Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4425
Peer-review started: April 4, 2023
First decision: May 12, 2023
Revised: May 25, 2023
Accepted: May 30, 2023
Article in press: May 30, 2023
Published online: June 26, 2023
Processing time: 83 Days and 15.7 Hours
Pneumocystis pneumonia (PCP) is a serious fungal infection usually seen in patients with human immunodeficiency virus, and it is more frequently found and has a high fatality rate in immunocompromised people. Surprisingly, it rarely occurs in immunocompetent patients. However, the clinical diagnosis of this pathogen is made more difficult by the difficulty of obtaining accurate microbiological evidence with routine tests. This case reports a PCP patient with normal immune function who was diagnosed through next-generation sequencing (NGS).
A 23-year-old female who had no special disease in the past was admitted to the hospital with a persistent fever and cough. Based on the initial examination results, the patient was diagnosed with bipulmonary pneumonia, and empirical broad-spectrum antibiotic therapy was administered. However, due to the undetermined etiology, the patient's condition continued to worsen. She was transferred to the intensive care unit because of acute respiratory failure. After the diagnosis of Pneumocystis jirovecii infection through NGS in bronchoalveolar lavage fluid and treatment with trimethoprim/sulfamethoxazole and caspofun
This case emphasizes that, for patients with normal immune function the possibility of PCP infection, although rare, cannot be ignored. NGS plays an important role in the diagnosis of refractory interstitial pneumonia and acute respiratory failure.
Core Tip: Although Pneumocystis pneumonia (PCP) in immunocompetent patients is rare, it should be considered in infected patients with sudden onset of respiratory failure. However, using routine clinical examination, it can be challenging to differentiate between colonizing bacteria and subclinical infections. Instead of traditional laboratory techniques, next-generation sequencing (NGS) was used to make the diagnosis in the current case report. Early diagnosis and fast treatment helped to avoid ineffective treatments and improve the prognosis. This case emphasized that PCP can arise in immunocompetent patients, and NGS may be an effective test method for the quick and accurate diagnosis of PCP.
- Citation: Huang JJ, Zhang SS, Liu ML, Yang EY, Pan Y, Wu J. Next-generation sequencing technology for the diagnosis of Pneumocystis pneumonia in an immunocompetent female: A case report. World J Clin Cases 2023; 11(18): 4425-4432
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4425.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4425
Pneumocystis pneumonia (PCP) is an opportunistic fungal infection caused by the invasion of the single-celled fungus Pneumocystis jirovecii, and it is more common in patients with human immunodeficiency virus (HIV) infection[1]. According to the most recent data, there are still over 500000 instances of PCP worldwide annually, with a death rate of up to 30%, although the incidence of HIV has decreased and can now be effectively prevented due to the deployment of highly active antiretroviral therapy[2,3]. Additionally, as the use of immunosuppressants has increased in recent years, PCP is more frequently found in patients with organ transplantation, malignant tumors, autoimmune diseases, and long-term corticosteroids, leading to an increase in nonHIV PCP immunocompromised individuals and eventually surpassing that of HIV patients[4]. Nonetheless, in the clinic, PCP without immunosuppression is incredibly rare[5]. In this case, a patient with a normal immune system developed severe pneumonia and acute progressive respiratory failure. Sputum cultures were negative many times, and treatment with conventional antibiotics was ineffective. Finally, PCP infection was detected in alveolar lavage fluid using next-generation sequencing (NGS) of bronchoalveolar lavage fluid (BALF). This case suggests that the possibility of PCP infection should not be ignored and the value of NGS in the diagnosis of refractory respiratory failure in patients with normal immune function and severe pneumonia.
A 23-year-old woman presented with a persistent fever (up to 40.5°C) accompanied by cough and sputum for a week.
The symptoms began 1 wk before the onset of the disease with recurrent fever due to cold with cough and sputum.
No particular disease was noted for the patient history.
No personal or family history of infectious disease, genetic disease, or other particular disease was noted.
The vital signs were as follows: Body temperature, 38.0°C; blood pressure, 96/63 mmHg; heart rate, 130 beats per min; and respiratory rate, 20 breaths per min. No other positive signs except slightly coarse breath sounds as well as moist rales in both lower lungs were observed during auscultation.
Laboratory tests showed that the main inflammatory parameters and (1-3)-β-D-glucan test (G test) level increased significantly (Table 1). Liver and kidney function, especially the lactate dehydrogenase (LDH) levels, deteriorated (Table 2). The results of the galactomannan test (GM test), purified protein derivative test, initial screening test for HIV antigens/antibodies and tests for other common respiratory pathogens, such as Mycoplasma pneumoniae, Chlamydia pneumoniae, parainfluenza virus, Epstein‒Barr virus, and influenza A and B, were negative. Based on the positive results of the G-test, increased inflammatory indicators, and elevated LDH levels, we preliminarily considered that the patient might have fungal infection. However, we could not determine the cause of respiratory failure and the specific source of infection with routine testing. Treatment with broad-spectrum antibiotics and ventilators could not prevent disease progression. To diagnose and save the patient more quickly and accurately, we conducted bronchoscopy examination and collected BALF specimens for NGS with the PMseq pathogen high-throughput sequencing platform MGISEQ-2000 for metagenomic sequencing of RNA pathogenic microorganisms at Complete Genomics (BGI, Shenzhen, China). With a sequencing depth of 31 M, BALF-NGS identified one fungus, Pneumocystis jirovecii (Table 3). The number of sequences was 12152.
| Item | Result on admission | Results at ICU | Reference range |
| WBC (× 109) | 4.31 | 2.64 | 3.5-10 |
| RBC (× 1012) | 2.49 | 2.10 | 3.5-5.5 |
| HGB (g/L) | 84 | 69 | 114-163 |
| NEUT (× 109) | 3.65 | 2.42 | 1.8-6.3 |
| %NEUT | 84.8 | 91.7 | 40-75 |
| MONO (× 109 ) | 0.04 | 0.04 | 0.1-0.6 |
| %MONO | 0.9 | 1.5 | 3-10 |
| LYMBP (× 109) | 0.60 | 0.17 | 1.1-3.2 |
| %LYMBP | 13.9 | 6.4 | 20-50 |
| PLT (× 109) | 114 | 69 | 125-350 |
| CRP (mg/L) | 7.51 | 37.69 | 0-5 |
| ESR (mm/h) | 47 | 120 | 0-20 |
| PCT (ng/mL) | 0.75 | > 100 | 0-0.046 |
| IL-6 (pg/mL) | 6.33 | 33.64 | < 7 |
| G test (pg/mL) | 572.42 | < 60 | |
| Item | Result on admission | Results at ICU | Reference range |
| TBIL (μmol/L) | 12.8 | 64.9 | 3.6-20.5 |
| TP (g/L) | 65.3 | 46.3 | 65-85 |
| ALB (g/L) | 37.3 | 22.2 | 40-55 |
| ALT (U/L) | 42 | 61 | 9-50 |
| AST (U/L) | 37 | 39 | 15-40 |
| sCrea (μmol/L) | 76 | 307 | 57-97 |
| Urea (mmol/L) | 8.53 | 31.25 | 3.1-8 |
| UA (μmol/L) | 314 | 629 | 210-420 |
| LDH(U/L) | 852 | 94-250 | |
| Genus | Number of sequences | Species | Number of sequences |
| Pneumocystis | 12297 | Pneumocystis jirovecii | 12152 |
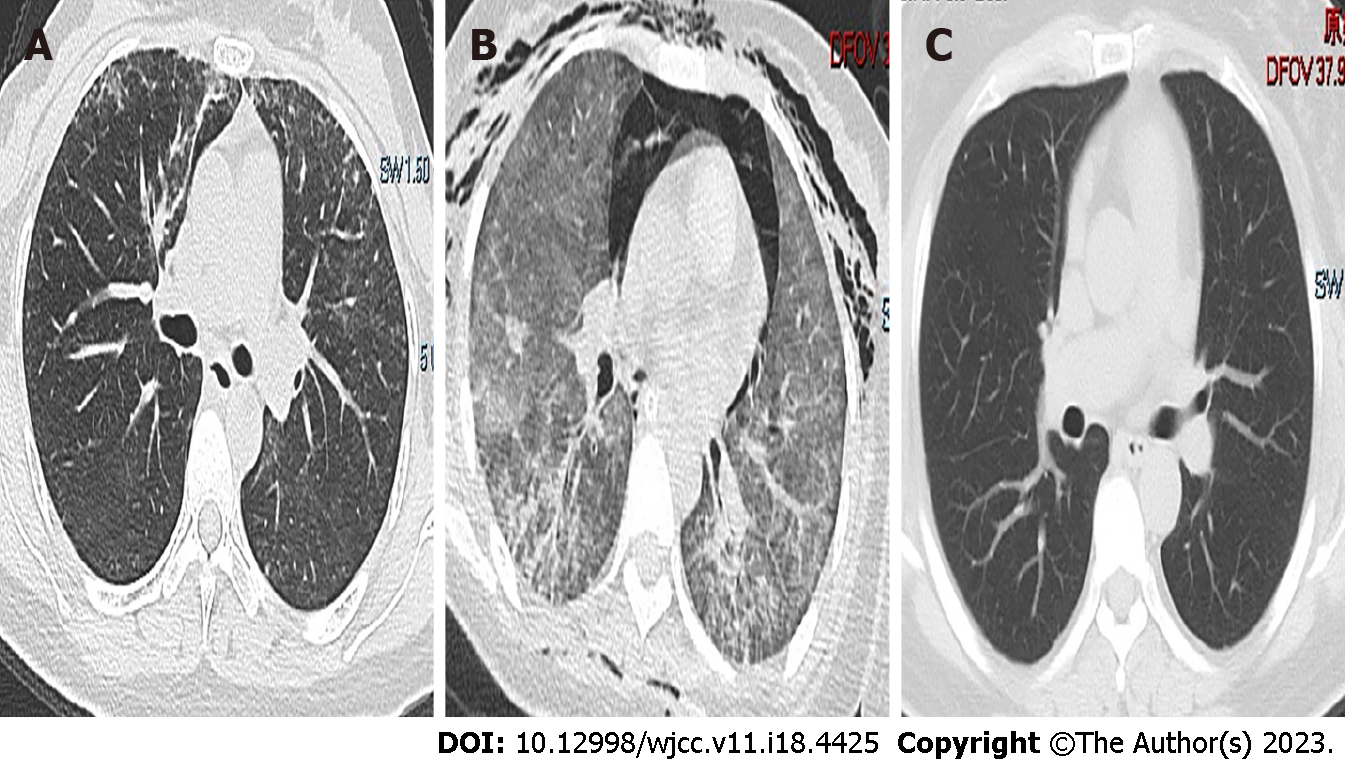
Chest imaging suggested inflammatory infiltration in both lungs (Figures 1 and 2).
According to the patient’s medical history, clinical characteristics, laboratory examinations and imaging results, we determined that Pneumocystis jirovecii was the causative pathogenic microorganism. The final diagnoses were bilateral interstitial pneumonia (Pneumocystis pneumonia), acute respiratory failure and multiple organ dysfunction syndrome.
Based on the initial test results, cefoperazone sulbactam (2.0 g, q12 h, intravenous drip) together with voriconazole (200 mg, q12 h, intravenous drip) were given. After being transferred to the intensive care unit (ICU), the patient was tracheally intubated and underwent prone ventilation (AC mode, FIO2 90%, PEEP 10 cm H2O). Broad-spectrum antibiotic therapies were administered, including meropenem (1 g, q8 h, intravenous drip), vancomycin (0.5 g, q6 h, intravenous drip), voriconazole (200 mg, q12 h, intravenous drip), and oseltamivir phosphate (75 mg, q12 h, oral administration). When the etiology was identified as Pneumocystis jirovecii, the antifungal therapies were adjusted to trimethoprim/sulfamethoxazole (TMP/SMX, TMP 0.8 g/SMZ 0.4 g, q6 h, oral administration) and caspofungin intravenously (50 mg, qd, intravenous drip).
After 14 d of standard anti-PCP and respiratory support treatments, the patient’s temperature returned to normal, and her respiratory condition improved significantly (SpO2 over 98%, PaO2/FIO2 452 mmHg). She was removed from the ventilator successfully. With almost all laboratory indicators returning to normal, the patient was transferred to the general ward for further treatments. Chest computed tomography indicated the basic absorption of inflammation at discharge. Follow-up indicated a good prognosis for the patient.
PCP is a serious, potentially fatal opportunistic illness that frequently applies to patients with compromised immune systems[6], and the incidence of PCP infection in non-HIV patients has increased recently. However, PCP in people with normal immune function is rare and difficult to clearly diagnose by permanent routine methods. Thus, the first startling aspect of this case is that PCP can arise in immunocompetent patients. Second, for bilateral interstitial pneumonia and acute respiratory failure, for which the etiology is difficult to determine, NGS may be an effective test method for quick and accurate diagnosis such that patients can receive appropriate treatments.
PCP is rarely found in immunocompetent patients, and the clinical manifestations of PCP in HIV and non-HIV patients are similar but different. First, most PCP patients experience fever, followed by chest tightness, shortness of breath, a dry cough, and less sputum as their chief complaints. As the disease progresses, hypoxia gradually worsens and eventually leads to progressive dyspnea; however, the pulmonary signs frequently do not correspond to the severity of symptoms[7]. PCP primarily has a subacute course in HIV-positive patients, whereas in non-HIV-positive patients the symptoms quickly progress to respiratory failure[8]. In this case, a PCP patient without HIV infection also experienced high fever, cough, shortness of breath, interstitial pneumonia, and rapid progression of respiratory failure. Second, in addition to differences in clinical manifestations, the severity of the disease and the proportion of patients who even need endotracheal intubation and mechanical ventilation between HIV and non-HIV PCP patients are different. In one cohort study, approximately 41% of patients with PCP needed to be admitted to the ICU, among whom 36% needed mechanical breathing[9]. With a mortality rate of 53% in non-HIV patients compared with 15% in HIV patients, 28% in HIV patients requiring invasive mechanical ventilation, and up to 60% in non-HIV invasive mechanical ventilation patients[10,11]. In general, HIV patients are admitted to the ICU less frequently than non-HIV patients. In addition to the rapidly progressing bilateral interstitial pneumonia, the acute exacerbation of respiratory failure was the main reason for the patient being transferred to the ICU in our case. Anti-PCP therapy and respiratory support therapy (tracheal intubation and prone ventilation) were the main effective treatments for this patient.
Except for the difference in clinical manifestations, it is challenging to make an early diagnosis of non-HIV PCP patients[12,13]. The gold standard for diagnosing PCP often uses microscopy for the detection of Pneumocystis jirovecii cysts or trophozoites in BALF by special staining and immunofluorescence methods. Because Pneumocystis jirovecii cannot be cultivated in vitro, BALF is particularly recommended clinically[14]. However, the sensitivity of special staining and immunofluorescence staining is not high, at approximately 50%, which is lower than that of the polymerase chain reaction (PCR) method. PCR is a simple and fast method for the clinical detection of Pneumocystis jirovecii in BALF and sputum specimens. However, due to its high sensitivity of 98%, it cannot easily differentiate between colonization and subclinical infection, leading to a limited number of true-positive results[15]. G test, which is a part of many fungal walls, is highly sensitive for the diagnosis of HIV PCP patients, but the specificity is not high in nonHIV PCP patients. Other invasive fungal infections, such as Candida, Aspergillus pneumoneum, and Fusarium infection, also present G test positivity. In our case, because the patient had no history of immune deficiency, a positive G test was more likely to be considered a common fungal infection rather than PCP infection. PCP diagnosis using traditional assays is still exceedingly difficult.
With the rapid advancement of molecular detection technology in recent years, BALF-NGS has emerged as a new diagnostic technique that is used in clinical settings for its high sensitivity and specificity (97.4% and 85.12%, respectively), particularly for the detection of special pathogen infections and mixed infections in immunosuppressed and immunocompetent patients[16]. It has the important advantages of being quicker, more accurate, and safer. Additionally, NGS was demonstrated to be significantly more sensitive than immunofluorescence staining (25%) and the G test (67.4%) in a retrospective study. It is important to note that the two were consistent in detecting Pneumocystis jirovecii when NGS was conducted separately using BALF and blood samples from the same patient[17]. In this case, the patient developed bilateral interstitial pneumonia and rapidly aggravated respiratory failure. In addition to a positive G test, increased inflammatory indicators and elevated levels of LDH, we did not obtain more effective laboratory positive test results. The pathogen causing pulmonary infection and respiratory failure has not been found, resulting in poor effects of antibiotics and respiratory support treatments. The results identified one fungus, Pneumocystis jirovecii, which was confirmed to be the pathogenic microorganism. After been diagnosed of PCP and given anti-PCP treatment, the patient recovered and had a good prognosis. No pneumocystis-specific analysis was performed for our case, which might be a limitation.
The standard suggested dose of meperidine 15-20 mg/kg/d and sulfamethoxazole 75-100 mg/kg/d given in 4 separate doses for 21 d is now the first-line treatment for PCP in all populations[18]. NonHIV PCP patients still experience high mortality rates and a high frequency of adverse events, such as rash, fever, gastrointestinal discomfort, leukopenia, hepatotoxicity, and renal insufficiency[19]. To remedy these problems, various complementary and alternative therapies are urgently needed. Caspofungin has received much attention as a novel class of echinocandin antifungal medication because it inhibits the synthesis of G test, which affects the creation of Pneumocystis jirovecii cell wall and ultimately causes Pneumocystis jirovecii death[20]. Despite the lack of prospective research, certain case reports have shown caspofungin's significant efficacy when used either alone or in conjunction with other treatments, and it can be used to treat PCP[21]. In this case, although the patient had no history of HIV infection, immune deficiency, hormone use or other diseases, we immediately administered standard anti-PCP treatment after the diagnosis of PCP through BALF-NGS. The patient's renal function and urinalysis were both normal at the time of admission, but with the aggravation of the disease, the patient's organ function, especially renal function, became abnormal. Considering the abnormality of renal function, in addition to the first-line treatment (TMP/SMX), we also used caspofungin as one of the anti-PCP treatments that has little impact on organ function, as reported in the literature[22]. Diseases of the immune system are a prominent factor in PCP.
In the past, studies have suggested corticosteroids as an adjuvant medication in addition to antibiotics. Research has revealed that corticosteroids dramatically lower mortality in HIV PCP patients[23]. However, it is debatable whether to treat nonHIV PCP with corticosteroids because the majority of patients are already taking them prior to the onset of pneumonia and because prolonged use of corticosteroids can cause immune deficiency, which is a significant contributing factor to non-HIV patients' susceptibility to PCP. Currently, the recommended course of treatment is a combination of corticosteroids and immunosuppressants based on supportive therapy. However, this course of treatment will also cause varying degrees of damage to the immune system, greatly increasing the risk of infection by pathogens such as fungi and bacteria. Therefore, corticosteroids and immunosuppressants were not administered in our case. Instead, supportive care was used to slow the progression of the disease.
In conclusion, we observed nonHIV PCP in individuals evaluated for the lack of underlying risk factors, which are rare and sometimes overlooked. Early application of NGS can help to quickly identify pathogens and buy time for patients who have sudden respiratory failure or a particular, atypical suspected infection. NGS is important in the identification of clinical infectious diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee YJ, Taiwan; Palmirotta R, Italy S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Sokulska M, Kicia M, Wesołowska M, Hendrich AB. Pneumocystis jirovecii--from a commensal to pathogen: clinical and diagnostic review. Parasitol Res. 2015;114:3577-3585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel). 2017;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1520] [Cited by in RCA: 1667] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 3. | Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017;17:e334-e343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 316] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 4. | Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget. 2017;8:59729-59739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Jacobs JL, Libby DM, Winters RA, Gelmont DM, Fried ED, Hartman BJ, Laurence J. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Roux A, Gonzalez F, Roux M, Mehrad M, Menotti J, Zahar JR, Tadros VX, Azoulay E, Brillet PY, Vincent F; Groupe de recherche respiratoire en réanimation en onco-hématologie (Grrr-OH). Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect. 2014;44:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Krajicek BJ, Thomas CF Jr, Limper AH. Pneumocystis pneumonia: current concepts in pathogenesis, diagnosis, and treatment. Clin Chest Med. 2009;30:265-278, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Mikaelsson L, Jacobsson G, Andersson R. Pneumocystis pneumonia--a retrospective study 1991-2001 in Gothenburg, Sweden. J Infect. 2006;53:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Schmidt JJ, Lueck C, Ziesing S, Stoll M, Haller H, Gottlieb J, Eder M, Welte T, Hoeper MM, Scherag A, David S. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit Care. 2018;22:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Fillatre P, Decaux O, Jouneau S, Revest M, Gacouin A, Robert-Gangneux F, Fresnel A, Guiguen C, Le Tulzo Y, Jégo P, Tattevin P. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am J Med. 2014;127:1242.e11-1242.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Guo F, Chen Y, Yang SL, Xia H, Li XW, Tong ZH. Pneumocystis pneumonia in HIV-infected and immunocompromised non-HIV infected patients: a retrospective study of two centers in China. PLoS One. 2014;9:e101943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Cillóniz C, Dominedò C, Álvarez-Martínez MJ, Moreno A, García F, Torres A, Miro JM. Pneumocystis pneumonia in the twenty-first century: HIV-infected vs HIV-uninfected patients. Expert Rev Anti Infect Ther. 2019;17:787-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Li WJ, Guo YL, Liu TJ, Wang K, Kong JL. Diagnosis of pneumocystis pneumonia using serum (1-3)-β-D-Glucan: a bivariate meta-analysis and systematic review. J Thorac Dis. 2015;7:2214-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 14. | Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 727] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 15. | Fan LC, Lu HW, Cheng KB, Li HP, Xu JF. Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PLoS One. 2013;8:e73099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Sun H, Wang F, Zhang M, Xu X, Li M, Gao W, Wu X, Han H, Wang Q, Yao G, Lou Z, Xia H, Shi Y, Li Q. Diagnostic Value of Bronchoalveolar Lavage Fluid Metagenomic Next-Generation Sequencing in Pneumocystis jirovecii Pneumonia in Non-HIV Immunosuppressed Patients. Front Cell Infect Microbiol. 2022;12:872813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Jiang J, Bai L, Yang W, Peng W, An J, Wu Y, Pan P, Li Y. Metagenomic Next-Generation Sequencing for the Diagnosis of Pneumocystis jirovecii Pneumonia in Non-HIV-Infected Patients: A Retrospective Study. Infect Dis Ther. 2021;10:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA; American Thoracic Society Fungal Working Group. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 392] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 19. | White PL, Price JS, Backx M. Therapy and Management of Pneumocystis jirovecii Infection. J Fungi (Basel). 2018;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Wyder MA, Johnston LQ, Kaneshiro ES. Evidence for DNA synthesis in Pneumocystis carinii trophozoites treated with the beta-1,3-glucan synthesis inhibitor pneumocandin L-693,989. J Eukaryot Microbiol. 2010;57:447-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Lee WS, Hsueh PR, Hsieh TC, Chen FL, Ou TY, Jean SS. Caspofungin salvage therapy in Pneumocystis jirovecii pneumonia. J Microbiol Immunol Infect. 2017;50:547-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Wu HH, Fang SY, Chen YX, Feng LF. Treatment of Pneumocystis jirovecii pneumonia in non-human immunodeficiency virus-infected patients using a combination of trimethoprim-sulfamethoxazole and caspofungin. World J Clin Cases. 2022;10:2743-2750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Wieruszewski PM, Barreto JN, Frazee E, Daniels CE, Tosh PK, Dierkhising RA, Mara KC, Limper AH. Early Corticosteroids for Pneumocystis Pneumonia in Adults Without HIV Are Not Associated With Better Outcome. Chest. 2018;154:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |