Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4377
Peer-review started: March 13, 2023
First decision: April 19, 2023
Revised: May 2, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: June 26, 2023
Processing time: 105 Days and 13.8 Hours
As hepatic myelolipoma is rarely encountered, its radiological diagnosis using ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) is challenging. Hepatic myelolipoma is similar to fat-contained hepatic lesions seen in hepatocellular carcinoma and angiomyolipoma. Therefore, further development of techniques to diagnose hepatic myelolipoma is warranted.
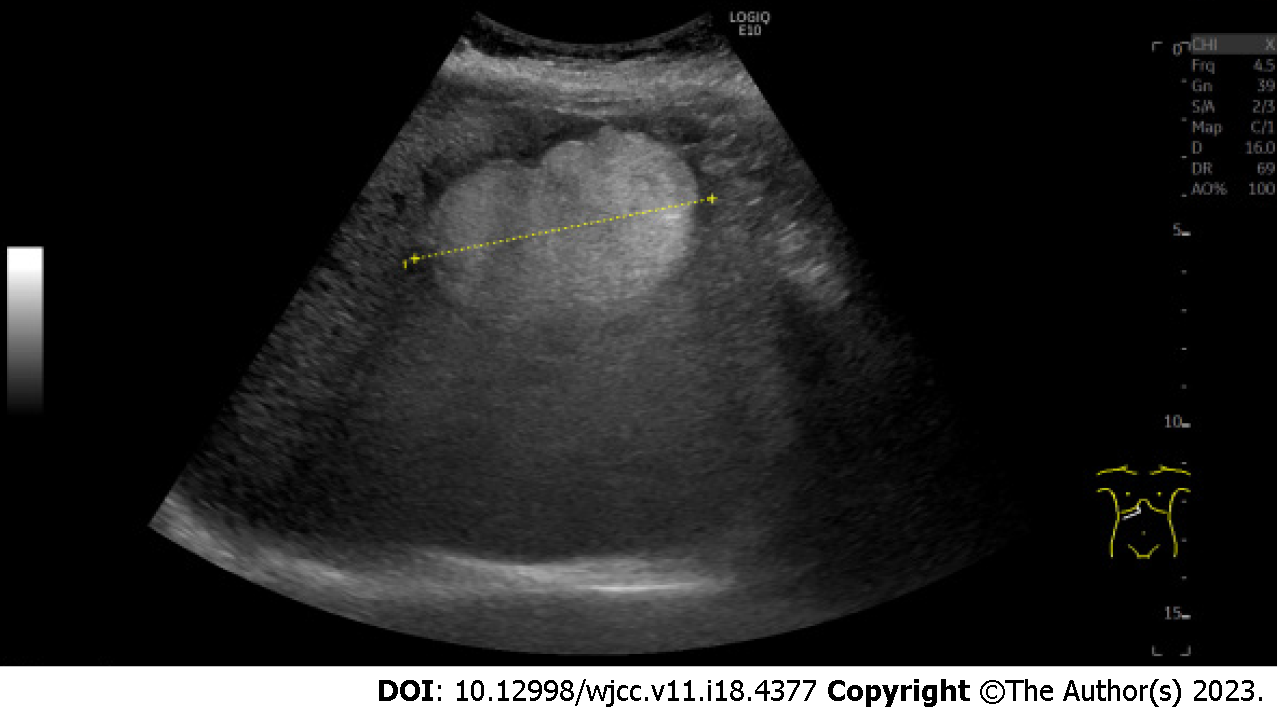
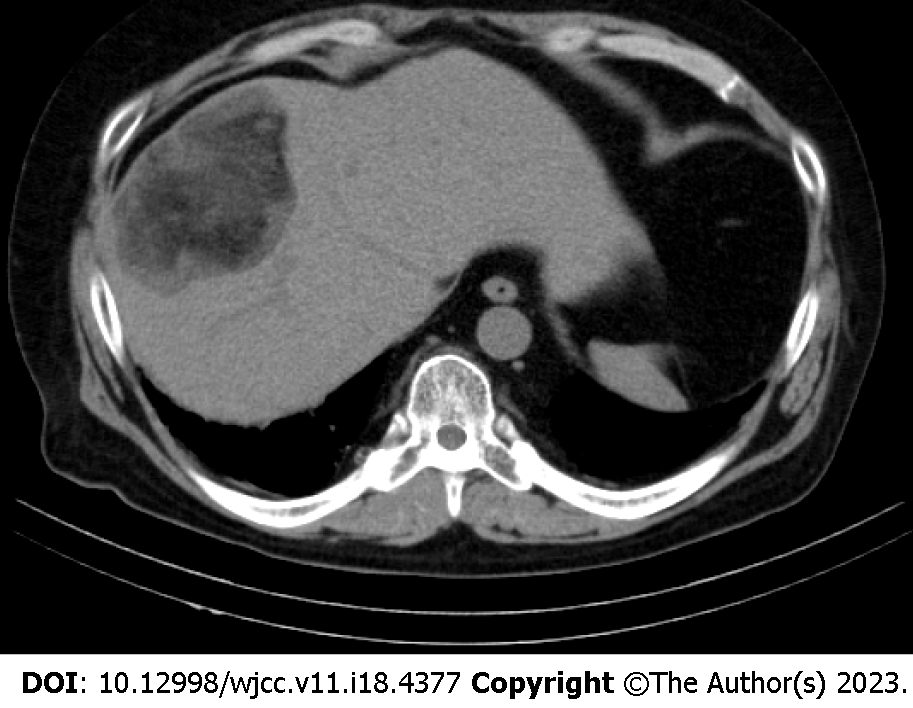
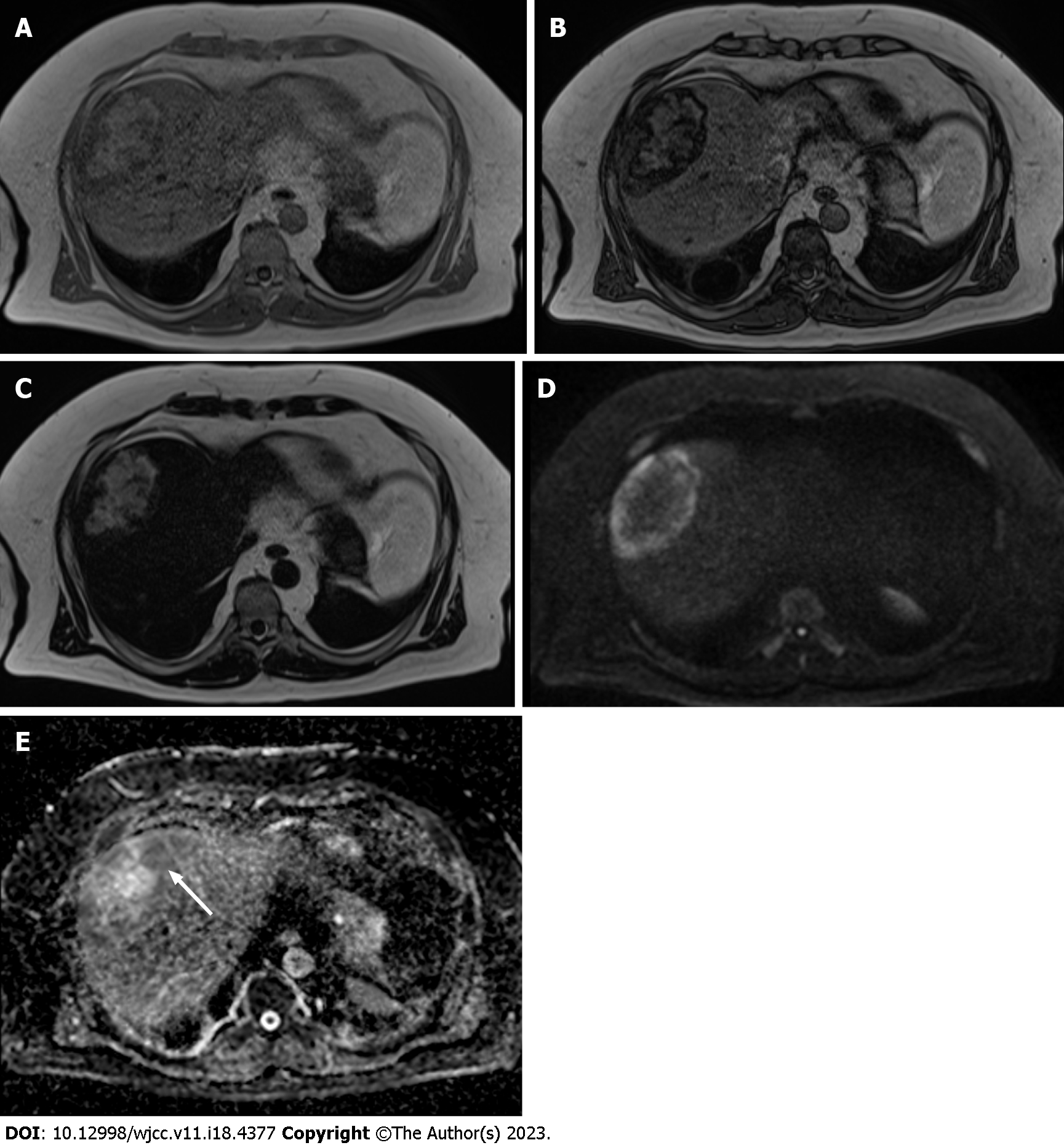
A 44-year-old obese man was found to have a hepatic lesion during his medical checkup. The lesion was 50 mm × 57 mm in size and was detected in segment 8 (S8) of the liver by US. The patient was diagnosed with hepatic lesion 20 years ago, but it was left unresolved. The patient had no symptoms, liver dysfunction, hepatitis virus antibody, or tumor marker elevation. Plain CT showed a well-defined lesion in S8 of the liver. The central and peripheral areas of the lesion primarily exhibited fat density and hypodensity, respectively. MRI revealed a capsule-like structure. Biopsy was performed to address the probability of hepatocellular carcinoma. The lesion was pathologically confirmed as a myelolipoma. Bone marrow scintigraphy performed using 111InCl3 revealed accumulation of the radiopharmaceutical in the soft tissue component, except in the fat-dominant part of the tumor, as well as in the surrounding liver parenchyma due to the presence of reticuloendothelial cells in the liver.
This is the first report on the diagnosis of hepatic myelolipoma using 111InCl3 scintigraphy. The effectiveness of bone marrow scintigraphy for diagnosing hepatic myelolipoma might be limited. As radiopharmaceuticals accumulate in both hematopoietic and reticuloendothelial cells, the accumulation of radiopharmaceuticals in the lesion is obscure.
Core Tip: We attempted to perform bone marrow scintigraphy for hepatic myelolipoma to determine whether 111InCl3 accumulates in the lesion. We found that the radiopharmaceutical accumulated in the soft tissue component, except for the fat-dominant part. However, the radiopharmaceutical also accumulated in the surrounding liver parenchyma, which comprised reticuloendothelial cells. Therefore, the effectiveness of bone marrow scintigraphy in diagnosing hepatic myelolipoma may be limited.
- Citation: Sato A, Saito K, Abe K, Sugimoto K, Nagao T, Sukeda A, Yunaiyama D. Indium chloride bone marrow scintigraphy for hepatic myelolipoma: A case report. World J Clin Cases 2023; 11(18): 4377-4383
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4377.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4377
Myelolipoma is a rare, nonfunctioning benign tumor that comprises mature fat tissue and hematopoietic cells. It is usually detected in the adrenal cortex and rarely outside the adrenal gland. When the lesion develops outside the adrenal gland, it is most often detected in the anterior sacral region[1]. According to previous reports, myelolipomas of liver origin are lesions with fatty and soft tissue density components accompanied by capsular-like structures[2-4]. Therefore, it is crucial to differentiate it from other tumors, including hepatocellular carcinoma. Yamamoto et al[5] reported that bone marrow scintigraphy is helpful in the diagnosis of adrenal myelolipoma as indium chloride (111InCl3) accumulates in hematopoietic cells in myelolipoma[5]. However, no study has diagnosed hepatic myelolipoma with bone marrow scintigraphy. In this study, we report a rare case of hepatic myelolipoma who underwent various imaging techniques, including bone marrow scintigraphy.
This study included a 44-year-old obese male (BMI 32.0) who showed no symptoms. Twenty years ago, he was diagnosed with a hepatic mass but the mass was not treated. In July 2020, during an abdominal ultrasound examination at a medical checkup, a 50 mm × 57 mm hyperechoic lesion was observed in segment 8 (S8) of the liver. He came to our hospital with suspected hepatocellular carcinoma.
The patient developed systemic lupus erythematosus and lupus nephritis aged 24 years, and type 2 diabetes and hypertension aged 32 years.
Physical examination was normal.
His blood tests showed mild inflammatory reaction and renal dysfunction but no liver dysfunction. The patient did not have hepatitis virus or tumor marker elevation. Urinalysis showed mildly elevated urine protein but no other abnormalities.
Ultrasonography (US) revealed a hyperechoic mass with a 79 mm oval halo in S8 of the liver (Figure 1). An abdominal plain computed tomography (CT) scan indicated a heterogeneous low-density mass with internal fat (Figure 2). The patient suffered from renal dysfunction and could not undergo contrast-enhanced CT. We performed abdominal magnetic resonance imaging (MRI) of the T1-weighted image using the Dixon method. The T1-weighted opposed phase image showed an apparent signal drop in the peripheral area of the lesion. The center of the lesion showed a predominant fat component, and the surrounding area showed a mixed fat component. The T2-weighted image showed hyperintensity, the diffusion-weighted image (DWI) showed a hyperintensity area in the peripheral region of the lesion, and the apparent diffusion coefficient (ADC) map showed hypointensity corresponding to the hyperintensity area on DWI, which indicated restricted diffusion. The central fat-predominant area showed hypointensity on the DWI and mixed hypo- and hyper-intensity on the ADC map (Figure 3).
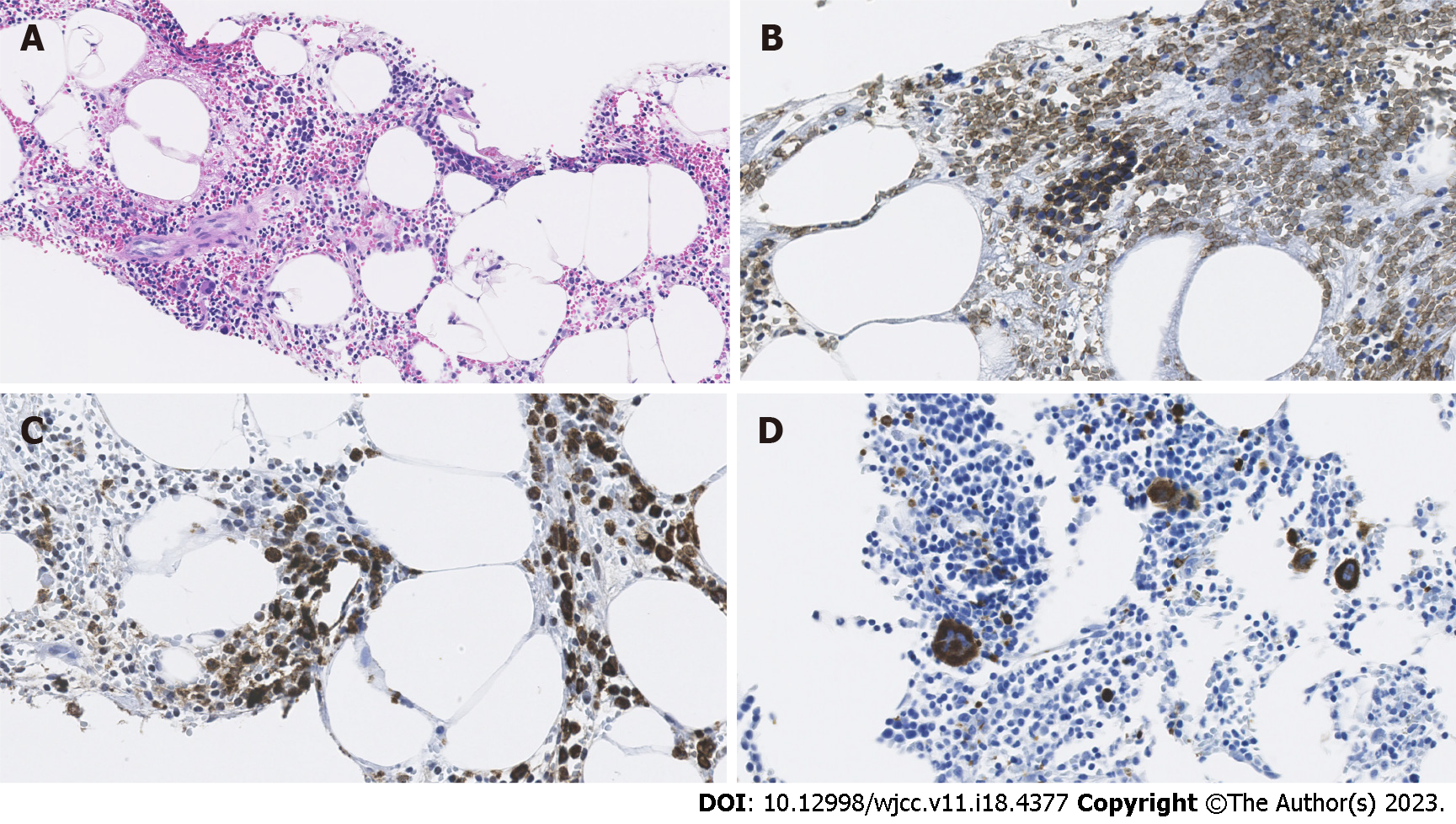
The lesion contained erythroblastosis cells on glycophorin C staining, granulocytic cells on myeloperoxidase staining, and megakaryocytes on CD61 immunostaining. Hematoxylin and eosin staining showed fat droplet deposition in the background liver tissue, which suggested chronic liver inflammation (Figure 4).
Hepatic myelolipoma with background tissue of nonalcoholic fatty liver disease.
The patient had no subjective symptoms and few objective symptoms. The clinician then had consent to follow-up with the patient.
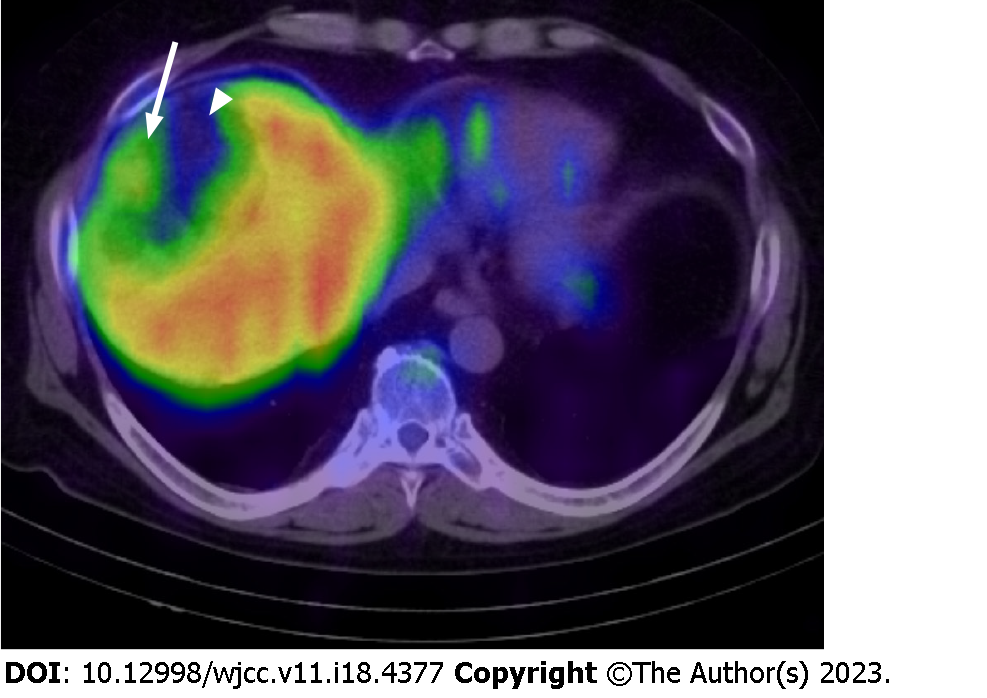
The patient was diagnosed with myelolipoma and underwent bone marrow scintigraphy with 111InCl3 to confirm the presence of bone-marrow elements radiologically (Table 1). 111InCl3 bone marrow scintigraphy showed a mild accumulation of radiopharmaceutical in areas of poor fatty tissue in the peripheral region of the lesion. The radiopharmaceutical accumulation was absent in the center of the mass corresponding to the fat-dominant part. 111InCl3 also mildly accumulated in the bone marrow and spleen (Figure 5). The tumor was in a stable condition and did not cause new symptoms.
| First indication | Twenty years ago |
| Second indication | One month ago |
| Referred to our hospital and ultrasound examination | Day 0 |
| Magnetic resonance cholangiopancreatography | Day 23 |
| Biopsy | Day 75 |
| Hepatic MRI | Day 287 |
| 111InCl3 scintigraphy and single-photon emission computed tomography/CT | Day 662 |
This is the first report to diagnose a case of hepatic myelolipoma with 111InCl3 scintigraphy. The etiology of hepatic myelolipoma is unknown, but several hypotheses exist. Among the most promising ideas are that it is due to an ectopic adrenal gland, an alteration of hepatocytes, or embryonic stem cells remaining in the liver[1,4]. Extra-adrenal myelolipomas tend to occur after middle age, with a male-to-female ratio of 1:2[4]. Myelolipoma is usually asymptomatic in the case of a small lesion, but spontaneous rupture due to mass effect, acute abdomen, and bleeding may occur as the lesion grows[1]. Resection is unnecessary unless the diagnosis is unclear or the lesion is symptomatic[6].
The radiological diagnosis of hepatic myelolipoma using US, CT, and MRI is challenging. Myelolipoma has a capsule-like structure at the lesion periphery and intratumoral fat[4]. These radiological findings are similar to hepatocellular carcinoma. Therefore, we used bone marrow scintigraphy to facilitate the distinction between these two entities[5,7].
Bone marrow scintigraphy showed accumulation of the radiopharmaceutical in the lesion’s soft tissue components, except for the fat-dominant part. Therefore, the efficacy of the radiopharmaceutical was confirmed. However, the conspicuity of the accumulation of radiopharmaceutical was weak because it accumulated in the surrounding liver parenchyma owing to the presence of reticuloendothelial cells[6].
111InCl3 radiopharmaceutical accumulates in bone marrow and the reticuloendothelial system in the liver parenchyma[8]. Therefore, 111InCl3 may mildly accumulate in well-differentiated hepatocellular carcinoma as in myelolipoma as it accumulates in the reticuloendothelial system. This prediction is based on previous reports indicating that superparamagnetic iron oxide (SPIO) accumulates in well-differentiated hepatocellular carcinoma with reticuloendothelial cells on SPIO-enhanced MRI[9]. Such hepatocellular carcinomas have less aggressiveness[10]. To our knowledge, no study has applied 111InCl3 to diagnose hepatocellular carcinoma. However, it could be suggested that hepatic lesions with an accumulation of 111InCl3 are less aggressive as the differential diagnosis includes myelolipoma and well-differentiated hepatocellular carcinoma.
Bone marrow scintigraphy has limited utility in diagnosing hepatic myelolipoma. As radiopharmaceuticals accumulate in both hematopoietic and reticuloendothelial cells, the accumulation of radiopharmaceuticals in the lesion is obscure.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, South Korea; Shuang W, China S-Editor: Li L L-Editor: Webster JR P-Editor: Li L
| 1. | Temizoz O, Genchellac H, Demir MK, Unlu E, Ozdemir H. Bilateral extra-adrenal perirenal myelolipomas: CT features. Br J Radiol. 2010;83:e198-e199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Xu SY, Xie HY, Zhou L, Zheng SS, Wang WL. Synchronous occurrence of a hepatic myelolipoma and two hepatocellular carcinomas. World J Gastroenterol. 2016;22:9654-9660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Kaurich JD, Coombs RJ, Zeiss J. Myelolipoma of the liver: CT features. J Comput Assist Tomogr. 1988;12:660-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Xin H, Li H, Yu H, Zhang J, Peng W, Peng D. MR imaging to detect myelolipomas of the liver: A case report and literature review. Medicine (Baltimore). 2019;98:e16497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Yamamoto T, Koizumi M, Kohno A, Numao N, Inamura K. A case report on 111In chloride bone marrow scintigraphy in management of adrenal myelolipoma. Medicine (Baltimore). 2019;98:e14625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Li KY, Wei AL, Li A. Primary hepatic myelolipoma: A case report and review of the literature. World J Clin Cases. 2020;8:4615-4623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Lilien DL, Berger HG, Anderson DP, Bennett LR. 111 In-chloride: a new agent for bone marrow imaging. J Nucl Med. 1973;14:184-186. [PubMed] |
| 8. | Gilbert EH, Earle JD, Goris ML, Kaplan HS, Kriss JP. The accuracy of 111InCl3 as a bone marrow scanning agent. Radiology. 1976;119:167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Imai Y, Murakami T, Yoshida S, Nishikawa M, Ohsawa M, Tokunaga K, Murata M, Shibata K, Zushi S, Kurokawa M, Yonezawa T, Kawata S, Takamura M, Nagano H, Sakon M, Monden M, Wakasa K, Nakamura H. Superparamagnetic iron oxide-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading. Hepatology. 2000;32:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Tanaka M, Nakashima O, Wada Y, Kage M, Kojiro M. Pathomorphological study of Kupffer cells in hepatocellular carcinoma and hyperplastic nodular lesions in the liver. Hepatology. 1996;24:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |