Published online Jul 6, 2023. doi: 10.12998/wjcc.v11.i19.4458
Peer-review started: December 24, 2022
First decision: March 23, 2023
Revised: May 9, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: July 6, 2023
Processing time: 187 Days and 18.1 Hours
Irritable bowel syndrome (IBS) is a chronic functional disorder which alters gastrointestinal (GI) functions, thus leading to compromised health status. Pathophysiology of IBS is not fully understood, whereas abnormal gut brain axis (GBA) has been identified as a major etiological factor. Recent studies are suggestive for visceral hyper-sensitivity, altered gut motility and dysfunctional autonomous nervous system as the main clinical abnormalities in IBS patients. Bidirectional signalling interactions among these abnormalities are derived through various exogenous and endogenous factors, such as microbiota population and diversity, microbial metabolites, dietary uptake, and psychological abnormalities. Strategic efforts focused to study these interactions including probiotics, antibiotics and fecal transplantations in normal and germ-free animals are clearly suggestive for the pivotal role of gut microbiota in IBS etiology. Additionally, neurotransmitters act as communication tools between enteric microbiota and brain functions, where serotonin (5-hydroxytryptamine) plays a key role in pathophysiology of IBS. It regulates GI motility, pain sense and inflammatory responses particular to mucosal and brain activity. In the absence of a better understanding of various interconnected crosstalks in GBA, more scientific efforts are required in the search of novel and targeted therapies for the management of IBS. In this review, we have summarized the gut microbial composition, interconnected signalling pathways and their regulators, available therapeutics, and the gaps needed to fill for a better management of IBS.
Core Tip: Irritable bowel syndrome (IBS) is a prevalent gastrointestinal disorder with a dysregulated gut brain communication. Gut microbiota functional characterization is still underappreciated but their roles have been found to be pivotal. Various microbial species and their metabolites with altered composition and diversity have been found to be specific to IBS. Clinical manipulation of these microbial species improved the symptom profile in IBS patients while the associated mechanisms have been identified for a bidirectional communication between gut microbiota and brain. This in turn seems promising for future treatments specific to microbiota manipulation and targeting various cross-talks for the management of IBS and associated symptoms.
- Citation: Singh SV, Ganguly R, Jaiswal K, Yadav AK, Kumar R, Pandey AK. Molecular signalling during cross talk between gut brain axis regulation and progression of irritable bowel syndrome: A comprehensive review. World J Clin Cases 2023; 11(19): 4458-4476
- URL: https://www.wjgnet.com/2307-8960/full/v11/i19/4458.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i19.4458
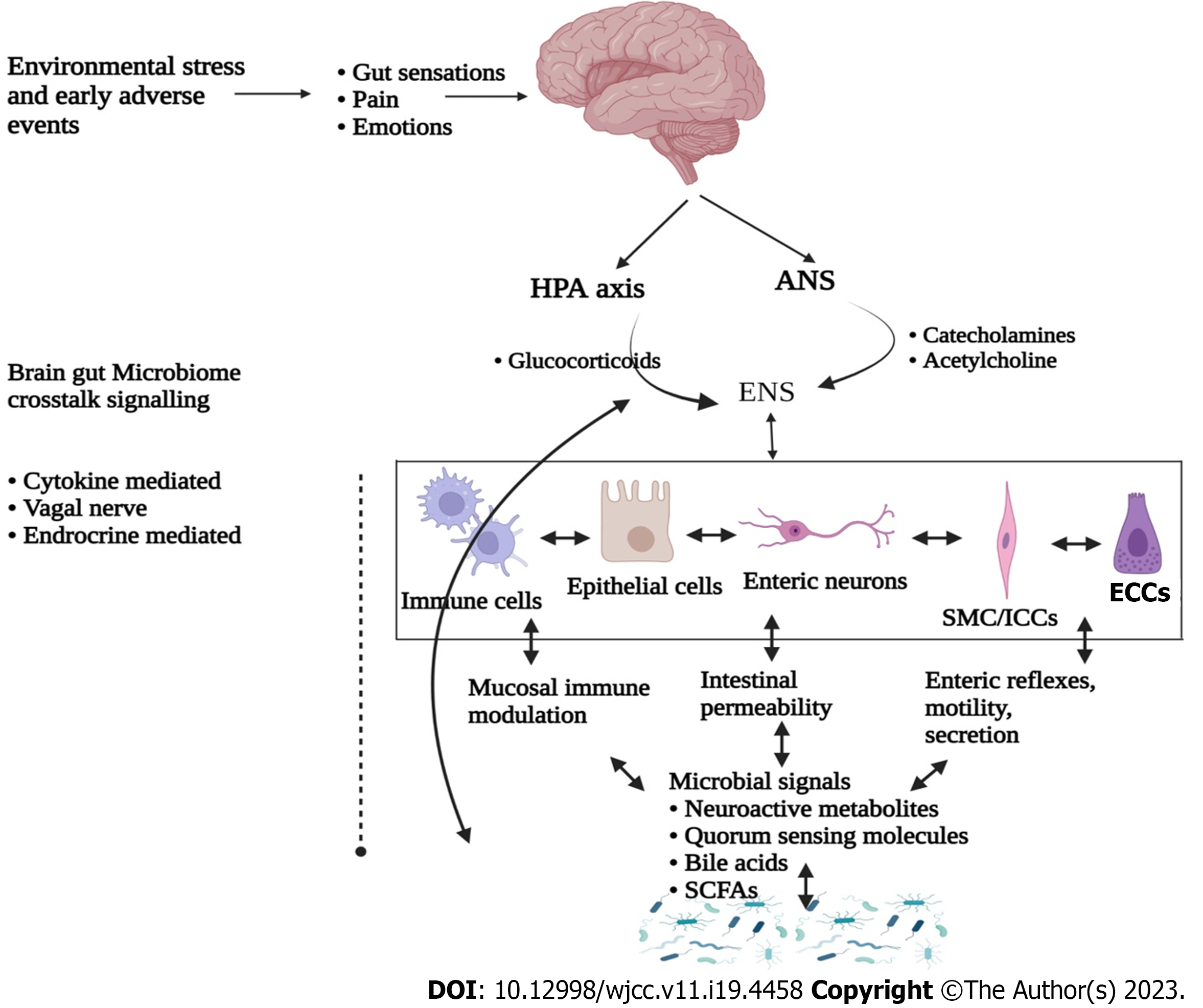
Human body consist of trillions of microbial cells, majority of which inhabit the gastrointestinal (GI) tract thus forming a microbial colonization with a dynamic ecological environment, commonly known as “microbiota”[1,2]. This microbiota is comprised of approximately 500 transient and indigenous species of bacteria, viruses, fungi and protozoa[3,4]. Among all, bacteria are the most abundant microbial community dominated with the members of Firmicutes and Bacteroidetes phyla[5,6]. In recent years, research focusing intestinal microbiota functional characterization led to the identification of a bidirectional crosstalk between brain and gut microbiota, thus forming a gut-brain axis (GBA)[7,8]. Moreover, GBA includes central nervous system (CNS), enteric nervous system (ENS), hypothalamic-pituitary-adrenal (HPA) axis, gut and its microbiota (Figure 1). Interestingly, these components have been identified to be interconnected through various coordinated signalling pathways[8,9]. Abnormalities in these pathways and their regulators have been identified for the etiology of irritable bowel syndrome (IBS). IBS is a common GI bowel disorder featured with altered GI motility, visceral hypersensitivity, post-infection reactivity, small intestinal bacterial overload, carbohydrate mal-absorption and intestinal inflammation[10,11]. These abnormalities result in dysbiosis, recurrent abdominal pain and distressed bowel habits.
IBS is affecting 10%-25% of global world population, especially in developed countries with a poorly deciphered pathophysiology[12]. Considering the predominant symptoms and bowel habits, IBS is further classified into four subtypes: diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), mixed IBS (IBS-M), and unclassified IBS[13,14]. Interestingly, women are more susceptible (1.67 times) than men for IBS. In recent years, IBS symptoms have been predominantly found to be associated with environmental factors such as, diet, enteric microbial communities, host genetics and psychology[15]. However, the ways in which these factors contribute to the etiology of IBS are not fully deciphered. The treatments available for IBS are also not very specific. Generally, dietary fiber supplementation for IBS-C, opioids for IBS-D and abdominal pain with bloating, and low doses of antidepressants are recommended for the management of various symptoms associated with IBS[12,13,15]. However, modulating the gut-brain axis seems to be a promising target for the development of novel therapeutic for IBS[16]. In addition to this, various metabolic disorders have also been identified to be massively regulated by gut microbiota and their metabolites[6,17,18]. Involvement of gut microbiota metabolites in maintaining homeostasis including host immunity, physiological functions (digestion and nutrition) and biosynthesis of vitamins have also been recently validated by various research groups[18,19]. Experimental sets of data intoning functional cruciality of microbiota in IBS advocate their widespread interactions not localized only with intestinal cells and ENS, but also directly with CNS through neuroendocrine and metabolic pathways[19,20]. The ENS composed of semiautonomous effector system is also connected to central autonomic network and modulated via afferent and efferent communications through parasympathetic and sympathetic nerves[21,22].
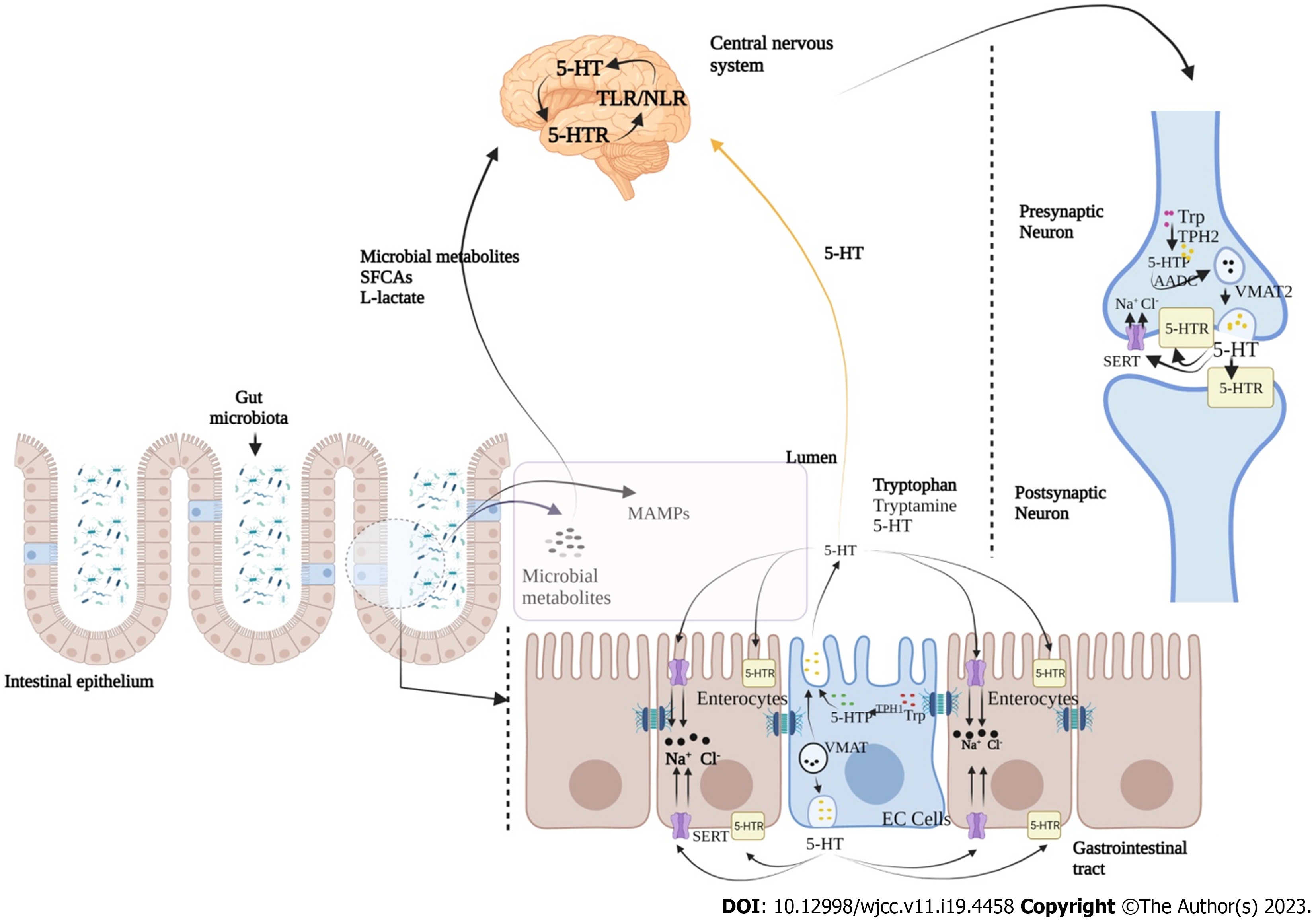
Ongoing bidirectional brain-gut interactions are significantly influenced by serotonergic [5-hydroxytryptamine (5-HT)] pathway, where serotonin also known as 5-HT is an important neurotransmitter and paracrine signalling molecule[23,24]. Serotonin is synthesized by enterochromaffin cells (EC) of the gut and by serotonergic neurons in the CNS[25]. Aberrant 5-HT signalling has been found to be accountable for various GI disorders, including IBS, diarrhea and chronic constipation, and functional dyspepsia[26,27]. Various components of serotonin signalling, including EC cells count, serotonin level, tryptophan hydroxylase activity, and expression of serotonin-selective reuptake transporters have also been found to be altered in IBS[28]. 5-HT signalling in between 5-HT receptors (on postsynaptic and presynaptic neurons at CNS and intestinal serotonergic neurons) and serotonin transporters (SERT) of various cell types of GI tract is crucial for proper functioning of gut-brain communication[29,30]. Additionally, 5-HT is produced by the chemical conversion of tryptophan to 5-hydroxytryptamine, a reaction catalyzed by enzyme tryptophan hydroxylase (TPH1 in EC cells and TPH2 in neurons)[31]. Stored into vesicles formed through the vesicular monoamine transporter (VMAT; VMAT1 in EC cells and VMAT2 in neurons), 5-HT is further released into the extracellular space where it binds to different serotonin receptors (5-HTR)[32]. Further, it is known to regulate peristaltic, secretory, vasodilatory, vagal and nociceptive reflexes (Figure 2)[26,27,33].
The present review summarizes recent updates consisting key components of microbiota derived GBA, molecular signalling during cross talk between GBA regulation and in the progression of IBS. Herein, we have also discussed the diagnostic and therapeutic implications of microbiota in the management of IBS and related symptoms.
IBS is a GI disorder characterized by chronic abdominal pain and altered bowel habits in the absence of any organic cause which significantly affects the quality of life[34]. It is the most diagnosed functional GI disorder accounting for approximately 30 percent of all referrals to gastroenterologists[35]. The pathophysiology of IBS is the result of aberrated and interconnected signalling networks, especially between gut microbiome and brain[34,35]. However, the associated mechanisms and pathways are still unclear.
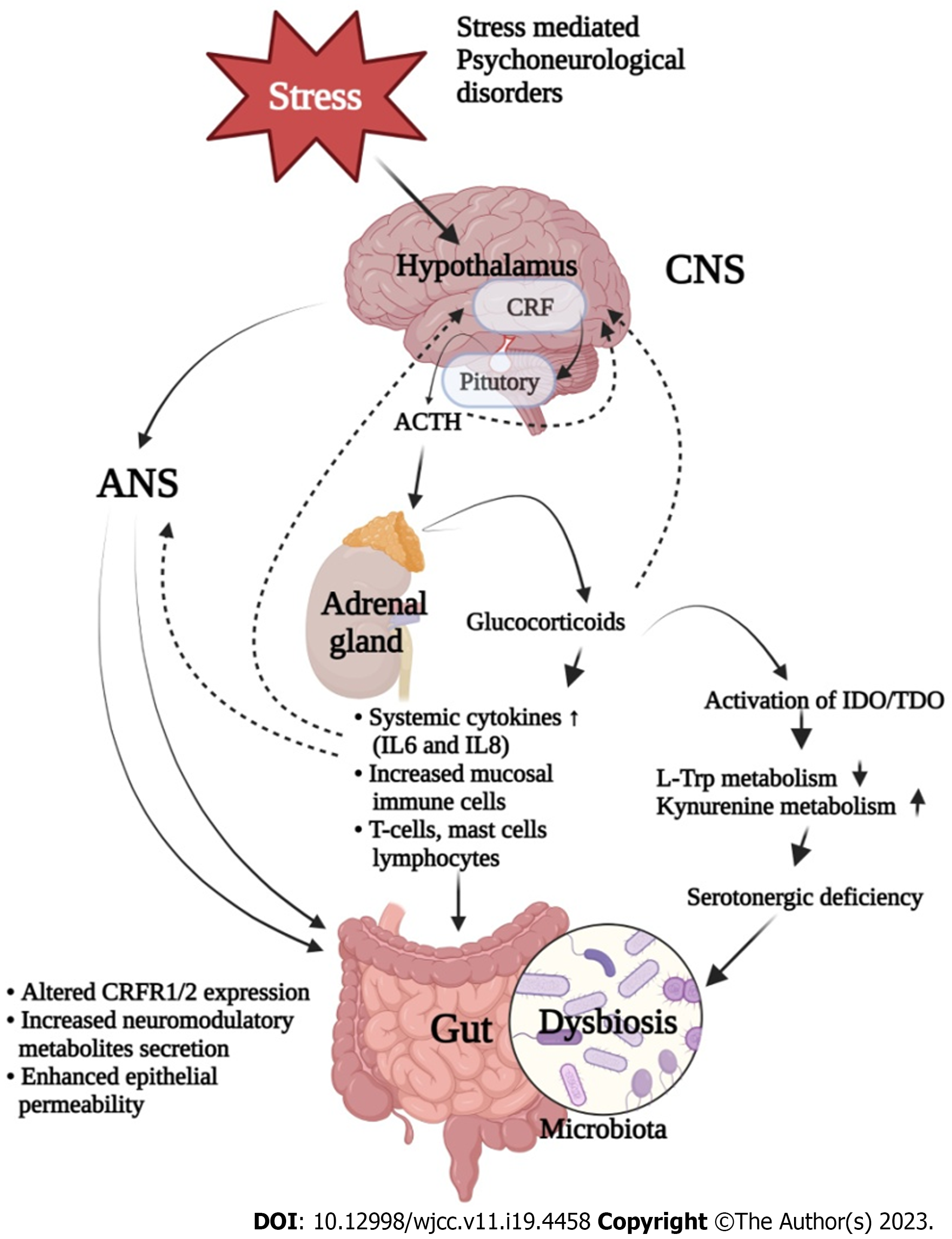
Traditionally, IBS was known to be associated with altered GI motility, visceral hypersensitivity and distorted pain perception[34,35]. Studies on IBS indicated the pivotal role of inflammation, alterations in fecal flora, and bacterial overgrowth[36]. IBS is also known as a psychosomatic illness since it is associated with mood disorders with abnormal psychiatric conditions[36,37]. Furthermore, mucosal immune activation, inflammatory cells and elevated inflammatory markers have been recorded in IBS patients[34,35]. Various studies associated with the effect of antibiotics in changing the gut microbiota diversity and complexities are co-related with gut microbiota profiles and IBS symptoms[34,38]. Perturbations in GBA have been also proposed as the main mechanisms in the pathophysiology of IBS. In a recent study, corticotrophin-releasing hormone (CRH) was also found to have an important role in IBS and in augmentation of intestinal mucosal inflammation[39-41].
Studies are suggestive for co-morbidity of stress-related psychiatric illness and IBS, since 50%-60% of IBS patients have been diagnosed with various psychosocial health issues[42,43]. Psychological stress plays an important role in the pathophysiology of IBS, since it critically influences gut-brain axis and its associated metabolism[44]. It is also known to regulate intestinal motility and permeability, visceral hypersensitivity, immune responses, and gut microbiota composition[45,46]. Among the possible mechanisms for this regulation, immune response dependent secretion of various proinflammatory cytokines seems to be the pivotal one[47]. These cytokines activate the HPA and hypothalamic-autonomic nervous system (ANS) axes along with the release of corticotropin releasing factor, adrenocorticotropic hormone and cortisol, and all these subsequently control the gut homeostasis (Figure 3)[48]. It is established that these also alter neurotransmitter release within the enteric nervous system which thereafter affect gut motility, secretion and epithelial permeability via tight junction dysregulation[49]. An abnormal progression of monoamine neurotransmitter systems, including 5-HT and noradrenaline have been noticed in many stress related disorders. Furthermore, serotonin is known for its pivotal role in emotional response and in pain management by modulating brain-gut-microbiota axis, which is also significantly affected by early life stress[50].
Human GI tract hosts various genera of bacteria that are present on its mucosal surface. The term “microbiota” refers to the ecological system composed of various commensal bacteria in the GI tract, particularly localized on the lining of mucosal surface[51]. The term “microbiome” refers to metagenome of the microbiota and their genetic architecture[52]. Mucosal surface is the only layer separating host tissues from various germ lines by which host immune system remains protective against various pathogens in GI tract[52,53].
Although, majority of microbiota is still uncharacterized, it consists of a very delicate community of commensal organisms that have evolved over millions of years. Among all, four bacterial phyla including gram-positive Firmicutes (Lactobacillus spp.) and Actinobacteria (Bifidobacterium spp.) along with gram-negative Bacteroides and Proteobacteria are the most prevalent[6,54,55]. In addition to bacterial strains, various viruses, protozoa, archae, and fungus also constitute GI microbiota. Multidisciplinary studies were also performed to characterize the gut microbiota, thus revealing the system as a state of symbiosis[56-59]. Homeostasis of host immune system and mucosal barrier are generally altered by dysbiosis, which in turn promotes the invasion and growth of pathogenic species. Microbiota also regulates gut inflammation with the presence of immune cells in gut wall[60]. Although, the complete sketch of a balanced microbiome is still unknown and each person is assumed to have a distinct microbiota (called as microbiome fingerprint), healthy individuals are generally known to have similar profile and distribution of bacterial phylotypes[61].
Microbial colonization is a vital early life phenomenon as a part of development of a healthy microbiota which seems to be crucial for the development of GBA[62,63]. Altered microbial colonization in human GI tract has been clearly identified to exhibit adverse health effects in the later years of the host[63]. The colonization process is also regulated with various factors such as birth mode and feeding of babies, since the breastfed and vaginally born babies were reported to be initially colonised by the member of Bifidobacteria, Lactobacilli, and Bacteroides species[64]. As compared to pre-term babies, full-term babies are reported for rapid maturation of gut microbiome in their first year of life. There is several evidence supporting the existence of a microbiota and foetal meconium, where the colonisation is reported to start even before the delivery, which suggest that these organisms could also colonise the foetal gut[65]. Various bacteria like Staphylococcus, Streptococcus, Bifidobacterium, and Lactobacillus are also reported in human milk[66]. Thus, colonization is significantly affected in neonatal period by human breast milk and the technique of delivery[67]. Moreover, mononuclear cells carrying bacterial population from the mother’s gut to the mammary gland through an entero-mammary pathway are also playing pivotal role in gut immunological maturation of the infant[68]. This mammary microbiome also possesses anti-infective, anti-inflammatory, immunomodulatory and metabolic activities which also constitute for microbiota colonization in neonatal gut[69,70].
The human microbiota play fundamental role in host physiology and pathology, and alteration in microbial population (dysbiosis) has been found to be significantly associated with various GI disorders[71]. A balanced interaction between microbiota and its host seems to be beneficial and essential for intestinal health and host metabolism. Under normal conditions, mucosal microbiota have been found to have significant role in digestion of food, vitamins synthesis, angiogenesis, epithelial cell and in development and maturation of the host defense system[72]. Recently, it has been evident that the intestinal bacterial population significantly affects the CNS physiology and gut inflammation, mainly by a bidirectional communication consisting of various signalling pathways. This communication commonly known as GBA[73], consists of multiple inter-connections including vagus nerve, immune components, and microbial metabolites[74,75].
An enhanced size of microbiota and neuronal development takes place generally in starting five years of an infant, where the neuronal development is mainly configured by maternal microbiota[76]. The effect of gut microbiota on neurodevelopment of an infant was also examined and proved using various experiments on germ-free (GF) or specific pathogen-free mice. These mice were treated with various antibiotics to alter the microbial diversity and thereby to study their importance in gut microbiome[77,78]. These experiments clearly suggested that various neurological problems appeared in the treated animals due to improper maturation of gut microbiome. Results of these studies are suggestive of exaggerated HPA axis in germ free animals with impaired social behaviors, reduced anxiety and increased motor activity[79,80]. In the same experiment, altered neuronal developments and behaviors were found to be improved when the newborn infants were supplemented with various microbial flora[81]. These altered behavioral phenotypes were also found to deregulate various genes and metabolites involved in HPA axis regulation. Among these, some common regulators are adrenaline, 5-hydroxytryptophan, postsynaptic density protein 95, dopamine and synaptophysin[82].
Scientific reports suggest that production of various neuroactive molecules by gut microbes directly or indirectly play critical role in GBA[83]. Among these neurotransmitters, acetylcholine, γ-aminobutyric acid and serotonin are released by various bacterial populations belonging to Bifidobacteria, Lactobacillus, Enterococcus, and Streptococcus species[84]. Interestingly, 90% of total serotonin is produced by gut microbiota, and used for mood disorders and functional regulation of CNS and GI tract[84,85]. Serotonin is known to bind to 5-HT receptors on microglial surface, thus inducing the release of inflammatory cytokines, which in turn play important role in the maintenance of gut inflammation[86]. In addition to 5-HT, tryptophan is also a serotonin precursor and known for influencing microglia activity in gut lumen. Moreover, tryptophan and its derivatives have been found to regulate CNS inflammation, particularly following an aryl hydrocarbon receptor (Ahr) mediated mechanism, which in turn is responsible for microglial activation and transcriptional regulation of astrocytes[87]. The vitality of tryptophan and its metabolism in maintaining CNS homeostasis has been reported recently, where GF animals were found to have augmented 5-HT levels and 5-hydroxyindoleacetic acid in the hippocampus and serum samples, compared to conventionally colonized control mice[87,88]. These findings are suggestive towards the ability of microbiota in maintaining CNS serotonergic neurotransmission, mainly through systemic circulation. These initial findings were also validated by colonizing these animals post-weaning, where they were found to have sufficient levels of tryptophan in peripheral samples and reduced anxiety. Interestingly, this colonization was found ineffective in reversing the CNS neurochemical consequences exhibited by GF animals[89]. In a recent study, one major finding was listed where an intact and diversified microbiota (post birth) was found to produce quinolinic acid and N-methyl-D-aspartate agonist, as a part of tryptophan metabolism regulated by microglial cells[90]. Both have a critical role in the pathogenesis of several neurological conditions such as Huntington’s disease and behavioral disorders[91]. In experimental setup where GF mice were recolonized with bacterial population belonging to Clostridium tyrobutyricum, they exhibited colonized mucus layer, and regulated immune and gut barrier homeostasis[92].
Furthermore, Lactobacillus rhamnosus (JB-1) containing probiotics supplementations in previously colonized mice were found to be able to reduce anxiety and depressive behavior[93]. Recently, researchers also found that microbiota dependent alterations in neural synapses along with fear extinction behavior are not mainly due to altered HPA axis, they are also due to a reduction in various neuroactive metabolites’ levels such as phenyl sulfate, pyrocatechol sulfate, and indoxyl sulfate[94,95]. This was further confirmed with the abraded levels of these metabolites in various samples collected from experimental animals, such as fecal sample, serum, and CSF. Collectively, gut microbiome and its associated bacterial populations were found to be pivotal in maintaining the level of various neuroactive metabolites through which they regulate the behavioral patterns in healthy subjects.
In humans, ENS is generally referred as the second brain which is located inside the digestive tract wall and shielded by a mucous membrane from gut lumen, having five times higher neurons and ganglia than spinal cord. An autonomous system regulating microbiome and GBA is known to be bidirectional whereas somatic sensory system received signals in pathological conditions causes abnormal feelings like discomfort, nausea, and pain which in turn exhibit bowel associated problems[96]. Herein, ANS transports afferent signals received from gut lumen to CNS through various routes such as enteric, spinal and vagal[96]. In these communications, vagus nerve plays a critical role as it has millions of nerve terminals (80% are afferent). Moreover, the same pathway is also used to send efferent signals from CNS to the gut wall[97]. In case of stress and anxiety, neuroendocrine system in response to stress regulates various vital body functions, including digestion and immunity which are mainly dependent on the HPA axis[98]. For example, generation of corticosteroids in response to environmental stress is also found to be dependent on HPA axis[97]. Collectively, neural and hormonal signals regulate brain and microbiome for further regulation of gut cell activity in controlled conditions.
In addition, signal transducing cells such as enterochromaffine cells (EC) and dendritic cells (DC) produce various neurotransmitters (Figure 2) viz., 5-HT, somatostatin, cholecystokinin and CRH[99-101]. All these are important for signal-based regulation of microbiota and CNS as these directly affect the microbial behavior. EC serve as luminal sensors for observing a great range of bacteria and microbial compounds in the gut[102]. These microbial populations have neurotransmitter receptors, and their activation is a key factor to understand the mode of microbiota functions and composition. A specialized process called as “inter-kingdom signalling” is a well-known regulatory pathway through which bacteria communicate with gut epithelial cells, mainly by using oligopeptides and monoamines which are also known for their neurotransmitter behavior[103,104]. Numerous neurotransmitters are produced by ENS exhibiting critical roles in GBA regulation, where each has a specific signalling pathway. Some neurotransmitters such as 5-HT, somatostatin, dopamine, neuropeptide Y, peptide YY, cholecystokinin, and corticotropin-releasing factor are also necessary for GBA regulation[96,101,104].
EC in GI tract produces majority of body’s 5-HT and dopamine. An increased synthesis of 5-HT has been found as a key regulator through which microbiota regulates HPA[97]. Additionally, gut microbiota senses the EC cells mainly via short-chain fatty acids (SCFA) (butyrate and acetate) to promote 5-HT synthesis that regulates GI motility, secretion and immunological responses[96]. Hence, a change in microbiota composition significantly affects the levels of 5-HT and its altered level contributes towards the pathophysiology of IBS. Interestingly, microbiota is also connected with CNS through TLR signalling pathway[105,106]. TLR is principally expressed on immune cells of gut wall and neurons of the ENS. Among microbial populations, gram-negative bacterial membrane component lipopolysaccharide (LPS) is selectively detected by TLR which in turn is responsible for the production of proinflammatory cytokines via NF-κB pathway[106]. In colonic biopsies of IBS-D patients, higher TLR expression has been recorded suggesting microbiota and host immune system interaction[102,104]. Furthermore, smooth muscle dysfunction and paralysis in septic ileus is also known to be significantly influenced by the number of cytokines produced by immune cells, in response to LPS through TLR mediated signalling[106].
5-HT is an important neurotransmitter and paracrine signalling molecule in the gut where its major role has been identified in the modulation of gut-brain communication and in functional GI disorders[21]. In previous studies, altered levels of 5-HT have been reported in various CNS related disorders including anxiety, depression, obsessive compulsive disorder, phobias and in other psychiatric disorders[67,69]. 5-HT modulators including SSRIs and specific 5-HT receptor agonists and antagonists have also been used to treat various metabolic disorders such as, migraine, nausea, obesity, chronic pain, hypertension, vascular disorders, and sexual dysfunction[107].
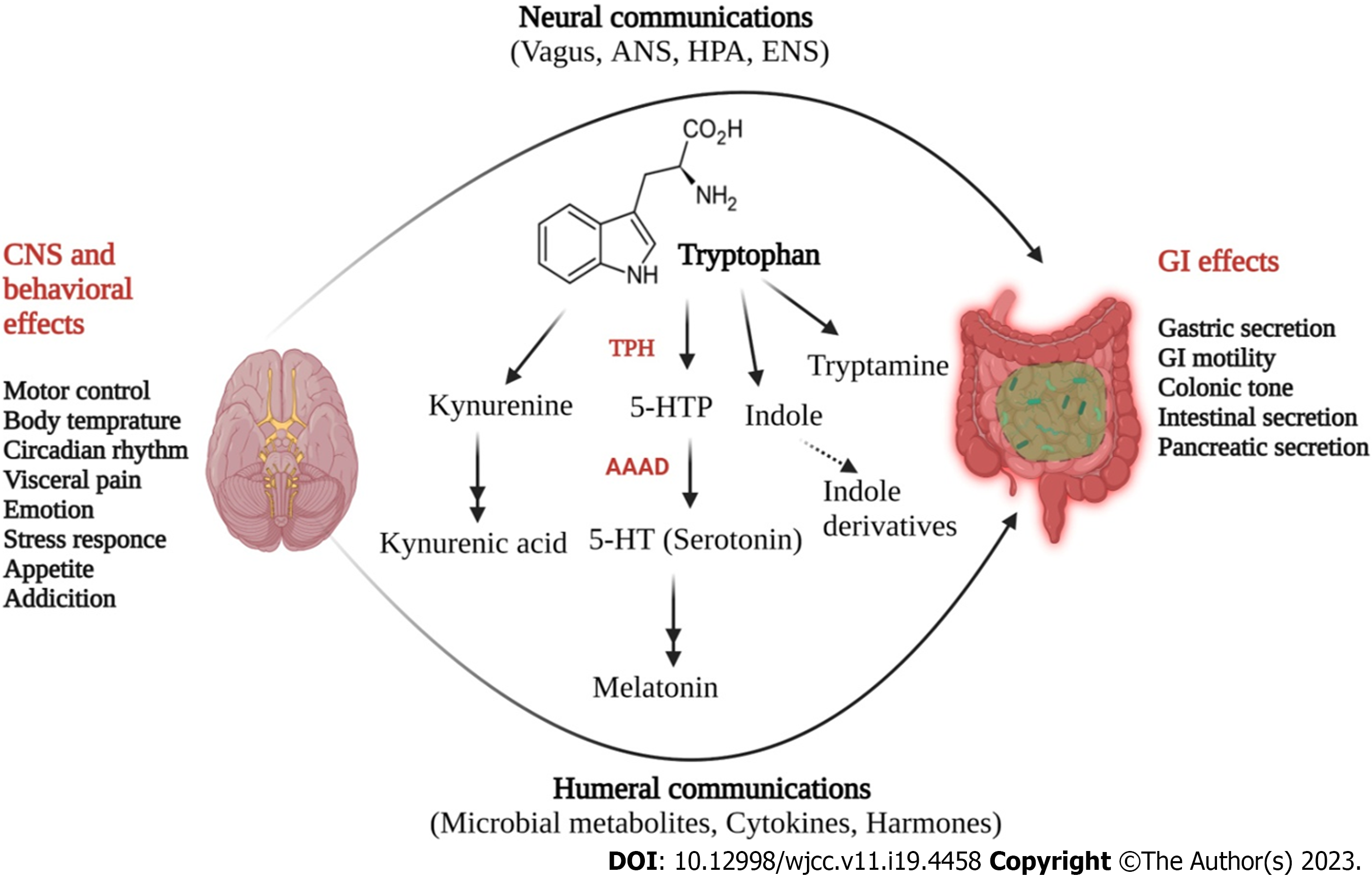
Among all the neurotransmitter-based regulations of GBA, 5-HT mediated signalling is primary where 5-HT mainly targets receptor subtypes of seven 5-HT family receptors, having 15 subtypes of recognition sites[85]. Additionally, seven types of 5-HT receptors have a wide range of biological tasks including enhancing mucosal permeability and visceral hypersensitivity, inflammation and immune cell activation, and gut motility[107]. Currently, 7 families of 5-HT receptors have been identified, where 5-HT3 and 5-HT4 receptors are in intestine, presynaptic positions and, in sensory and mesenteric neurons[107]. In clinical practices, medications that are known to target these receptors have been frequently used for the management of IBS complications. Enteric microbiota has also been found to control the expression of 5-HT receptors[107]. 5-HT mainly activates peristaltic reflexes in GI tract (Figure 4) thus causing ascending contractile and descending relaxant limbs. 5-HT has been also found to control segmental motor patterns in small intestine. A study performed in tryptophan hydroxylase deficient (Tph2-/- mice) mice demonstrated the functional aspects of 5-HT in GI motility mainly by attributing with significant reductions in contractile complexes and synaptic transmission due to lower level of 5-HT[108,109]. This is further indicative for the significance of serotonergic neurons, comparing to EC cells for constitutive GI motility. Additionally, ENS dopaminergic neurons were found to be immature in Tph2-/- mice, which are responsible for homeostatic GI movement[110]. Moreover, in vivo and in vitro studies have shown decreased intestinal motility in mice with SERT Ala56 mutation which was restored by 5-HT4 receptor antagonists[111]. A SERT antagonist named ‘fluoxetine’ was found to improve GI motility in SERT-/- mice, along with a higher level of 5-HT, thus indicating the importance of 5-HT in GI motility, by targeting SERT[112].
Various antibiotic treatments have been found to impede GI motility along with a reduced production of peripheral 5-HT level, which was also confirmed by GF animal studies where a slower GI transit was recorded in these animals compared to control mice having normal gut microbiota[66,112]. In GF mice, treatment with pharmacological blockers of 5-HT4 receptors significantly improved GI transit along with a higher level of luminal 5-HT level[113]. Additionally, 5-hydroxyindole has been produced as a key metabolite of serotonin metabolic pathway (Figure 4), mainly by increasing the colonic motility, especially by activating L-type calcium channels[114]. Recently, in a BTBR (BTBR T+ Itpr3tf/J) mice model of autism spectrum disorder, an altered serotonergic pathway has been found along with downregulation of Tph1 gene, while SERT expression was upregulated along with a reduced level of 5-HT and its producing host (Blautia spp)[115]. A list of various enteric microbiota and their mode of regulating serotonergic pathway are listed in Table 1. Additionally, 5-HT level in combination with gut microbial stimulus can also lift the proportion of M20000 macrophages, which are known to be stimulatory for GI motility particularly in the colonic muscle layer located nearby ENS[117]. Various microbial byproducts such as SCFAs were also found to enhance colonic transit with the release of 5-HT intra-luminally, mainly by targeting GPR43 receptor located on mucosal mast cells[117]. Therefore, these findings clearly suggest that through various mechanisms enteric microbial population controls 5-HT level and associated signalling for the control of GI motility, and dysregulation in this system resulted in abnormal GI movement, which is linked with IBS symptoms[116,117]. It has been widely accepted that IBS patients have altered motor and stool patterns, where enhanced GI motility produce abnormal gut contractions resulting in abdominal pain and discomfort.
| Microbiota spp. | 5-HT pathway | Mechanisms of action and observations | Ref. |
| Akkermansia muciniphila (Amuc_1100) | Upregulation | Promote intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling | [129] |
| Akkermansia muciniphila (extracellular vesicles) | Upregulation | Increase expression of the Htr4 gene, and decreases expression of the Htr2B, Htr3B, and Htr7 genes | [129] |
| Bacteriodes thetaiotaomicron | Upregulation | Restore 5-HT+ EC cells and shape EC networks in the GI tract of GF mice by producing SCFAs | [130] |
| Bifidobacterium dentium | Upregulation | Increase intestinal 5-HT level; expressions of 5-HTra receptors 2a and 4, and SERT by producing acetate | [131] |
| Bifidobacterium longum and | Downregulation | Upregulate SERT expression | [132] |
| Lactobacillus acidophilus | |||
| Bifidobacterium pseudolongum | Downregulation | Diminish EC cells | [133] |
| Clostridium ramosum | Upregulation | Promote 5-HT synthesis in colonic EC cells and program differentiation of intestinal stem progenitors toward a secretory 5-HT-producing lineage | [134] |
| Corynebacterium spp., Enterococcus spp., Streptococcus spp. | Upregulation | Enable the direct production of 5-HT | [135] |
| Escherichia coli Nissle 1917 | Upregulation | Enhance 5-HT bioavailability in ileal tissue through interaction with compounds secreted from host tissue | [136] |
| Indigenous spore-forming bacteria | Upregulation | Enhance colonic 5-HT pathway by upregulation of Htr4 | [137] |
| Lactobacillus acidophilus | Down regulation | Upregulate SERT expression | [138] |
| Lactobacillus plantarum IS-10506 | Upregulation | Increase gut 5-HT production along with brain 5-HTT, neurotrophin, and brain-derived neurotrophic factor | [139] |
| Lactobacillus plantarum PS128 | Upregulation | Increase 5-HT+ cells in the gut and alter expression levels of Tph1, Chga, Slc6a4, and Htr4 | [140] |
| Lactobacillus rhamnosus | Down regulation | Upregulate gene and protein level of SERT | [141] |
| SadA-expressing Staphylococci, Trichinella spiralis and Campylobacter jejuni (pathogens) | Upregulation | Promote converting 5-HTP into 5-HT; increase EC cell number and reduce SERT expression | [141] |
Chronic abdominal pain is a crucial aspect of IBS which is also influenced by intestinal gut microbiota[118]. Animal studies are further suggestive for the ability of fecal microbiota to transmit hypersensitivity to colonic distension in rats[119]. These results suggest that abnormal pain perception associated with IBS is also critically derived from gut microbial components[118,119]. Additionally, neurotransmitters derived from gut microbiota have been also found to be decisive for the perception of visceral discomfort. Among the other neurotransmitters, a preferential role of 5-HT has been found to modulate the intestinal pain by activating mesenteric sensory nerve fibers along with the activation of vagal and spinal afferent fibers[120]. Microbial colonization and their commensal activities have been reported to be essential for promoting excitability of the gut sensory neurons, which are responsible for the development of homeostatic pain sensitivity[121]. In GF mice, where a little mucosal inflammation has been found to be associated with visceral hypersensitivity caused by abnormal pain processing in the brain, it can be corrected by fecal microbiota transplantation (FMT) therapies derived from conventional mice[122]. FMTs are also known to stimulate primary nociceptive neurons in the dorsal root ganglia (DRG) and various gut microbial components such as TLR ligands, formyl peptide receptor 1 agonists and SCFAs[122]. These can modulate direct or indirect enhancement of visceral pain sensitivity. Additionally, microbiota derived kynurenic acid, serine proteases and bile acids can reduce pain sensitivity by inactivating DRG neurons or indirectly by releasing opioid-like factors, mainly from the mucosal immune cells[123,124].
Interestingly, the mode of action of 5-HT mediated signalling depends on the type of 5-HT receptor activation and the release of enteric 5-HT, which has been significantly linked to the intensity of abdominal discomfort in IBS patients[125]. The etiology of IBS also includes visceral hypersensitivity along with the nociceptive process and these are mainly regulated with 5-HT3 receptor activation, where it is mainly expressed on peripheral terminals of spinal afferent nerves and vagal afferent nerve endings in stomach[125]. 5-HT3 receptor has been also identified to influence other neurotransmitters in the brain, where a 5-HT3 receptor antagonist (Ramosetron) has been found to lower the visceral hypersensitivity and modifies GI transit in IBS-D patients[126]. Additionally, 5-HT3 receptor antagonists have demonstrated anti-inflammatory effects particularly to the enhanced permeability of gut and mucosal inflammation reported to be caused by various microbial populations[125]. Moreover, an impaired intestinal barrier linked to dysfunctional 5-HT metabolism in IBS patients has been observed recently[126]. Collectively, these findings are indicative of the vitality of gut microbiota in 5-HT-mediated pain perception, where visceral hypersensitivity resulted from the dysfunction of serotonergic pathway has been linked to enteric dysbiosis as reported in IBS cases[127,128].
Among the various pathways underpinning the IBS pathogenesis, a persistent and low-grade mucosal inflammation has been reported in majority of clinical cases, with aberrant immune cell activation[90-129]. In IBS patients, abdominal pain has been characterized with a significant infiltration of mast cells in the colonic mucosa, which is also accompanied with an augmented level of mucosal 5-HT[28,29]. Although, exact underlying mechanisms and causes of mucosal inflammation are not completely known, dysbiosis of the gut microbiota along with altered serotonergic signalling have been identified as the important contributors for the onset of IBS. Indeed, augmented numbers of mucosal and EC cells have been recorded in various inflammatory diseases, such as inflammatory bowel disease (IBD), where a range of 5-HT receptors are expressed to stimulate intestinal inflammation through serotonergic signalling[118]. In colitis, 5-HT directs proinflammatory cytokines generations by stimulating T cells, peritoneal macrophages and splenic DCs in NFκB-dependent manner[130].
Moreover, enhanced 5-HT availability has been recorded in SERT-deficient animals sensitive to gut mucosal inflammation whereas Tph1-/- mice with a lower level of 5-HT have been found to be resistant to experimental colitis[131]. These results suggest that intestinal inflammation is significantly regulated by enteric 5-HT, mainly by acting as pro-inflammatory immune regulator. Various results derived from a post-inflammatory IBS rat model also reveal the onset of visceral hypersensitivity along with fecal microbial dysbiosis, along with an elevated serum level of 5-HT[132]. Additionally, in recent studies lamina propria mast cells (MC) were found to be significantly co-related with an enhanced and spontaneous release of 5-HT in IBS patients[133]. Interestingly, histamine has also been identified as an important biogenic amine found to have pathophysiological role in IBS, where the exact mechanism is still not fully deciphered.
Usually, histamine played critical roles in regulating GI motility, gastric acid and mucosal ion secretion[49,133]. Histamine H1 receptors (H1R) are known to be involved in mediating sensorineural signalling and vascular dilatation, where their activation is known to regulate food and water intake and diurnal feeding rhythm. In addition to this, stimulation of histamine H2 receptors (H2R) have been known for the degranulation of MCs and production of various antibodies, T helper (Th) 1 cytokines, and for T-cell proliferation[133,134]. However, evidences are indicative for the overproduction of histamine by MCs that has been known to cause diarrhea, with an increased neuronal secretomotor function. In constipation, histamine also induces altered enteric neuron function resulting in an excessive segmental contractile colonic motor activity[50,135]. Although, evidences suggested that use of various agents targeting the histamine receptors (HRs) have been found to be potential therapeutic option for IBS patients[134].
In addition to this, upregulated expression of various colonic receptors of 5-HT (5-HT3A/5-HT2B) along with impaired junction proteins has been found in test animals[136]. Additionally, 5-HT3A receptor antagonist administration or FMT derived from the faeces of normal healthy rats, were found to be able to alleviate IBS-like symptoms[136]. Moreover, SCFAs from the gut microbiota have been appeared to have a dual nature directing GI mucosal immunity and inflammation, which are known to be crucial for maintaining gut homeostasis[58]. These cause the over expression of G-protein coupled receptors and induction of regulatory T cells in the gut which in turn increases the integrity of epithelial cell barrier, and thus exhibiting anti-inflammatory effects[136]. In parallel to this, SCFAs are known to cause mucosal inflammation by promoting mucosal 5-HT synthesis (serotonergic pathway) and upregulation of TPH1 transcription[137].
Various preclinical and clinical data have demonstrated the critical role of 5-HT derived from gut in glucose and lipid metabolism, and in various metabolic disorders[138,139]. Various antagonist specific to 5-HT receptors have been beneficial in reversing the clinical manifestations received by altered 5-HT metabolism. For example, fluoxetine was used to prevent T. sanguinis population by lowering serum triglyceride levels and changing the expression of lipid metabolism genes[139]. Additionally, the gut was found to be significantly regulated by its microbiota member ”Clostridium ramosum”which is found to be associated with lipid transport and storage functions in animals. Furthermore, glucose homeostasis is also controlled by various members of enteric microbiota, mainly with the modulation of EC cell’s 5-HT production where this was mainly targeted with the inhibition or genetic depletion of TPH1[140]. Purine metabolism was also found to be regulated with host-microbial metabolic route in IBS patients[141]. These results are clearly indicative for the 5-HT-dependent regulation of various metabolic pathways in the pathogenesis of IBS.
The population of gut microbiota has been reported to be massive along with a modular genome which in turn reported to be benefitting the host and adapted to gut environment. These microbes carry out a variety of tasks, such as xenobiotic metabolism, vitamin production, pathogen defense, and dietary fiber fermentation[115,136,142]. Microbiota derived metabolites are small substances synthesized as intermediate or end products of microbial metabolisms, which are also the principal regulators through which gut microbiota plays an important role in host specific metabolism[136]. These metabolites may be produced directly by bacteria, by alteration of host molecules like bile acids, or through bacterial metabolism of food components. Immunological maturation, immune homeostasis, host energy metabolism, and mucosal integrity maintenance are all identified to be influenced by microbial metabolites mediated endocrine signalling[11,12,14]. IBD’s pathophysiology has been principally linked to certain groups of metabolites, such as bile acids, SCFAs, and tryptophan which have been found in a variety of biological tissues, including faeces, urine, serum, liver, and cerebrospinal fluid, thus having significant impact on the physiology of the host[17,136]. Additionally, some metabolites have been also found to be specific to Crohn’s disease patients and non-IBD controls, where individuals with IBD were found to have higher amounts of bile acids, amino acids, and sphingolipids[143]. Interestingly, these individuals have lower levels of cholesterols, phenylbenzodioxanes, indoles, tetrapyrroles and long-chain fatty acids[144]. SCFAs are derived from carbohydrates and are localized to microbiome, regulating the function of intestinal macrophages, appetite, fat accumulation, intestinal motility and energy metabolism[145]. Other SCFAs produced by microbial fermentation include acetate, propionate, and butyrate, as well as the gases methane, hydrogen sulphide and some other intermediates[145,146]. Butyrate, along with other SCFAs, inhibits epithelial stem cells and promotes epithelial homeostasis by producing IL-18 through inflammasome activation, which is known to be the main source of energy in colonic epithelial cells[147].
Like SCFAs, medium-chain fatty acid levels have been found to be depleted in IBD. Thus, it is hypothesized that certain fatty acids, such as conjugated linoleic acid might exert some anti-inflammatory effects by activating the peroxisome proliferator-activated receptor-γ (PPAR-γ)[148]. Additionally, bile acids also influence host metabolism and immune system via interacting with receptors, such as the transmembrane G protein-coupled receptor 5 (TGR5) and farnesoid X receptor (FXR)[149]. By activating type 2 iodothyronine deiodinase, TGR5 enhances insulin sensitivity (via GLP1), thus regulating energy expenditure (in muscle and brown adipose tissue), and gall bladder relaxation[149]. Activation of bile acid receptor (FXR) also affects host metabolism through various ways, including decreased lipogenesis, hepatic gluconeogenesis, and liver regeneration, as well as by generating antimicrobial peptides for liver regeneration[150,151]. Constitutional makeup of gut microbiota is also significantly influenced by bile acid composition. Furthermore, activation of small intestine FXR also reduces bacterial translocation and growth[152].
In addition to other metabolic intermediates, tryptophan is a well-known precursor for the synthesis of several metabolites such as serotonin, melatonin, nicotinamide and vitamin B3[153]. Gut is the main site of tryptophan synthesis and metabolism, where it is converted by commensal microbiota into indoles agonists of pregnane X receptor and AhR, which have pivotal roles in mucosal immunity and homeostasis[154,155]. Moreover, dysbiosis in IBD also causes a significant decrease in microbial tryptophan activation, which in turn increases metabolism (Figure 4)[148,149]. Indole also stimulates GLP1 release while other indole derivatives [such as indoleacetic acid, indole-3-acetaldehyde, indole-3-aldehyde, indoleacrylic acid (IA) and IPA] are also known to act as agonists for AhR[156]. Additionally, AhR also act as a transcription factor which having developmental and tissue-dependent effects on T cell immunity[157]. Reduced AhR expression has been noted in inflamed mucosal samples from Crohn’s disease patients whereas serum samples from these patients were also found to have altered xenobiotic metabolism[158]. Moreover, enhanced catabolism of fatty acids, reduced level of amino acid metabolites including serotonergic and indole derivatives of tryptophan, as well as decreased levels of phenylalanine and histidine metabolite ergothioneine has been also found in these patients[153,155,158].
Restoring the gut microbiome seems to be promising and therapeutic strategy for the management of IBS with diversified clinical scenarios. Moreover, maintaining the GI microbiome to a level of optimal health with the ideal fecal product is known to be challenging and beneficial to human being. In recent years, FMT has been introduced to yield promising results in various gut disorders such as in Clostridioides difficile infection, where FMT has shown high cure rates, compared to other standard therapies[55,159,160]. This initial lead has been also implanted with microbiome manipulation for the treatment of IBD, ulcerative colitis and Crohn’s disease, with a higher rate of cure effectively[161,162]. However, designing FMT formulations as the treatment for IBS is very challenging, since the optimal microbiome composition in healthy people is poorly defined with a higher degree of variability[163]. In addition to this, disrupted microbiota which would be ideally restored via FMT is known to be highly variable.
Interestingly, dysbiosis of several inflammatory markers is known to induce the expansion of facultative anaerobes, where the mechanisms by which microbiota are disrupted remain unclear. At the same time, GI tract is known to be contributory for key determinants of FMT success whereas, some patients that received FMT also developed systemic infections owing to the adverse effects of FMT[61,63,148]. To avoid the side effects received from FMT application, one more way is to administer autologous FMT (auto-FMT also known as microbiome restoration), where patient sample is screened for various pathogens, banked and then re-administered to the same patient after disrupting microbiome. In this case, restoring the microbiome samples for a long period of time has storage and safety issues, leading to future health problems. Meanwhile, several IBS therapeutics (Table 2) have been developed which are known to target the gut microbiota and target secondary consequences of alterations in the gut microbiota[164].
| Therapy | Description | Proposed mechanism(s) of action |
| Prebiotics | Ingested compounds targeted to stimulate gut microbiota | Mechanism of action undefined, but may include: Anti-inflammatory effects; inhibition of pathogen adherence; and growth of intestinal mucosal layer |
| Probiotics | Ingested microorganisms (e.g., bacteria) | Mechanism of action undefined, but may include: Inhibition of pathogenic microorganism colonization; support intestinal barrier integrity and function; production of beneficial micronutrients; and activation and augmentation of the enteric nervous system |
| Rifaximin | Nonabsorbable, bile-soluble antibiotic indicated for the treatment of adults with IBS-D | Antibacterial against Gram-positive and Gram-negative bacteria: Modulation of gut-immune signalling; inhibition of bacterial translocation; SIBO eradication (in some patients); causing decreases in GI methane concentrations in combination; and with the antibiotic neomycin (in patients with IBS-C) |
| SBI | Prescription medical food for patients with IBS-D | Modulation of gut microbiota: Causing decreases in GI permeability |
| SYN-010 | Derivative of the HMG-CoA reductase inhibitor lovastatin lactone; currently in development for the treatment of patients with IBS-C | Inhibition of methane production by Methanobrevibacter smithii |
| Dietary modification | Variable; one example is the low FODMAP diet | Causing decreases in GI gas production |
| Causing decreases in intra-luminal fluid production |
IBS is a prevalent GI disorder where mechanisms regulating the interconnected cross talk between brain and gut microbiota have been found to be altered. Various metabolic pathways and diseases have also been found to be associated with gut microbiota architecture. The pathophysiology of IBS is still not fully deciphered, whereas a complex network of interaction between various genes, metabolic pathways, behavioral events, host immune response and psychosocial factors has been found to be contributory for IBS and its associated symptoms. Interestingly, serotonin (5-HT) as a neurotransmitter secreted by EC cells regulates GI motility, secretion, and sensation, whereas altered 5-HT signalling has been found contributory in the pathophysiology of IBS. Furthermore, environmental stress has a significant impact on IBS etiology where it significantly regulates the neuroendocrine system and gut functions, mainly through immune system mediated mechanisms. The dietary composition and its intake have a pivotal role in the regulation of IBS, hence usage of quality foods which are gluten free, low fat and FODMAP content, tryptophan and fiber rich may be prominent approach for the management of IBS.
Shiv Vardan Singh acknowledges University Grants Commission (UGC), New Delhi, India for Dr DS Kothari Fellowship. Risha Ganguly, Kritika Jaiswal and Ramesh Kumar acknowledge financial support from UGC/Council of Scientific and Industrial Research, New Delhi, India in the form of Junior and Senior Research Fellowships. Aditya Kumar Yadav acknowledges financial support from UGC in the form of CRET fellowship. All the authors also acknowledge DST-FIST and UGC-SAP facilities of the Department of Biochemistry, University of Allahabad, Prayagraj, India.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Losurdo G, Italy; Yu YB, China S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Grace-Farfaglia P, Frazier H, Iversen MD. Essential Factors for a Healthy Microbiome: A Scoping Review. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | León ED, Francino MP. Roles of Secretory Immunoglobulin A in Host-Microbiota Interactions in the Gut Ecosystem. Front Microbiol. 2022;13:880484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Yan X, Si H, Zhu Y, Li S, Han Y, Liu H, Du R, Pope PB, Qiu Q, Li Z. Integrated multi-omics of the gastrointestinal microbiome and ruminant host reveals metabolic adaptation underlying early life development. Microbiome. 2022;10:222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | He J, Yi L, Hai L, Ming L, Gao W, Ji R. Characterizing the bacterial microbiota in different gastrointestinal tract segments of the Bactrian camel. Sci Rep. 2018;8:654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Leite G, Pimentel M, Barlow GM, Chang C, Hosseini A, Wang J, Parodi G, Sedighi R, Rezaie A, Mathur R. Age and the aging process significantly alter the small bowel microbiome. Cell Rep. 2021;36:109765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 6. | Kumar Singh A, Cabral C, Kumar R, Ganguly R, Kumar Rana H, Gupta A, Rosaria Lauro M, Carbone C, Reis F, Pandey AK. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 7. | Guo P, Lei M, Hu S, Xu Z, Zhou Y, Zhou P, Huang R. Long-term LDR exposure may induce cognitive impairments: A possible association through targeting gut microbiota-gut-brain axis. Ecotoxicol Environ Saf. 2023;249:114351. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Muhammad F, Fan B, Wang R, Ren J, Jia S, Wang L, Chen Z, Liu XA. The Molecular Gut-Brain Axis in Early Brain Development. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 9. | Dai CL, Liu F, Iqbal K, Gong CX. Gut Microbiota and Immunotherapy for Alzheimer's Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Kraaij R, Schuurmans IK, Radjabzadeh D, Tiemeier H, Dinan TG, Uitterlinden AG, Hillegers M, Jaddoe VWV, Duijts L, Moll H, Rivadeneira F, Medina-Gomez C, Jansen PW, Cecil CAM. The gut microbiome and child mental health: A population-based study. Brain Behav Immun. 2023;108:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Dothel G, Barbaro MR, Di Vito A, Ravegnini G, Gorini F, Monesmith S, Coschina E, Benuzzi E, Fuschi D, Palombo M, Bonomini F, Morroni F, Hrelia P, Barbara G, Angelini S. New insights into irritable bowel syndrome pathophysiological mechanisms: contribution of epigenetics. J Gastroenterol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Shrestha B, Patel D, Shah H, Hanna KS, Kaur H, Alazzeh MS, Thandavaram A, Channar A, Purohit A, Venugopal S. The Role of Gut-Microbiota in the Pathophysiology and Therapy of Irritable Bowel Syndrome: A Systematic Review. Cureus. 2022;14:e28064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 13. | Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 404] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 14. | Osadchuk AM, Osadchuk MA, Kvetnoĭ IM. [Irritated bowel syndrome: clinico-morphological types]. Klin Med (Mosk). 2007;85:46-50. [PubMed] |
| 15. | Sheptulin AA, Vize-Khripunova MA. [NEWS IN ETIOLOGY AND PATHOGENESIS OF IRRITATED BOWEL SYNDROME]. Klin Med (Mosk). 2016;94:92-96. [PubMed] |
| 16. | Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4573] [Cited by in RCA: 3698] [Article Influence: 205.4] [Reference Citation Analysis (0)] |
| 17. | Hu Y, Chen Z, Xu C, Kan S, Chen D. Disturbances of the Gut Microbiota and Microbiota-Derived Metabolites in Inflammatory Bowel Disease. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 18. | Singh AK, Singla RK, Pandey AK. Chlorogenic Acid: A Dietary Phenolic Acid with Promising Pharmacotherapeutic Potential. Curr Med Chem. 2023;30:3905-3926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 19. | Camilleri M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA. 2021;325:865-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 20. | Basseri RJ, Weitsman S, Barlow GM, Pimentel M. Antibiotics for the treatment of irritable bowel syndrome. Gastroenterol Hepatol (N Y). 2011;7:455-493. [PubMed] |
| 21. | Wood JD. Enteric nervous system, serotonin, and the irritable bowel syndrome. Curr Opin Gastroenterol. 2001;17:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Holland AM, Bon-Frauches AC, Keszthelyi D, Melotte V, Boesmans W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol Life Sci. 2021;78:4713-4733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, Beyder A. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A. 2018;115:E7632-E7641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 24. | Dickson I. Gut mechanosensors: enterochromaffin cells feel the force via PIEZO2. Nat Rev Gastroenterol Hepatol. 2018;15:519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Young SN. How to increase serotonin in the human brain without drugs. J Psychiatry Neurosci. 2007;32:394-399. [PubMed] |
| 26. | Noddin L, Callahan M, Lacy BE. Irritable bowel syndrome and functional dyspepsia: different diseases or a single disorder with different manifestations? MedGenMed. 2005;7:17. [PubMed] |
| 27. | Gwee KA, Chua AS. Functional dyspepsia and irritable bowel syndrome, are they different entities and does it matter? World J Gastroenterol. 2006;12:2708-2712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Sangkuhl K, Klein TE, Altman RB. Selective serotonin reuptake inhibitors pathway. Pharmacogenet Genomics. 2009;19:907-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Lukiw WJ, Li W, Bond T, Zhao Y. Facilitation of Gastrointestinal (GI) Tract Microbiome-Derived Lipopolysaccharide (LPS) Entry Into Human Neurons by Amyloid Beta-42 (Aβ42) Peptide. Front Cell Neurosci. 2019;13:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Guzel T, Mirowska-Guzel D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 31. | Maffei ME. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 32. | Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252-265. [PubMed] |
| 33. | Saatçi̇Oğlu Ö, BuketTomruk N. Antidepressant Treatment Strategies in the Perinatal Period with a Focus on SSRI use. NPA. 2013;50:93-94. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Shukla R, Ghoshal U, Ranjan P, Ghoshal UC. Expression of Toll-like Receptors, Pro-, and Anti-inflammatory Cytokines in Relation to Gut Microbiota in Irritable Bowel Syndrome: The Evidence for Its Micro-organic Basis. J Neurogastroenterol Motil. 2018;24:628-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Fukudo S. [Irritable Bowel Syndrome, Emotion Regulation, and Gut Microbiota]. Brain Nerve. 2016;68:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. 2017;312:G52-G62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 37. | Bennet SM, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver. 2015;9:318-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 39. | Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42 Suppl 17:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 41. | Singh AK, Bishayee A, Pandey AK. Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 42. | Torii A, Toda G. Management of irritable bowel syndrome. Intern Med. 2004;43:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Levy RL, Cain KC, Jarrett M, Heitkemper MM. The relationship between daily life stress and gastrointestinal symptoms in women with irritable bowel syndrome. J Behav Med. 1997;20:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Qin HY, Cheng CW, Tang XD, Bian ZX. Impact of psychological stress on irritable bowel syndrome. World J Gastroenterol. 2014;20:14126-14131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 171] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (6)] |
| 46. | Alonso C, Guilarte M, Vicario M, Ramos L, Ramadan Z, Antolín M, Martínez C, Rezzi S, Saperas E, Kochhar S, Santos J, Malagelada JR. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163-172.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Patacchioli FR, Angelucci L, Dellerba G, Monnazzi P, Leri O. Actual stress, psychopathology and salivary cortisol levels in the irritable bowel syndrome (IBS). J Endocrinol Invest. 2001;24:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Posserud I, Agerforz P, Ekman R, Björnsson ES, Abrahamsson H, Simrén M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 49. | Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tόth J, Holvoet L, Farré R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 444] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 50. | Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 52. | Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. 2019;10:1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 53. | González-Castro AM, Martínez C, Salvo-Romero E, Fortea M, Pardo-Camacho C, Pérez-Berezo T, Alonso-Cotoner C, Santos J, Vicario M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J Gastroenterol Hepatol. 2017;32:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 656] [Article Influence: 72.9] [Reference Citation Analysis (1)] |
| 55. | Devanarayana NM, Rajindrajith S. Irritable bowel syndrome in children: Current knowledge, challenges and opportunities. World J Gastroenterol. 2018;24:2211-2235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (3)] |
| 56. | Sinagra E, Morreale GC, Mohammadian G, Fusco G, Guarnotta V, Tomasello G, Cappello F, Rossi F, Amvrosiadis G, Raimondo D. New therapeutic perspectives in irritable bowel syndrome: Targeting low-grade inflammation, immuno-neuroendocrine axis, motility, secretion and beyond. World J Gastroenterol. 2017;23:6593-6627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 57. | Alnoman A, Badeghiesh AM, Baghlaf HA, Dahan MH. Pregnancy, delivery, and neonatal outcomes among women with irritable bowel syndrome (IBS) an evaluation of over 9 million deliveries. J Matern Fetal Neonatal Med. 2022;35:5935-5942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Talavera JIR, Parrill AM, Elsayad C, Fogel J, Riggs JC, Peng B. The association between ectopic pregnancy and inflammatory bowel disease, irritable bowel syndrome, and celiac disease: A systematic review. J Obstet Gynaecol Res. 2021;47:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Ganguly R, Gupta A, Pandey AK. Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: A review. World J Gastroenterol. 2022;28:3047-3062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (2)] |
| 60. | Nabavi-Rad A, Sadeghi A, AsadzadehAghdaei H, Yadegar A, Smith SM, Zali MR. The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management. Gut Microbes. 2022;14:2108655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 61. | Farzaei MH, Singh AK, Kumar R, Croley CR, Pandey AK, Coy-Barrera E, Kumar Patra J, Das G, Kerry RG, Annunziata G, Tenore GC, Khan H, Micucci M, Budriesi R, Momtaz S, Nabavi SM, Bishayee A. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 62. | Cozma-Petruţ A, Loghin F, Miere D, Dumitraşcu DL. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J Gastroenterol. 2017;23:3771-3783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (6)] |
| 63. | Senn V, Bassler D, Choudhury R, Scholkmann F, Righini-Grunder F, Vuille-Dit-Bile RN, Restin T. Microbial Colonization From the Fetus to Early Childhood-A Comprehensive Review. Front Cell Infect Microbiol. 2020;10:573735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 64. | Cerdó T, García-Santos JA, Rodríguez-Pöhnlein A, García-Ricobaraza M, Nieto-Ruíz A, G Bermúdez M, Campoy C. Impact of Total Parenteral Nutrition on Gut Microbiota in Pediatric Population Suffering Intestinal Disorders. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 611] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 66. | Wennerberg J, Sharma S, Nilsson PM, Ohlsson B. A possible association between early life factors and burden of functional bowel symptoms in adulthood. Scand J Prim Health Care. 2021;39:506-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765-74; quiz 775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 68. | Collado MC, Cernada M, Neu J, Pérez-Martínez G, Gormaz M, Vento M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res. 2015;77:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 69. | Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum Dev. 2012;88 Suppl 1:S41-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 70. | Cassir N, Simeoni U, La Scola B. Gut microbiota and the pathogenesis of necrotizing enterocolitis in preterm neonates. Future Microbiol. 2016;11:273-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 71. | Sproat T, Payne RP, Embleton ND, Berrington J, Hambleton S. T Cells in Preterm Infants and the Influence of Milk Diet. Front Immunol. 2020;11:1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Singh P, Alm EJ, Kelley JM, Cheng V, Smith M, Kassam Z, Nee J, Iturrino J, Lembo A. Effect of antibiotic pretreatment on bacterial engraftment after Fecal Microbiota Transplant (FMT) in IBS-D. Gut Microbes. 2022;14:2020067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 73. | Singh P, Lembo A. Emerging Role of the Gut Microbiome in Irritable Bowel Syndrome. Gastroenterol Clin North Am. 2021;50:523-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 74. | Zaman S, Lippman SI, Schneper L, Slonim N, Broach JR. Glucose regulates transcription in yeast through a network of signaling pathways. Mol Syst Biol. 2009;5:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 75. | Amabebe E, Anumba DOC. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front Immunol. 2020;11:2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 76. | Hurley E, Mullins D, Barrett MP, O'Shea CA, Kinirons M, Ryan CA, Stanton C, Whelton H, Harris HMB, O'Toole PW. The microbiota of the mother at birth and its influence on the emerging infant oral microbiota from birth to 1 year of age: a cohort study. J Oral Microbiol. 2019;11:1599652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Sears MR, Scott JA, Kozyrskyj AL; Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators. Roles of Birth Mode and Infant Gut Microbiota in Intergenerational Transmission of Overweight and Obesity From Mother to Offspring. JAMA Pediatr. 2018;172:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 78. | De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, Umeh G, Miranda PM, Pigrau Pastor M, Sidani S, Pinto-Sanchez MI, Philip V, McLean PG, Hagelsieb MG, Surette MG, Bergonzelli GE, Verdu EF, Britz-McKibbin P, Neufeld JD, Collins SM, Bercik P. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 79. | Craven LJ, Silverman M, Burton JP. Transfer of altered behaviour and irritable bowel syndrome with diarrhea (IBS-D) through fecal microbiota transplant in mouse model indicates need for stricter donor screening criteria. Ann Transl Med. 2017;5:490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Juncadella AC, Moss A. Fecal microbiota transplantation as a possible treatment of irritable bowel syndrome. Ann Transl Med. 2017;5:506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9292] [Cited by in RCA: 8012] [Article Influence: 616.3] [Reference Citation Analysis (2)] |
| 82. | Vlasova AN, Kandasamy S, Chattha KS, Rajashekara G, Saif LJ. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet Immunol Immunopathol. 2016;172:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 83. | Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017;7:987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 84. | Kolodziejczak M, Béchade C, Gervasi N, Irinopoulou T, Banas SM, Cordier C, Rebsam A, Roumier A, Maroteaux L. Serotonin Modulates Developmental Microglia via 5-HT2B Receptors: Potential Implication during Synaptic Refinement of Retinogeniculate Projections. ACS Chem Neurosci. 2015;6:1219-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 85. | Rothhammer V, Kenison JE, Li Z, Tjon E, Takenaka MC, Chao CC, Alves de Lima K, Borucki DM, Kaye J, Quintana FJ. Aryl Hydrocarbon Receptor Activation in Astrocytes by Laquinimod Ameliorates Autoimmune Inflammation in the CNS. Neurol Neuroimmunol Neuroinflamm. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 86. | Chen Y, Palm F, Lesch KP, Gerlach M, Moessner R, Sommer C. 5-hydroxyindolacetic acid (5-HIAA), a main metabolite of serotonin, is responsible for complete Freund's adjuvant-induced thermal hyperalgesia in mice. Mol Pain. 2011;7:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255-264, e119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 931] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 88. | Lugo-Huitrón R, Ugalde Muñiz P, Pineda B, Pedraza-Chaverrí J, Ríos C, Pérez-de la Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev. 2013;2013:104024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 429] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 89. | Alharthi A, Alhazmi S, Alburae N, Bahieldin A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 90. | Chernikova MA, Flores GD, Kilroy E, Labus JS, Mayer EA, Aziz-Zadeh L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 91. | Rutsch A, Kantsjö JB, Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front Immunol. 2020;11:604179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 459] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 92. | Dobson GP, Letson HL, Biros E, Morris J. Specific pathogen-free (SPF) animal status as a variable in biomedical research: Have we come full circle? EBioMedicine. 2019;41:42-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. Am J Psychiatry. 2011;168:1255-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 94. | Ahmed H, Leyrolle Q, Koistinen V, Kärkkäinen O, Layé S, Delzenne N, Hanhineva K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes. 2022;14:2102878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 177] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 95. | Doroszkiewicz J, Groblewska M, Mroczko B. The Role of Gut Microbiota and Gut-Brain Interplay in Selected Diseases of the Central Nervous System. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 96. | Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 97. | Zubcevic J, Richards EM, Yang T, Kim S, Sumners C, Pepine CJ, Raizada MK. Impaired Autonomic Nervous System-Microbiome Circuit in Hypertension. Circ Res. 2019;125:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 98. | Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34:468-483. [PubMed] |
| 99. | Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW. Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol Metab. 2018;11:70-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 100. | Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 884] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 101. | Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ. The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 102. | Jones LA, Sun EW, Martin AM, Keating DJ. The ever-changing roles of serotonin. Int J Biochem Cell Biol. 2020;125:105776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 103. | Li Q, Ren Y, Fu X. Inter-kingdom signaling between gut microbiota and their host. Cell Mol Life Sci. 2019;76:2383-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 104. | Gupta A, Singh AK, Kumar R, Jamieson S, Pandey AK, Bishayee A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv Nutr. 2021;12:1211-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 105. | Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1293] [Article Influence: 161.6] [Reference Citation Analysis (0)] |
| 106. | Cox LM, Weiner HL. Microbiota Signaling Pathways that Influence Neurologic Disease. Neurotherapeutics. 2018;15:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 107. | Pithadia AB, Jain SM. 5-Hydroxytryptamine Receptor Subtypes and their Modulators with Therapeutic Potentials. J Clin Med Res. 2009;1:72-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012;367:2444-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 109. | Zaniewska M, Mosienko V, Bader M, Alenina N. Tph2 Gene Expression Defines Ethanol Drinking Behavior in Mice. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Koopman N, Katsavelis D, Hove AST, Brul S, Jonge WJ, Seppen J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 111. | Gaster LM, King FD. Serotonin 5-HT3 and 5-HT4 receptor antagonists. Med Res Rev. 1997;17:163-214. [PubMed] [DOI] [Full Text] |
| 112. | Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson GM, Snyder I, Veenstra-VanderWeele J, Blakely RD, Gershon MD. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest. 2016;126:2221-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 113. | Blatter LA, McGuigan JA. Estimation of the upper limit of the free magnesium concentration measured with Mg-sensitive microelectrodes in ferret ventricular muscle: (1) use of the Nicolsky-Eisenman equation and (2) in calibrating solutions of the appropriate concentration. Magnesium. 1988;7:154-165. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 114. | Waclawiková B, Bullock A, Schwalbe M, Aranzamendi C, Nelemans SA, van Dijk G, El Aidy S. Gut bacteria-derived 5-hydroxyindole is a potent stimulant of intestinal motility via its action on L-type calcium channels. PLoS Biol. 2021;19:e3001070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 115. | Taniya MA, Chung HJ, Al Mamun A, Alam S, Aziz MA, Emon NU, Islam MM, Hong SS, Podder BR, Ara Mimi A, Aktar Suchi S, Xiao J. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front Cell Infect Microbiol. 2022;12:915701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 116. | Wang J, Xu W, Wang R, Cheng R, Tang Z, Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021;12:3597-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 117. | Modasia A, Parker A, Jones E, Stentz R, Brion A, Goldson A, Defernez M, Wileman T, Ashley Blackshaw L, Carding SR. Regulation of Enteroendocrine Cell Networks by the Major Human Gut Symbiont Bacteroides thetaiotaomicron. Front Microbiol. 2020;11:575595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Engevik MA, Luck B, Visuthranukul C, Ihekweazu FD, Engevik AC, Shi Z, Danhof HA, Chang-Graham AL, Hall A, Endres BT, Haidacher SJ, Horvath TD, Haag AM, Devaraj S, Garey KW, Britton RA, Hyser JM, Shroyer NF, Versalovic J. Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol Gastroenterol Hepatol. 2021;11:221-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 119. | Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 120. | Tsuruta T, Saito S, Osaki Y, Hamada A, Aoki-Yoshida A, Sonoyama K. Organoids as an exvivo model for studying the serotonin system in the murine small intestine and colon epithelium. Biochem Biophys Res Commun. 2016;474:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 121. | Tatsuoka M, Osaki Y, Ohsaka F, Tsuruta T, Kadota Y, Tochio T, Hino S, Morita T, Sonoyama K. Consumption of indigestible saccharides and administration of Bifidobacterium pseudolongum reduce mucosal serotonin in murine colonic mucosa. Br J Nutr. 2022;127:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Mandić AD, Woting A, Jaenicke T, Sander A, Sabrowski W, Rolle-Kampcyk U, von Bergen M, Blaut M. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci Rep. 2019;9:1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 123. | Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2337] [Article Influence: 233.7] [Reference Citation Analysis (0)] |
| 124. | Nzakizwanayo J, Dedi C, Standen G, Macfarlane WM, Patel BA, Jones BV. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci Rep. 2015;5:17324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 125. | Shajib MS, Wang H, Kim JJ, Sunjic I, Ghia JE, Denou E, Collins M, Denburg JA, Khan WI. Interleukin 13 and serotonin: linking the immune and endocrine systems in murine models of intestinal inflammation. PLoS One. 2013;8:e72774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 126. | Cao YN, Feng LJ, Wang BM, Jiang K, Li S, Xu X, Wang WQ, Zhao JW, Wang YM. Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J Gastroenterol. 2018;24:59-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 127. | Ranuh R, Athiyyah AF, Darma A, Risky VP, Riawan W, Surono IS, Sudarmo SM. Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: role of intestinal microbiota on the gut-brain axis. Iran J Microbiol. 2019;11:145-150. [PubMed] |
| 128. | Chen CM, Wu CC, Huang CL, Chang MY, Cheng SH, Lin CT, Tsai YC. Lactobacillus plantarum PS128 Promotes Intestinal Motility, Mucin Production, and Serotonin Signaling in Mice. Probiotics Antimicrob Proteins. 2022;14:535-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 129. | Cao YN, Feng LJ, Liu YY, Jiang K, Zhang MJ, Gu YX, Wang BM, Gao J, Wang ZL, Wang YM. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World J Gastroenterol. 2018;24:338-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 130. | Yip JLK, Balasuriya GK, Spencer SJ, Hill-Yardin EL. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell Mol Gastroenterol Hepatol. 2021;12:1701-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 131. | Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit viaintraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1269-R1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 316] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 132. | Mishima Y, Ishihara S. Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 133. | Crouzet L, Gaultier E, Del'Homme C, Cartier C, Delmas E, Dapoigny M, Fioramonti J, Bernalier-Donadille A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25:e272-e282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 134. | Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 578] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 135. | Luczynski P, Tramullas M, Viola M, Shanahan F, Clarke G, O'Mahony S, Dinan TG, Cryan JF. Microbiota regulates visceral pain in the mouse. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 136. | Bai T, Zhang L, Wang H, Qian W, Song J, Hou X. Fecal Microbiota Transplantation Is Effective in Relieving Visceral Hypersensitivity in a Postinfectious Model. Biomed Res Int. 2018;2018:3860743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 137. | Ciapała K, Mika J, Rojewska E. The Kynurenine Pathway as a Potential Target for Neuropathic Pain Therapy Design: From Basic Research to Clinical Perspectives. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 138. | Staats Pires A, Tan VX, Heng B, Guillemin GJ, Latini A. Kynurenine and Tetrahydrobiopterin Pathways Crosstalk in Pain Hypersensitivity. Front Neurosci. 2020;14:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 139. | Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 793] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 140. | Min YW, Rhee PL. The clinical potential of ramosetron in the treatment of irritable bowel syndrome with diarrhea (IBS-D). Therap Adv Gastroenterol. 2015;8:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 141. | Machu TK. Therapeutics of 5-HT3 receptor antagonists: current uses and future directions. Pharmacol Ther. 2011;130:338-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 142. | Carco C, Young W, Gearry RB, Talley NJ, McNabb WC, Roy NC. Increasing Evidence That Irritable Bowel Syndrome and Functional Gastrointestinal Disorders Have a Microbial Pathogenesis. Front Cell Infect Microbiol. 2020;10:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 143. | Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (2)] |
| 144. | Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 145. | Kwon YH, Wang H, Denou E, Ghia JE, Rossi L, Fontes ME, Bernier SP, Shajib MS, Banskota S, Collins SM, Surette MG, Khan WI. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell Mol Gastroenterol Hepatol. 2019;7:709-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 146. | Li N, Ghia JE, Wang H, McClemens J, Cote F, Suehiro Y, Mallet J, Khan WI. Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol. 2011;178:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 147. | Lucarini E, Di Pilato V, Parisio C, Micheli L, Toti A, Pacini A, Bartolucci G, Baldi S, Niccolai E, Amedei A, Rossolini GM, Nicoletti C, Cryan JF, O'Mahony SM, Ghelardini C, Di Cesare Mannelli L. Visceral sensitivity modulation by faecal microbiota transplantation: the active role of gut bacteria in pain persistence. Pain. 2022;163:861-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 148. | Morita H, Mochiki E, Takahashi N, Kawamura K, Watanabe A, Sutou T, Ogawa A, Yanai M, Ogata K, Fujii T, Ohno T, Tsutsumi S, Asao T, Kuwano H. Effects of 5-HT2B, 5-HT3 and 5-HT4 receptor antagonists on gastrointestinal motor activity in dogs. World J Gastroenterol. 2013;19:6604-6612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Melhem H, Kaya B, Ayata CK, Hruz P, Niess JH. Metabolite-Sensing G Protein-Coupled Receptors Connect the Diet-Microbiota-Metabolites Axis to Inflammatory Bowel Disease. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 150. | Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1053-G1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 151. | Ślifirski G, Król M, Turło J. 5-HT Receptors and the Development of New Antidepressants. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 152. | Liu N, Sun S, Wang P, Sun Y, Hu Q, Wang X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 153. | Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, Vavilina A, McGinn J, Rendon T, Forrest LR, Hsiao EY. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol. 2019;4:2064-2073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 297] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 154. | Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. CurrOpin Endocrinol Diabetes Obes. 2013;20:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 450] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 155. | Ahluwalia B, Iribarren C, Magnusson MK, Sundin J, Clevers E, Savolainen O, Ross AB, Törnblom H, Simrén M, Öhman L. A Distinct Faecal Microbiota and Metabolite Profile Linked to Bowel Habits in Patients with Irritable Bowel Syndrome. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 156. | Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1802] [Article Influence: 300.3] [Reference Citation Analysis (1)] |
| 157. | Bromke MA, Krzystek-Korpacka M. Bile Acid Signaling in Inflammatory Bowel Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 158. | Ma C, Vasu R, Zhang H. The Role of Long-Chain Fatty Acids in Inflammatory Bowel Disease. Mediators Inflamm. 2019;2019:8495913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 159. | Heimerl S, Moehle C, Zahn A, Boettcher A, Stremmel W, Langmann T, Schmitz G. Alterations in intestinal fatty acid metabolism in inflammatory bowel disease. Biochim Biophys Acta. 2006;1762:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 160. | Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 970] [Cited by in RCA: 2141] [Article Influence: 356.8] [Reference Citation Analysis (0)] |
| 161. | Gasaly N, Hermoso MA, Gotteland M. Butyrate and the Fine-Tuning of Colonic Homeostasis: Implication for Inflammatory Bowel Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 162. | Feng Z, Long W, Hao B, Ding D, Ma X, Zhao L, Pang X. A human stool-derived Bilophilawadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog. 2017;9:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 163. | Lefever S, Van Den Bossche D, Van Moerkercke W, D'Hondt M, Alegret Pampols MDC, Struyve M, De Bel A, Boudewijns M. Ruminococcus gnavus bacteremia, an uncommon presentation of a common member of the human gut microbiota: case report and literature review. Acta Clin Belg. 2019;74:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 164. | Barrett E, Fitzgerald P, Dinan TG, Cryan JF, Ross RP, Quigley EM, Shanahan F, Kiely B, Fitzgerald GF, O'Toole PW, Stanton C. Bifidobacterium breve with α-linolenic acid and linoleic acid alters fatty acid metabolism in the maternal separation model of irritable bowel syndrome. PLoS One. 2012;7:e48159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |