Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4498
Peer-review started: December 28, 2022
First decision: March 28, 2023
Revised: April 26, 2023
Accepted: May 15, 2023
Article in press: May 15, 2023
Published online: July 6, 2023
Processing time: 184 Days and 8.7 Hours
Monkeypox (mpox) has been a public health emergency of international concern that emerged in mid-2022 and has spread to 110 countries. The clinical findings of the disease vary according to the seriousness of the cases. Although its case fatality risk has not been high, a significant percentage of patients require hospitalization. In this context, local initiatives were taken to extend the limited supply of vaccines against the disease; however, such measures have not been sufficient to contain the spread of cases and ensure an equitable distribution of health resources. As a result, endemic regions of low-income countries continue to have insufficient access to mpox vaccination. Despite this and considering the global scope of the disease, there is still little discussion in the literature about the difficulties in achieving adequate vaccination coverage rates for the target population of interest. In this article, we briefly discussed general aspects of the disease, including its surveillance, the current global context of challenges for mpox vaccination, and issues on global allocation of health resources as well as proposed related recommendations.
Core Tip: The development and implementation of new global public health policies, to ensure greater equity in global health financing in the short term, may lead to positive impacts in the global distribution of monkeypox vaccines. This would provide safer and more appropriate management of both this outbreak and future pandemics.
- Citation: Tovani-Palone MR, Doshi N, Pedersini P. Inequity in the global distribution of monkeypox vaccines. World J Clin Cases 2023; 11(19): 4498-4503
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4498.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4498
Along with the ongoing coronavirus disease 2019 (COVID-19) pandemic, a recent public health threat has put the world on high alert: the global monkeypox (mpox) outbreak. According to World Health Organization (WHO) data, confirmed cases of the disease exceeded 86000 in April 2023, with a spread across 110 countries and a total of over 110 deaths. Based on WHO regions, the Americas and Europe have been the two most affected regions, accounting for over 90% of all cases. However, as an endemic disease in West and Central Africa, greater attention from local governments and health authorities is needed during the current period[1].
In an attempt to gain greater support, sufficient production of vaccines, more equitable access by the target population, and the implementation of medical countermeasures for coordinated global action aimed at combating the spread of cases of the disease, the WHO declared mpox a public health emergency of international concern in July 2022[2,3]. In light of this, local initiatives were taken to extend the limited supply of vaccines during the early outbreak period. Although dose-saving approaches have been implemented in some countries including the United States, such measures have not been sufficient to contain the spread of the disease[4] and ensure equity in the global distribution of mpox vaccines. As a result, African countries in particular have had insufficient access to these vaccines[5,6].
Although the number of cases reported globally peaked in August 2022[7], an unequal distribution of health resources, negating any equitable prospects, could have the capacity to potentially threaten different regions of the world with significant limitations in this regard. Despite this and considering the global scope of the disease, there is still little discussion in the literature about the difficulties in achieving adequate vaccination coverage rates for the target population of interest. Here, we briefly discussed general aspects of the disease, including its surveillance, the current global context of challenges for mpox vaccination, and issues on global allocation of health resources as well as proposed related recommendations.
Mpox is a zoonotic viral infection caused by the mpox virus (MPXV). Patients present different symptoms and clinical involvement, which can lead to death in severe cases. Although a smallpox-like rash can be characteristically observed in affected patients[5], the mpox transmission and its case fatality rates are substantially lower compared to smallpox infection[1].
MPXV, in turn, corresponds to a double-stranded DNA orthopoxvirus, belonging to the same genus as smallpox, vaccinia, and cowpox viruses[5]. During the outbreak in 2022, a new lineage and sub-lineages of MPXV were identified. The B.1 lineage, classified as clade IIb for its close relationship to clade II, has been predominant in the worldwide spread of mpox[8,9]. Although this suggests that the current outbreak has a major source of infection[9], knowing how such variants might affect transmissibility, virulence and human adaptation remains a challenge.
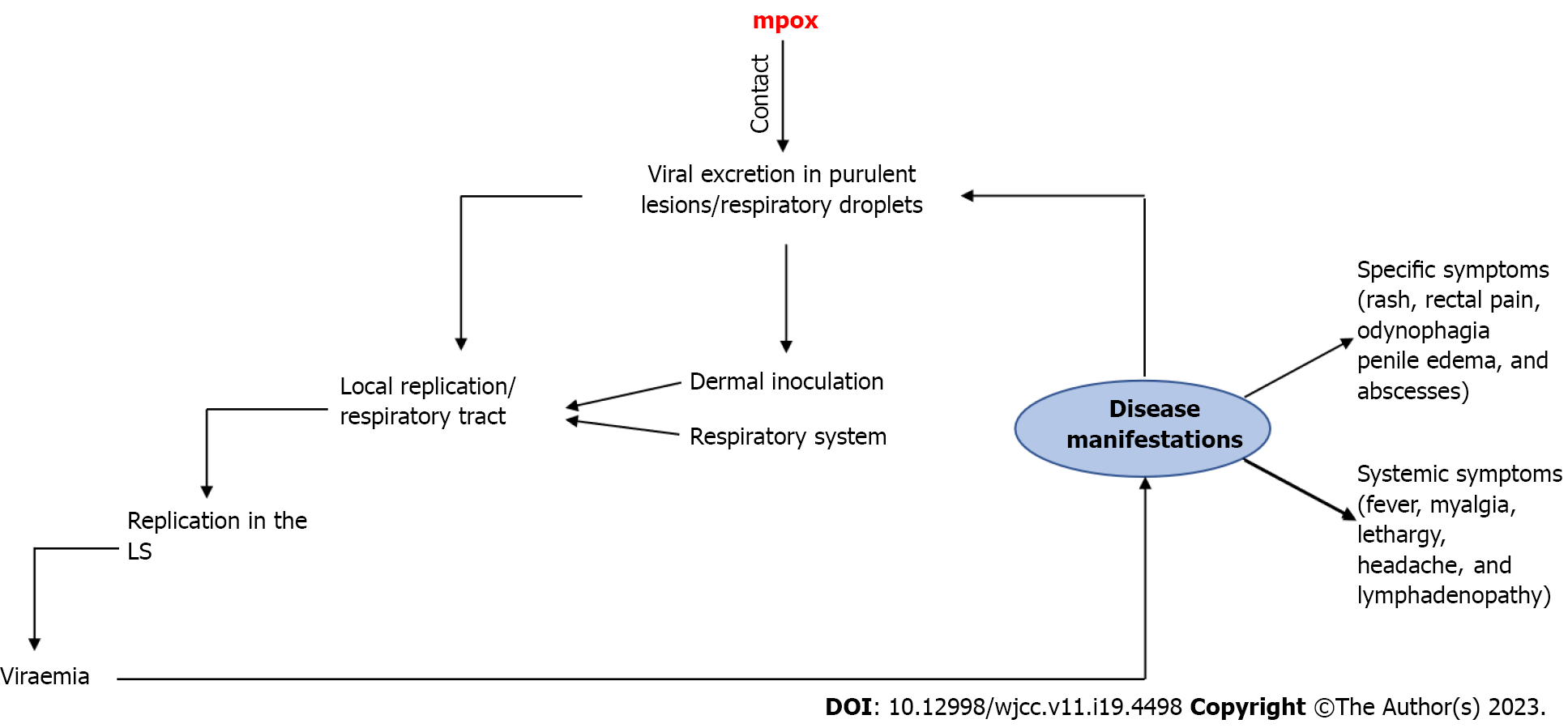
Among the means of transmission of MPXV, the most common include skin-to-skin and sexual contact[2]. Similar to severe acute respiratory syndrome coronavirus 2, the diagnosis of MPXV infection is primarily made by polymerase chain reaction, using lesion swabs or body fluids[5,10]. Different studies report incubation periods for MPXV ranging from 7-10 d, with most affected patients having systemic disease and approximately 40% progress to complications. Although mpox-related mortality rates are less than 1%, about 1%-13% of cases may require hospitalization, which is expected to significantly burden health systems[5,11]. Figure 1[2,5,11] illustrates a proposed general mechanism for the trans
According to the latest WHO recommendations published in 2022, the following actions of response to the multi-country outbreak of mpox should be prioritized and implemented: (1) Provide accurate information to people who may be at increased risk of simianpox virus infection; (2) Ensure access to pre- and post-exposure mpox vaccination for at-risk populations; (3) Prevent the spread of MPXV; and (4) Protect vulnerable individuals and frontline healthcare workers. From this perspective, it is imminent that doctors working in private or public settings, including hospital surveillance services, immediately report suspected cases to local and national public health authorities and to the WHO. In suspected cases of the disease, the investigation should consist of a clinical examination of the patient in a well-ventilated single room, using appropriate personal protective equipment. Patients should be questioned about possible sources of exposure, and a safe specimen collection should be obtained and forwarded to the respective laboratories[7].
As soon as a suspected case is identified, identification and tracing of all possible contacts should be initiated. Contacts of probable and confirmed cases must be monitored or self-monitored daily for any signs or symptoms for a period of 21 d from the last contact with confirmed cases or contaminated materials during the infectious period. However, in the absence of symptoms, quarantine or exclusion from work is not required during the monitoring period, which in turn differs from initial recommendations for COVID-19 surveillance. In this stage, asymptomatic contacts are encouraged to practice hand hygiene and respiratory etiquette and to avoid contact with children, immunocompromised individuals, and pregnant women. Moreover, known contacts are advised to avoid sexual contact with others during this period. This is due to the fact that the evidence on the possible transmission of MPXV before the onset of symptoms continues to be reviewed. Therefore, regardless of whether or not symptoms are present, it is strongly encouraged that this advice is followed. Finally, non-essential travel is also discouraged[7].
In the current context of the global mpox outbreak, the non-replicating modified vaccinia Ankara (MVA) vaccine has been one of the most used for both pre- and post-exposure prophylaxis for people at high risk for mpox. This vaccine is commonly administered subcutaneously in two doses, with a 4-wk interval between them. However, given the shortage of vaccine supplies, different countries have authorized single-dose intradermal administration of the MVA vaccine for adults. Thus, a possible increase in vaccine coverage in target groups could be achieved. In this case, only one-fifth of the volume of the subcutaneous route is needed[5]. Recent research conducted by Bertran et al[12] demonstrated that a single dose of the attenuated MVA-Bavaria Nordic smallpox vaccine is highly protective against symptomatic mpox, making it a useful tool for controlling disease outbreaks when rapid protection is required[12]. Moreover, the use of the intradermal route may increase immunogenicity and induce antibody responses similar to the subcutaneous route[5].
The main target groups for mpox vaccination in newly affected countries are homosexuals, bisexuals, and others at a higher risk of contracting mpox. Health professionals and people who have had contact with infected individuals have also been included due to the possibility of medium and high risk of exposure to MPXV infection. However, despite the worldwide concern of mpox vaccination for vulnerable populations, adequate access to both antiviral treatment and vaccines remains an obstacle in different endemic countries[5,6]. Even with strong recommendations for the need to increase the coverage of mpox vaccines on a global scale from the Global Alliance for Vaccines and Immunization, the Vaccine Alliance, and the Global Fund to Fight AIDS, Tuberculosis and Malaria, their administration has been made available for the most part to populations in North America and Europe, with a severe limitation of supplies to low-income countries[5].
Although the outbreak has subsided in countries such as the United Kingdom and the United States due to the roll-out of vaccines and therapies as well as changes in awareness and social behavior, the same has not occurred in countries in West and Central Africa. According to the only announcement made in 2022 by the African Centers for Disease Control and Prevention regarding the shipment of mpox vaccines, the African Union should receive a donation of only 50000 doses from South Korea by then. These vaccines may be the first in the continent not intended for research, but the number of vaccines is too small for the target population of mpox vaccination campaigns across the continent[6]. In addition to vaccine shortages, delays in the access of tecovirimat, an antiviral authorized for emergency use to treat severe mpox[13], have also been reported in African countries[6]. It is therefore plausible to affirm that Africa still lacks adequate access to vaccination and antiviral treatment[5,6], which is essential for patients with severe manifestations and people at risk of serious disease[5].
However, this was similar to the events that happened during the COVID-19 pandemic when countries in Africa received low numbers of vaccines at a delayed timeframe[14]. It is also possible that cases were underreported[15]. The existence of inequity in the global distribution of mpox vaccines and possible underreporting[6] together with the increase in vaccine hesitancy since the beginning of the COVID-19 pandemic[16] and the resurgence of other previously eradicated infectious diseases[17,18] should call for swift and urgent action. Although mass vaccination of the population is neither required nor recommended for mpox, efforts to contain this global outbreak must focus primarily on ensuring equitable vaccine distribution, with concern focused on low-income and middle-income countries. In addition, other relevant measures must be taken, such as massive global support to enable affected countries to respond equitably and effectively to the outbreak, including increased funding for mpox screening centers. This last action is expected to strengthen the disease surveillance system and contribute to access to both adequate treatment and preventive health services. In short, to effectively control the spread of mpox, broad and efficient measures involving a variety of specific measures (Table 1)[2,4,9] should be implemented.
| No. | Key points |
| 1 | Massive fight against misinformation |
| 2 | Strong information campaign strategy |
| 3 | Support from global health agencies |
| 4 | Effective collaboration with local laboratories, hospitals, and universities |
| 5 | Commitment of Ministries of Health of affected countries and partners |
| 6 | Implementing genome surveillance |
| 7 | Standardized and robust pharmacovigilance |
| 8 | Collaborative efficacy studies of vaccines with standardized protocols and data collection tools |
The lack of equity in the global allocation of health resources may impede the implementation of many relevant actions, especially in low-income and middle-income countries. The conclusions of a recent evidence-based review demonstrate that as global health expenditures continue to grow, they remain unequally distributed around the world[19].
As a result of this issue, a worse situation for the mpox outbreak may be expected in several countries beyond what has been reported, particularly in low-income countries. This is due to the potential for underreporting of cases, which may be related to the occurrence of the disease in rural areas[20] in addition to the scarcity of health resources. This context, added to the persistent weaknesses of many health systems, should generate additional limitations that strongly impact the capacity of surveillance systems and the implementation of effective vaccination programs. In order to improve the situation, comprehensive actions, including a planned allocation of financial resources aimed at implementing new interventional measures and surveillance programs using cheap and quick strategies, such as wastewater monitoring of MPXV DNA[9], are also essential.
In addition to countries in Africa not receiving enough mpox vaccines in a timely manner, the recent knowledge gained about MPVX did not change the course of the outbreak in Africa. Therefore, even though there was an increase in world attention focused on MPXV, the resulting benefits were observed, at least initially, in developed countries only. Most research on this topic has been published by researchers based in high-income countries[6], which implies the need for more investments as well as better-targeted funding towards robust and globalized scientific advances aimed at the proper management of the disease and the control of its spread.
Furthermore, even though many uncertainties remain about mpox therapies and comparative data on safety and efficacy related to the treatment of affected individuals, this should be yet another viable opportunity to fund the development of predesigned adaptive trial protocols that may enable faster discovery of effective drugs against viruses with pandemic potential[21].
In light of the current situation, the WHO together with other international alliances could play a key role in the development and implementation of global public health policies to ensure greater equity in global health funding in the short term, which should positively impact the global distribution of mpox vaccines as well as provide better management of this outbreak and future health emergencies. Only after building better health systems will be possible to satisfactorily control this and other imminent global emergencies and move closer to meeting the related sustainable development goals[22].
We thank the Italian Ministry of Health-Ricerca Corrente 2023 and Saveetha Institute of Medical and Technical Sciences for supporting this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gajdács M, Hungary; Giacomelli L, Italy; Haque N, Bangladesh S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | World Health Organization. 2022 Mpox (Monkeypox) outbreak: global trends [Internet]. [cited 11 April 2023]. Available from: https://worldhealthorg.shinyapps.io/mpx_global/. |
| 2. | Ren SY, Li J, Gao RD. 2022 Monkeypox outbreak: Why is it a public health emergency of international concern? World J Clin Cases. 2022;10:10873-10881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Wenham C, Eccleston-Turner M. Monkeypox as a PHEIC: implications for global health governance. Lancet. 2022;400:2169-2171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Gruber MF. Current status of monkeypox vaccines. NPJ Vaccines. 2022;7:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Mitjà O, Ogoina D, Titanji BK, Galvan C, Muyembe JJ, Marks M, Orkin CM. Monkeypox. Lancet. 2023;401:60-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 285] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 6. | Kozlov M. WHO may soon end mpox emergency - but outbreaks rage in Africa. Nature. 2023;614:600-601. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | World Health Organization. Surveillance, case investigation and contact tracing for mpox (monkeypox): interim guidance, 22 December 2022 [Internet]. [cited 11 April 2023]. Available from: https://www.who.int/. |
| 8. | Patiño LH, Guerra S, Muñoz M, Luna N, Farrugia K, van de Guchte A, Khalil Z, Gonzalez-Reiche AS, Hernandez MM, Banu R, Shrestha P, Liggayu B, Firpo Betancourt A, Reich D, Cordon-Cardo C, Albrecht R, Pearl R, Simon V, Rooker A, Sordillo EM, van Bakel H, García-Sastre A, Bogunovic D, Palacios G, Paniz Mondolfi A, Ramírez JD. Phylogenetic landscape of Monkeypox Virus (MPV) during the early outbreak in New York City, 2022. Emerg Microbes Infect. 2023;12:e2192830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Gao L, Shi Q, Dong X, Wang M, Liu Z, Li Z. Mpox, caused by the MPXV of the clade IIb lineage, goes global. Trop Med Infect Dis. 2023;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Tan NK, Madona CP, Taylor JF, Fourali LH, Sehmi JK, Stone MJ, Pond MJ, Cliff PR, Pope CF. Performance evaluation of the Viasure PCR assay for the diagnosis of monkeypox: a multicentre study. J Clin Virol. 2023;158:105350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 11. | Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, Palich R, Nori A, Reeves I, Habibi MS, Apea V, Boesecke C, Vandekerckhove L, Yakubovsky M, Sendagorta E, Blanco JL, Florence E, Moschese D, Maltez FM, Goorhuis A, Pourcher V, Migaud P, Noe S, Pintado C, Maggi F, Hansen AE, Hoffmann C, Lezama JI, Mussini C, Cattelan A, Makofane K, Tan D, Nozza S, Nemeth J, Klein MB, Orkin CM; SHARE-net Clinical Group. Monkeypox virus infection in humans across 16 Countries - April-June 2022. N Engl J Med. 2022;387:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 1236] [Article Influence: 412.0] [Reference Citation Analysis (0)] |
| 12. | Bertran M, Andrews N, Davison C, Dugbazah B, Boateng J, Lunt R, Hardstaff J, Green M, Blomquist P, Turner C, Mohammed H, Cordery R, Mandal S, Campbell C, Ladhani SN, Ramsay M, Amirthalingam G, Bernal JL. Effectiveness of one dose of MVA-BN smallpox vaccine against mpox in England using the case-coverage method: an observational study. Lancet Infect Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 13. | Tempestilli M, Mondi A, Matusali G, Mariotti D, Pinnetti C, Beccacece A, Cimini E, Mazzotta V, Carletti F, Faccendini P, Maggi F, Girardi E, Nicastri E, Vaia F, Antinori A. Tecovirimat concentrations and viral suppression in seminal fluid from patients with mpox. Lancet Infect Dis. 2023;23:531-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Uwishema O, Onyeaka H, Alshareif BAA, Omer MEA, Sablay ALR, Tariq R, Mohamed RIH, Zahabioun A, Yousif MYE, Chalhoub E, Tovani-Palone MR. Current context of pneumonia amidst the COVID-19 pandemic in Africa. J Contemp Stud Epidemiol Public Health. 2021;2:ep21007. [DOI] [Full Text] |
| 15. | Tovani-Palone MR, Garoli F, Shah PA, Kamal MA, Nawaz FA. Underreporting of cases during the COVID-19 pandemic: a worrying warning for Africa. J Contemp Stud Epidemiol Public Health. 2022;3:ep22001. [DOI] [Full Text] |
| 16. | Khawaja UA, Franchi T, Pedersini P, Tovani-Palone MR. Declining rates of global routine vaccination coverage amidst the COVID-19 syndemic: a serious public health concern. Einstein (Sao Paulo). 2022;19:eED6552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Hill M, Pollard AJ. Detection of poliovirus in London highlights the value of sewage surveillance. Lancet. 2022;400:1491-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | The Lancet. Polio eradication: falling at the final hurdle? Lancet. 2022;400:1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Global Burden of Disease 2020 Health Financing Collaborator Network. Tracking development assistance for health and for COVID-19: a review of development assistance, government, out-of-pocket, and other private spending on health for 204 countries and territories, 1990-2050. Lancet. 2021;398:1317-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 20. | Moore MJ, Rathish B, Zahra F. Mpox (Monkeypox). 2022 Nov 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 21. | Lindholm DA, Kalil AC. Déjà vu all over again? Monkeypox and the urgent need for randomised controlled trials. Lancet Infect Dis. 2023;23:e56-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | United Nations. Sustainable Development Goals. The 17 goals [Internet]. [cited 23 April 2023]. Available from: https: https://sdgs.un.org/goals. |