Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4446
Peer-review started: April 29, 2023
First decision: May 15, 2023
Revised: May 23, 2023
Accepted: May 26, 2023
Article in press: May 26, 2023
Published online: June 26, 2023
Processing time: 58 Days and 4.2 Hours
Cholangiocarcinoma and small intestine cancer are common clinical malignancies, but metastatic cholangiocarcinoma and small intestine cancer are rare, especially simultaneous metastatic cholangiocarcinoma and small intestine cancer from breast cancer. Since the clinical presentation of metastatic cholangiocarcinoma and small intestine cancer does not differ from primary tumor, it may lead to misdiagnosis preoperatively.
A 66-year-old woman was admitted to our hospital for further treatment due to abdominal pain and jaundice. Abdominal magnetic resonance imaging and magnetic resonance cholangiopancreatography showed an occupying lesion of the bile duct, considering a high possibility of primary bile duct tumor. Therefore, we performed a radical bile duct cancer surgery and cholecystectomy, and multi
Simultaneous metastatic cholangiocarcinoma and small intestine cancer from breast cancer are rare and the prognosis is extremely poor. Improving preoperative diagnostic accuracy is beneficial to avoid excessive surgical treatment. Treatment should be aimed at relieving biliary obstruction and abdominal pain, and then supplemented with chemotherapy and targeted therapy to control tumor progression and prolong the patient’s life.
Core Tip: Simultaneous metastatic cholangiocarcinoma and small intestine cancer from breast cancer are rare. We present a rare case of bile duct and small intestine metastasis from breast cancer in an older patient who died 5 mo after undergoing radical cholangiocarcinoma surgery due to extensive abdominal metastases and sepsis. Improving preoperative accuracy is necessary, and treatment should be aimed at relieving biliary obstruction and abdominal pain.
- Citation: Jiao X, Zhai MM, Xing FZ, Wang XL. Simultaneously metastatic cholangiocarcinoma and small intestine cancer from breast cancer misdiagnosed as primary cholangiocarcinoma: A case report. World J Clin Cases 2023; 11(18): 4446-4453
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4446.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4446
Breast cancer is a common malignant tumor in women. The metastatic pathways of breast cancer are mainly local spread (invasion of Cooper’s ligament and skin), lymphatic metastasis, and hematogenous metastasis, and the most common distant metastases are bone, lung and liver in that order[1]. Metastasis of breast cancer to the bile duct and small intestine is rare, and definitive preoperative diagnosis is difficult due to the lack of specific clinical presentation and sensitive investigations. We report a case of metastatic cholangiocarcinoma and small intestine cancer from breast cancer that was misdiagnosed as primary cholangiocarcinoma in our hospital. Misdiagnosis of diseases may lead to excessive examination and treatment, which is not helpful for the overall prognosis of patients. Metastasis of breast cancer to the bile duct and small intestine has been reported in a few cases, while simultaneous metastasis to the bile duct and small intestine has not. We believe that our report will broaden the diagnostic thinking when we encounter similar cases and improve diagnostic accuracy.
A 66-year-old woman was admitted to our hospital for further treatment with a history of right upper abdominal pain and progressively increasing jaundice for 1 mo. The abdominal pain was paroxysmal and dull, mainly after meals.
The patient suffered from persistent abdominal pain and vomiting occurred when the pain was severe. The pain usually lasted for 3-5 min and can be relieved on its own. The patient had no other significant discomfort, such as nausea, vomiting, fever, chills, fatigue or melena.
The patient underwent a right total mastectomy and axillary lymph node dissection because of right breast cancer 2 years ago and the pathology was invasive ductal carcinoma. After surgery, she was given eight courses of chemotherapy with a TC regimen [docetaxel: 75 mg/m2, d1, intravenous (iv) drip; cyclophosphamide: 600 mg/m2, d1, intravenous (iv) drip. Every 21 d is 1 cycle of chemotherapy]. The patient did not apply trastuzumab due to personal financial reasons.
There was no personal or family history of acute or chronic disease (hypertension, diabetes, heart disease, etc). There was no family history of tumors.
Physical examination revealed obvious jaundice in the skin and sclera, absence of right breast, flat abdomen, soft abdominal muscles, right upper abdominal pressure pain without rebound pain, negative Murphy sign, and no obvious masses were palpated in the abdomen. The liver and spleen were not touched. Bowel sounds are 5 times per minute.
Laboratory results showed liver function impairment. Alanine aminotransferase and aspartate amino
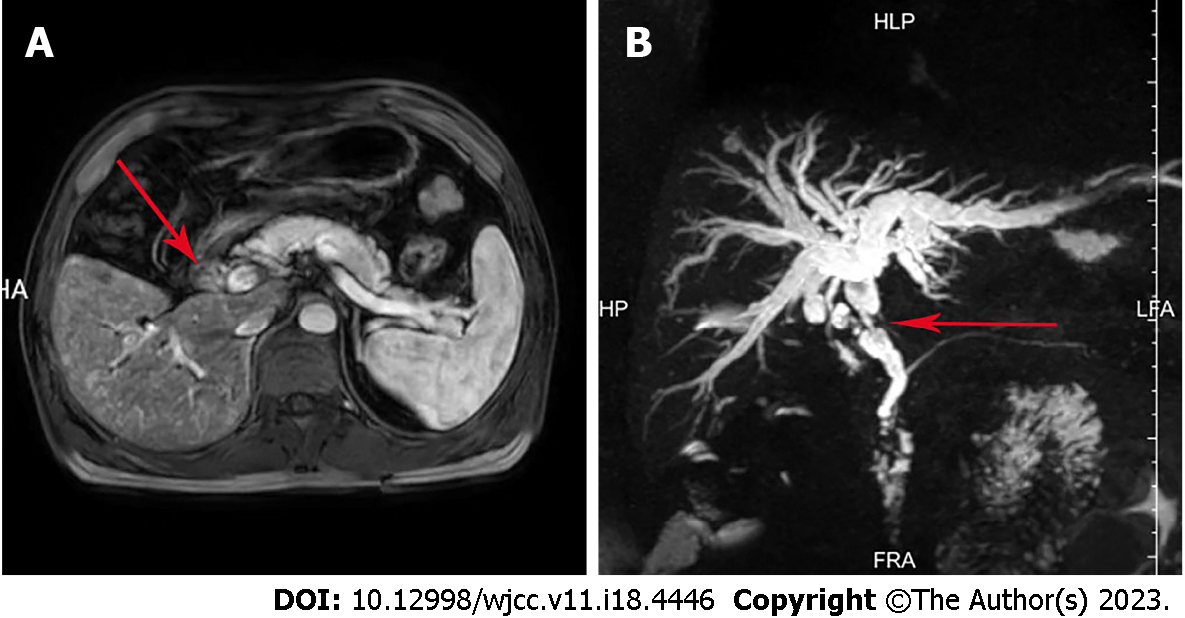
Abdominal magnetic resonance imaging (Figure 1A) and magnetic resonance cholangiopancreatography (Figure 1B) showed an occupying lesion of the bile duct, significant stenosis of the bile duct, and dilatation of the intrahepatic and extrahepatic bile ducts. The tumor was located in the bile ducts of the porta hepatis, measured 1.5 cm × 1.0 cm. Chest computed tomography (CT) showed no significant abnormalities and no occupying lung lesions. Due to the patient’s previous history of breast cancer, a whole-body bone scan was performed and no bone metastasis was observed.
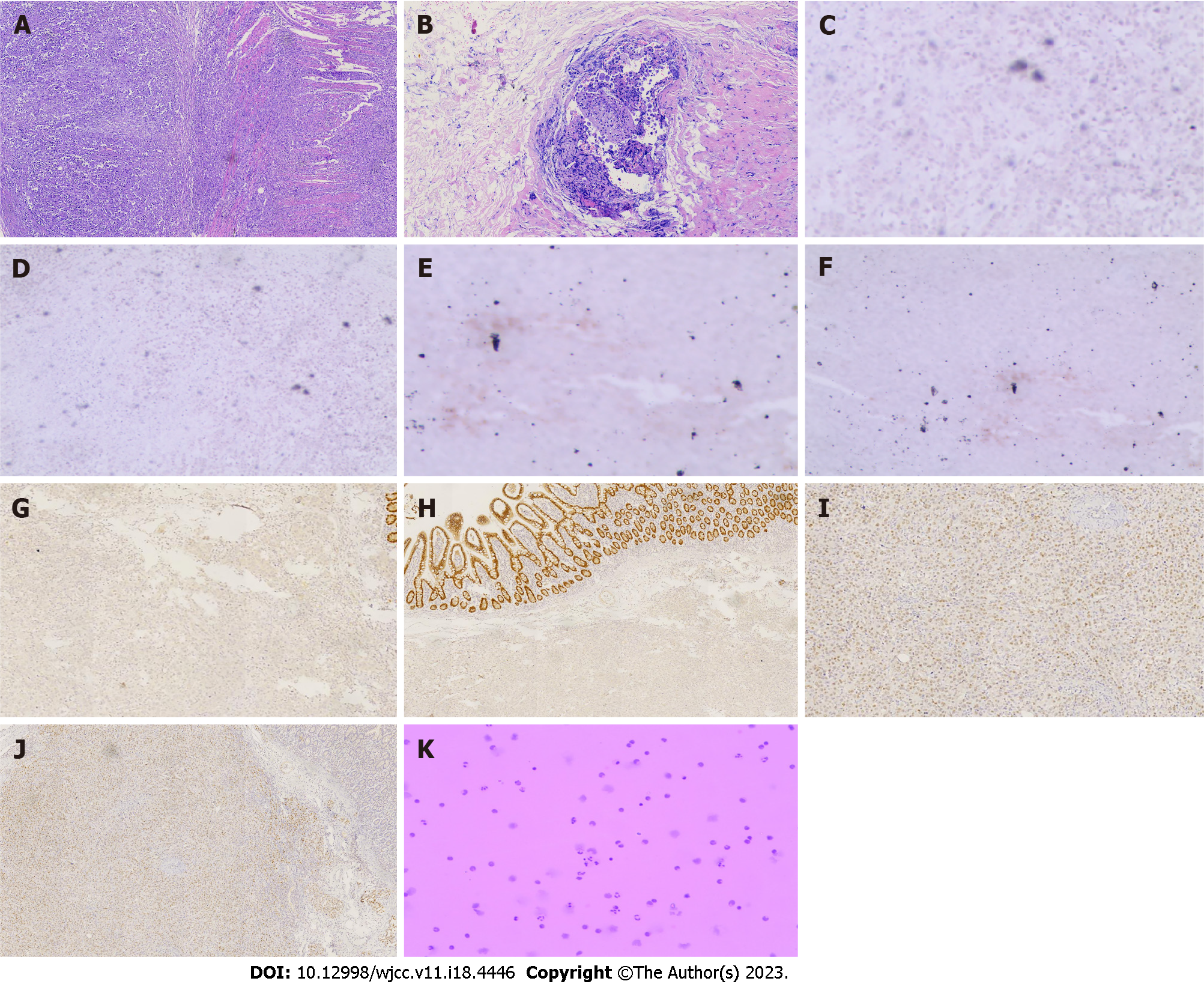
Combining the patient’s clinical manifestations, elevated tumor markers, and imaging examinations, the patient was initially diagnosed with bile duct tumor and a history of breast cancer. It was more likely that the bile duct tumor was a primary tumor. Due to the presence of jaundice in the patient, removal of biliary obstruction was necessary. After obtaining the consent of the patient and her family, surgery was performed in June 2021. Intraoperatively, the tumor was located in the upper part of the bile duct, with a hard texture, with compression of the bile duct causing luminal narrowing, marked dilatation of the proximal bile duct, and chronic inflammatory changes with thickening of the gallbladder wall. The tumor was 40 cm, 70 cm and 130 cm from the flexural ligament with a size of about 2 cm × 2 cm, 1 cm × 2 cm and 2 cm × 2 cm, respectively, with a hard texture, and invaded the whole intestinal wall, resulting in incomplete obstruction of the small intestine. The nature of the tumor was not clear, but there was biliary and intestinal obstruction, so radical bile duct cancer surgery, cholecystectomy and small intestine tumor resection were performed successfully. Postoperative pathology confirmed that the small intestine tumor was poorly differentiated adenocarcinoma (Figure 2A), with cancerous tissue infiltrating the entire intestinal wall, and a cancerous thrombus was found in the vasculature with nerve invasion. The bile duct tumor was poorly differentiated adenocarcinoma with most of the bile duct epithelium missing (Figure 2B), replaced by inflammatory exudate and necrotic tissue, and one metastatic lymph node was found in the surrounding fatty tissue. Immunohistochemistry of small intestine tumor and bile duct tumor was negative for cytokeratin (CK)20, lymphocyte common antigen (LCA), caudal-related homeobox transcription factor 2 (CDX-2) (Figures 2C and D), villin (Figures 2E and F), estrogen receptor (ER) and progesterone receptor (PR), and positive for gross cystic disease fluid protein (GCDFP)-15 (Figures 2G and H), GATA binding protein-3 (GATA3) (Figures 2I and J), CK7, CK8, CK19 and HER-2(++++).
Medical history and immunohistochemistry showed that the bile duct and small intestine tumors were derived from breast cancer. Pathological examination confirmed preoperative misdiagnosis and revised the clinical diagnosis to metastatic cholangiocarcinoma and metastatic small intestine cancer from breast cancer.
Postoperatively, the patient received four cycles of chemotherapy and targeted therapy with TXH [docetaxel: 75 mg/m2, d1, intravenous (iv) drip; capecitabine: 1000 mg/m2, po, bid, d1-d14 and trastu
The patient gradually recovered after surgery, abdominal pain was relieved, jaundice gradually reduced, aspartate aminotransferase and alanine aminotransferase gradually decreased to normal, CA125 significantly decreased to 35.8 U/mL and CA19-9 increased to 34.4 U/mL 1 mo after surgery. Laboratory results showed that CEA increased to 15.3 U/mL, CA125 started to increase again at 66.8 U/mL after dropping to normal, and CA19-9 decreased to normal at 16.3 U/mL after four cycles of chemotherapy and targeted therapy. The patient was readmitted to our hospital for further treatment for abdominal pain and distention in November 2021, and CEA, CA125 and CA19-9 were significantly elevated to 30.5 U/mL, 251 U/mL and 394 U/mL, respectively. CT of the chest and abdomen showed large amounts of hydrothorax and ascites. Thoracentesis and abdominocentesis were performed, and tumor cells were detected in the hydrothorax and ascites (Figure 2K). The patient eventually died from tumor progression, thoracoabdominal infection, and sepsis 5 mo after surgery.
Breast cancer is the most common malignancy in women worldwide. The most common metastases from breast cancer are axillary lymph nodes, bone, lung and liver. Intestinal metastases from breast cancer have been reported[2-4], and a few cases of bile duct metastases from breast cancer[5-7] are also reported in Reference Citation Analysis (https://www.referencecitationanalysis.com/), PubMed and Embase. However, simultaneous metastastic bile duct and intestine cancer has not been reported. In the few previous studies, 24% of cases of metastatic breast cancer involved the common bile duct, 66% the extrahepatic duct or lymph nodes extending directly into bile ducts and ampulla of Vater, and 10% of cases were uncertain[8]. Primary small intestine cancer is uncommon, and metastatic intestine cancer is even rarer. The pathogenesis of primary small intestine cancer is unclear, but it is still believed to be a genetic disease, and the loss of brain and muscle arnt-like 1 increases tumor initiation in the intestine[9]. In our case, small intestinal tumor was not detected on preoperative imaging due to its small size, but was found during surgery. We found both bile duct and small intestinal metastases from breast cancer, which are rare in clinical practice. However, unfortunately, we were unable to confirm the diagnosis preoperatively. Elevated tumor markers may assist in tumor diagnosis, but it is difficult to detect tumor markers associated with the primary tumor in metastatic cholangiocarcinoma. CA19-9 and CEA were elevated in 40% and 85% of patients with cholangiocarcinoma, respectively[10], and a-fetoprotein is usually negative, which is not specific for the diagnosis of metastatic cholangiocarcinoma. Imaging examination is difficult to determine the nature and origin of cholangiocarcinoma. Preoperative diagnosis is difficult and metastatic cholangiocarcinoma was mostly diagnosed after surgery, such as cholangioduodenectomy or pancreatoduodenectomy for malignant bile duct stenosis of unknown origin in previous studies[11]. Pathology is the gold standard for tumor diagnosis, so preoperative pathology may be necessary, but most tumors like cholangiocarcinoma and pancreatic cancer are often not diagnosed through pathology before surgery. Endoscopic retrograde cholangiopancreatography (ERCP) and a transpapillary biliary biopsy were performed to confirm bile duct tumors derived from breast cancer by Suzuki et al[12] and Tang et al[8] for patients with a history of breast cancer. However, negative results may also be obtained due to a small amount of tissue, superficial tissue, or implantation of the metastatic cells in the submucosa. ERCP is not used as a routine preoperative procedure for bile duct cancer. Fine-needle aspiration biopsy may also be used for preoperative pathological examination, but successful puncture may also be failed due to obscuration of surrounding organs and small bile duct tumors. Cochrane and Schlepp[13] demonstrated metastatic breast cancer in the mid-portion of the common bile duct with fine-needle aspiration biopsy under endoscopic ultrasound. In our case, we did not perform ERCP or fine-needle aspiration, thus we misdiagnosed primary bile duct cancer. Positron emission tomography (PET)/CT examination may reveal smaller metastases in the small intestine and may be helpful in the detection of metastases in other organs. PET/CT scans can show bile duct cancer as small as 1 cm. PET/CT is accurate in evaluating primary tumors, lymph node metastases, and distant metastases in patients with bile duct cancer. Accurate preoperative diagnosis can have an impact on treatment.
GATA3 is an important indicator of primary breast cancer; however, CDX-2 and villin are important indicators of enterogenous cancer. CK 7, CEA, ER, PR and GCDFP-15 are usually positive in metastatic breast cancer[14]. CK 7 and CEA are also positive in gastrointestinal tumors, so they are nonspecific[15]. CK7, CK8, CK19, CA19-9, CEA and CDX2 are usually positive in cholangiocarcinoma as well as in breast cancer, thus they are also nonspecific. However, CK 20 is almost invariably present in gastro
Breast tumor cells may metastasize to the abdominal lymph nodes through lymphatic reflux or to the bile ducts and small intestine through hematogenous metastasis. This indicates that the tumor has metastasized distantly and the prognosis is extremely poor. The time interval between the diagnosis of breast cancer and secondary bile duct cancer can be as long as 21 years and as short as 2 years[8], and the time interval between the diagnosis of breast cancer and secondary colon cancer can be as long as 10 years and as short as 4 mo. Breast cancer with simultaneous metastasis to the intestine and bile ducts is not currently reported in the literature. Surgical resection of primary small intestine cancer and primary cholangiocarcinoma is useful to improve quality of life and prolong overall survival, but patients with metastatic tumors cannot benefit from surgery. Preoperative diagnosis is obtained in the minority and most of them are treated surgically, but patient prognosis is poor and does not contribute to overall survival.
Due to our misdiagnosis, we performed surgery on our patient. Tumor markers showed a decreasing trend after surgery, but the level of CEA, CA125 and CA199 increased to 30.5 U/mL, 251 U/mL and 394 U/mL, respectively, at 5 mo after surgery. CT of the chest and abdomen showed a large amount of fluid accumulated in the thoracic and abdominal cavities, and tumor cells were detected in the hydrothorax and ascites. Following disease progression, infection of hydrothorax and ascites, and uncontrollable growth of tumor cells, the patient died of multiple organ failure. The initial decrease in level of tumor markers was due to tumor resection, but ultimately tumor progression could not be controlled. The patient did not benefit from surgery. This patient was admitted to our hospital mainly for jaundice and abdominal pain. If the nature and origin of the bile duct cancer had been determined preoperatively, surgery would have been avoided. In general, patients with pancreaticobiliary malignancies, metastatic common bile duct cancer and external biliary compression by lymph nodes should be implanted with plastic or self-expanding metal stents to relieve related jaundice and abdominal pain[17]. Percutaneous transhepatic biliary drainage is another effective method to relieve jaundice. Adjuvant treatment of metastatic tumors should be based on the primary tumor. Treatment of metastatic breast cancer is influenced by multiple factors; most importantly, expression of ER, PR and HER2, treatment history and prognostic indicators (i.e., short disease-free interval, presence of visceral metastases, performance status, and severity of symptoms)[18]. For example, patients with hormone-receptor-positive tumors are expected to benefit from hormonal therapy, while patients with HER2-positive tumors are treated with chemotherapy in combination with targeted therapy. However, treatment of patients with triple-negative breast cancer relies only on cytotoxic chemotherapy[18]. In our case, the patient could not have benefited from hormonal therapy due to the negative expression ER and PR. Chemotherapy combined with targeted therapy would be preferred because of positive expression of HER2. Adjuvant treatment with chemotherapy and targeted therapy should be applied to control tumor progression. Our patient died 5 mo after surgery, and surgery may have been unnece
Simultaneous metastasis of breast cancer to the bile duct and small intestine is rare and the prognosis is extremely poor. We should combine PET/CT, ERCP and fine-needle aspiration biopsy to improve the accuracy of preoperative diagnosis, to avoid excessive surgical treatment without improving prognosis. Unnecessary surgical treatment is not helpful for the overall survival time of the patient. Treatment should be aimed at relieving biliary obstruction and abdominal pain symptoms, such as percutaneous transhepatic biliary drainage and biliary stent implantation, and then supplemented with chemotherapy and targeted therapy to control tumor progression and prolong the patient’s life. It is believed that effective treatment of patients with metastatic breast cancer should be determined by further research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkhateeb A, Jordan; wu K, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Imai J, Hanamura T, Kawanishi A, Ueda T, Mishima Y, Ito A, Shirataki Y, Morimachi M, Kodama T, Sato H, Kaneko M, Sano M, Teramura E, Monma M, Tsuda S, Tsuruya K, Mizukami H, Arase Y, Fujisawa M, Miyahara S, Nakamura N, Suzuki T, Matsushima M, Suzuki H, Takashimizu S, Kagawa T, Nishizaki Y. A case of breast cancer with extensive colon metastasis. DEN Open. 2023;3:e189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 2. | Law WL, Chu KW. Scirrhous colonic metastasis from ductal carcinoma of the breast: report of a case. Dis Colon Rectum. 2003;46:1424-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Gómez-Sánchez T, Ayllón Gámez S, Pacheco García JM. Intestinal obstruction secondary to metastases of undiagnosed breast cancer. Cir Esp (Engl Ed). 2020;98:104-105. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Franceschini G, Manno A, Mulè A, Verbo A, Rizzo G, Sermoneta D, Petito L, D'alba P, Maggiore C, Terribile D, Masetti R, Coco C. Gastro-intestinal symptoms as clinical manifestation of peritoneal and retroperitoneal spread of an invasive lobular breast cancer: report of a case and review of the literature. BMC Cancer. 2006;6:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Titus AS, Baron TH, Listinsky CM, Vickers SM. Solitary breast metastasis to the ampulla and distal common bile duct. Am Surg. 1997;63:512-515. [PubMed] |
| 6. | Budimir I, Sabol Pusic M, Nikolic M, Dorosulic Z, Ljubicic N, Stajduhar E, Mise I, Vazdar L, Sarcevic B. Obstructive Jaundice as an Uncommon Manifestation of Metastatic Breast Cancer. World J Oncol. 2015;6:297-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Coletta M, Montalti R, Pistelli M, Vincenzi P, Mocchegiani F, Vivarelli M. Metastatic breast cancer mimicking a hilar cholangiocarcinoma: case report and review of the literature. World J Surg Oncol. 2014;12:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Tang J, Zhao GX, Deng SS, Xu M. Rare common bile duct metastasis of breast cancer: A case report and literature review. World J Gastrointest Oncol. 2021;13:147-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Stokes K, Nunes M, Trombley C, Flôres DEFL, Wu G, Taleb Z, Alkhateeb A, Banskota S, Harris C, Love OP, Khan WI, Rueda L, Hogenesch JB, Karpowicz P. The Circadian Clock Gene, Bmal1, Regulates Intestinal Stem Cell Signaling and Represses Tumor Initiation. Cell Mol Gastroenterol Hepatol. 2021;12:1847-1872.e0. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H; British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6:VI1-VI9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Papo M, Fernandez J, Quer JC, Sirvent JJ, Richart C. Metastatic breast carcinoma presenting as obstructive jaundice. Am J Gastroenterol. 1996;91:2240-2241. [PubMed] |
| 12. | Suzuki Y, Hoshi K, Tominaga K, Inaba Y, Yoshinaga T, Kojimahara S, Maki R, Nemoto R, Tetsuka Y, Kawata Y, Yamamiya A, Sugaya T, Iso Y, Takada-Owada A, Ishida K, Goda K, Irisawa A. A case of obstructive jaundice caused by metastasis of breast cancer to the intra/extrahepatic bile duct. DEN Open. 2023;3:e144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Cochrane J, Schlepp G. Metastatic Breast Cancer to the Common Bile Duct Presenting as Obstructive Jaundice. Case Rep Gastroenterol. 2015;9:278-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 14. | Tohfe M, Shami P, Aftimos G, Saade M. Gastrointestinal metastases from breast cancer: a case report. South Med J. 2003;96:624-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Hsieh PS, Yeh CY, Chen JR, Changchien CR. Ileocecal breast carcinoma metastasis. Int J Colorectal Dis. 2004;19:607-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Tot T. The role of cytokeratins 20 and 7 and estrogen receptor analysis in separation of metastatic lobular carcinoma of the breast and metastatic signet ring cell carcinoma of the gastrointestinal tract. APMIS. 2000;108:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Fernandez Y Viesca M, Arvanitakis M. Early Diagnosis And Management Of Malignant Distal Biliary Obstruction: A Review On Current Recommendations And Guidelines. Clin Exp Gastroenterol. 2019;12:415-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 18. | Smith NZ. Treating metastatic breast cancer with systemic chemotherapies: current trends and future perspectives. Clin J Oncol Nurs. 2012;16:E33-E43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Zhou L, Rueda M, Alkhateeb A. Classification of Breast Cancer Nottingham Prognostic Index Using High-Dimensional Embedding and Residual Neural Network. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |