results of 18 cases diagnosed with LBWC in early pregnancy to explore the ultrasound features of prenatal diagnosis of LBWC in early pregnancy and enhance the understanding of LBWC.
To explore the value of prenatal ultrasound in the diagnosis of fetal LBWC syndrome during early pregnancy.
The ultrasonographic data and follow-up results of 18 cases of fetal LBWC diagnosed by prenatal ultrasound during early pregnancy (11-13+6 wk) were retrospectively analyzed, and their ultrasonographic characteristics were analyzed.
Among the 18 fetuses with limb wall abnormalities, there were spinal dysplasia (18/18, 100%), varying degrees of thoracoschisis and gastroschisis (18/18, 100%), limb dysplasia in 6 cases (6/18, 33%), craniocerebral malformations in 4 cases (4/18, 22%), thickening of the transparent layer of the neck in 5 cases (5/18, 28%), and umbilical cord abnormalities in 18 cases (18/18, 100%), single umbilical artery in 5 cases.
Prenatal ultrasound in early pregnancy can detect LBWC as early as possible, and correct prenatal evaluation provides important guidance value for pregnancy decision-making and early intervention.
As LBWC is a lethal disease, enhancing early detection is of utmost importance. Prenatal ultrasound should be well-acquainted with the sonographic features of LBWC, such as thoracoschisis and gastroschisis, severe spinal scoliosis, neural tube defects, limb abnormalities, and absence or shortening of the umbilical cord. Furthermore, when spinal scoliosis and omphalocele are observed, the likelihood of LBWC should be strongly considered. In conclusion, early pregnancy ultrasound can effectively diagnose LBWC, and precise prenatal evaluation holds significant value for informing parental decision-making.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guerriero S, Italy; Matute JD, United States S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Mandrekar SR, Amoncar S, Banaulikar S, Sawant V, Pinto RG. Omphalocele, exstrophy of cloaca, imperforate anus and spinal defect (OEIS Complex) with overlapping features of body stalk anomaly (limb body wall complex). Indian J Hum Genet. 2014;20:195-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Baruah P, Ray Choudhury P. Limb body wall complex with sacrococcygeal mass and agenesis of external genitalia. Case Rep Med. 2013;2013:218626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Wang J, Chen L, Zhou C, Wang L, Xie H, Xiao Y, Yin D, Zeng Y, Tang F, Yang Y, Zhu H, Chen X, Zhu Q, Liu Z, Liu H. Identification of copy number variations among fetuses with ultrasound soft markers using next-generation sequencing. Sci Rep. 2018;8:8134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Yang T, Li R, Liang N, Li J, Yang Y, Huang Q, Li Y, Cao W, Wang Q, Zhang H. The application of key feature extraction algorithm based on Gabor wavelet transformation in the diagnosis of lumbar intervertebral disc degenerative changes. PLoS One. 2020;15:e0227894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Van Allen MI, Curry C, Walden CE, Gallagher L, Patten RM. Limb-body wall complex: II. Limb and spine defects. Am J Med Genet. 1987;28:549-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Daskalakis G, Sebire NJ, Jurkovic D, Snijders RJ, Nicolaides KH. Body stalk anomaly at 10-14 weeks of gestation. Ultrasound Obstet Gynecol. 1997;10:416-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Hartwig NG, Vermeij-Keers C, De Vries HE, Kagie M, Kragt H. Limb body wall malformation complex: an embryologic etiology? Hum Pathol. 1989;20:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Torpin R. Amniochorionic Mesoblastic Fibrous Strings And Amnionic Bands: Associated Constricting Fetal Malformations Or Fetal Death. Am J Obstet Gynecol. 1965;91:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 274] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Russo R, Vecchione R. Limb body wall complex: craniofacial defects as a distinctive factor. Birth Defects Orig Artic Ser. 1996;30:157-164. [PubMed] |
| 10. | Van Allen MI, Curry C, Gallagher L. Limb body wall complex: I. Pathogenesis. Am J Med Genet. 1987;28:529-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 183] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Paul C, Zosmer N, Jurkovic D, Nicolaides K. A case of body stalk anomaly at 10 weeks of gestation. Ultrasound Obstet Gynecol. 2001;17:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Sahinoglu Z, Uludogan M, Arik H, Aydin A, Kucukbas M, Bilgic R, Toksoy G. Prenatal ultrasonographical features of limb body wall complex: a review of etiopathogenesis and a new classification. Fetal Pediatr Pathol. 2007;26:135-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Russo R, D'Armiento M, Angrisani P, Vecchione R. Limb body wall complex: a critical review and a nosological proposal. Am J Med Genet. 1993;47:893-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Pumberger W, Schaller A, Bernaschek G. Limb-body wall complex: a compound anomaly pattern in body-wall defects. Pediatr Surg Int. 2001;17:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Colpaert C, Bogers J, Hertveldt K, Loquet P, Dumon J, Willems P. Limb-body wall complex: 4 new cases illustrating the importance of examining placenta and umbilical cord. Pathol Res Pract. 2000;196:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Akinmoladun J, Bello O. Prenatal sonographic diagnosis of limb body wall complex: A rare lethal fetal anomaly. Sahel Med J. 2019;22:226. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Becker R, Runkel S, Entezami M. Prenatal diagnosis of body stalk anomaly at 9 weeks of gestation. Case report. Fetal Diagn Ther. 2000;15:301-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Mulita F, Parchas N, Solou K, Tchabashvili L, Gatomati F, Iliopoulos F, Maroulis I. Postoperative Pain Scores After Open Inguinal Hernia Repair: Comparison of Three Postoperative Analgesic Regimens. Med Arch. 2020;74:355-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Jayaram PR, Pereira FD, Barrett JA. Evaluation of dynamic ultrasound scanning in the diagnosis of equivocal ventral hernias with surgical comparison. Br J Radiol. 2018;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Daemen JHT, Loonen TGJ, Lozekoot PWJ, Maessen JG, Maal TJJ, Hulsewé KWE, Vissers YLJ, de Loos ER. Optical imaging versus CT and plain radiography to quantify pectus severity: a systematic review and meta-analysis. J Thorac Dis. 2020;12:1475-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Lain A, Garcia L, Gine C, Tiffet O, Lopez M. New Methods for Imaging Evaluation of Chest Wall Deformities. Front Pediatr. 2017;5:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Daskalakis G, Pilalis A, Papadopoulos D, Antsaklis A. Body stalk anomaly diagnosed in the 2nd trimester. Fetal Diagn Ther. 2003;18:342-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Daniilidis A, Balaouras D, Chitzios D, Balaouras G, Capilna M, Asimakopoulos E. Variation of ultrasound findings in the first trimester examination of recurrent cases with trisomy 21. J Clin Med Res. 2015;7:495-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Chen CP, Lin CJ, Chang TY, Hsu CY, Tzen CY, Wang W. Second-trimester diagnosis of limb-body wall complex with literature review of pathogenesis. Genet Couns. 2007;18:105-112. [PubMed] |
| 26. | Stein W, Haller F, Hawighorst T, Emons G. Pentalogy of Cantrell vs. limb body wall complex: differential diagnosis of a severe malformation in early pregnancy. Ultraschall Med. 2009;30:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |