Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4368
Peer-review started: March 20, 2023
First decision: April 20, 2023
Revised: May 3, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: June 26, 2023
Processing time: 98 Days and 5.8 Hours
It is difficult and risky for patients with a single lung to undergo thoracoscopic segmental pneumonectomy, and previous reports of related cases are rare. We introduce anesthesia for Extracorporeal membrane oxygenation (ECMO)-assisted thoracoscopic lower lobe subsegmental resection in a patient with a single left lung.
The patient underwent comprehensive treatment for synovial sarcoma of the right lung and nodules in the lower lobe of the left lung. Examination showed pulmonary function that had severe restrictive ventilation disorder, forced expiratory volume in 1 second of 0.72 L (27.8%), forced vital capacity of 1.0 L (33%), and maximal voluntary ventilation of 33.9 L (35.5%). Lung computed tomography showed a nodular shadow in the lower lobe of the left lung, and lung metastasis was considered. After multidisciplinary consultation and adequate preoperative preparation, thoracoscopic left lower lung lobe S9bii+S10bii combined subsegmental resection was performed with the assistance of total intravenous anesthesia and ECMO intraoperative pulmonary protective ventilation. The patient received postoperative ICU supportive care. After surgical treatment, the patient was successfully withdrawn from ECMO on postoperative Day 1. The tracheal tube was removed on postoperative Day 4, and she was discharged from the hospital on postoperative Day 15.
The multi-disciplinary treatment provided maximum medical optimization for surgical anesthesia and veno-venous ECMO which provided adequate protection for the patient's perioperative treatment.
Core Tip: The patient was a 50-year-old woman with synovial sarcoma of the right lung. A left pulmonary nodule was found more than 6 mo after surgery. The pulmonary function tests showed that lung function was severely limited. Performing anesthesia and surgery in this patient was difficult. Multidisciplinary treatment provided maximum medical optimization for the surgical anesthesia in this case; the combined protocol of total intravenous anesthesia, veno-venous extracorporeal membrane pulmonary oxygenation, goal-directed fluid therapy, perioperative pulmonary protection and rehabilitation, and multimodal analgesia provided adequate protection for the patient's perioperative treatment. The patient recovered and was discharged from the hospital.
- Citation: Wang XF, Li ZY, Chen L, Chen LX, Xie F, Luo HQ. Anesthesia for extracorporeal membrane oxygenation-assisted thoracoscopic lower lobe subsegmental resection in a patient with a single left lung: A case report. World J Clin Cases 2023; 11(18): 4368-4376
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4368.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4368
It is difficult and risky for patients with a single lung to undergo thoracoscopic segmental pneumonectomy, and previous reports of related cases are rare[1]. The patient in this report underwent comprehensive treatment for synovial sarcoma of the right lung and nodules in the lower lobe of the left lung. Examination showed pulmonary function that had severe restrictive ventilation disorder, and her surgical risk was high. Interventional treatment was unsuitable due to the high risk of intraoperative respiratory failure. Following multidisciplinary discussion it was decided that after adequate preoperative preparation, subsegmental pneumonectomy was planned with the assistance of extracorporeal membrane oxygenation (ECMO).
Ensuring the patient’s perioperative safety was a difficult problem for the surgical team. ECMO has provided the opportunity for many complex thoracic surgeries, but its operation is complicated, and its perioperative management difficulties should not be underestimated. Through multidisciplinary collaboration, the thoracoscopic subsegmental resection was performed with the assistance of total intravenous anesthesia and ECMO. Intraoperative pulmonary protective ventilation, hemodynamic monitoring and goal-directed fluid therapy (GDFT) were performed. The patient recovered and was discharged from the hospital.
A 50-year-old female complained of right lung synovial sarcoma more than 6 years after surgery, and a left lung nodule was present for 6 mo.
The patient visited our hospital for "right chest and back pain for more than 2 mo" more than 6 years before admission and underwent "thoracoscopic-assisted wedge resection of the upper lobe of the right lung + resection of the middle lobe of the right lung + internal fixation of a rib fracture" on February 29, 2016. The postoperative pathology report showed a "right upper lobe lung mass" monophasic (spindle cell) synovial sarcoma. On December 13, 2016, "right thoracic adhesion decomposition + right residual pneumonectomy + total pleurectomy + mediastinal lymph node dissection" was performed. On July 24, 2020, the patient underwent thoracoscopic-assisted right chest wall tumor resection and postoperative camrelizumab immunotherapy. Six months previously, an external examination revealed a nodule in the lower lobe of the left lung, and computed tomography (CT) of the lung (May 9, 2022) revealed the following: Right chest wall tumor + right lung lesion changed by total pneumonectomy. The patient had a nodular shadow in the lower lobe of the left lung, suggesting lung metastasis and right pleural encapsulated effusion. She was admitted to the outpatient clinic with "right lung synovial sarcoma after comprehensive treatment and a left lung lower lobe nodule".
Six months before presentation, she was diagnosed with hypothyroidism at the local hospital and started oral levothyroxine tablets (75 µg qd) and prednisone (2.5 mg bid).
Locally born and long-term resident, the patient had no history of tobacco, alcohol, drugs or other substance use, and no industrial poison, dust, or radioactive material contact history. Her parents, brothers and sisters are in good health, and there are no similar diseases in the family.
A surgical scar approximately 20 cm in length was seen on the right chest. The patient had an asymmetric thorax, there was no pressure pain on the sternum, and no breath sounds were heard in the right lung. The respiratory motion of the left lung was normal, the respiratory sound of the left lung was clear on auscultation, and no abnormal respiratory sound was heard.
Complete biochemical examination (2022-05-26) showed the following: total protein: 55.8 g/L ↓, albumin: 34.2 g/L ↓, the remaining examinations were normal. Plasma D-dimer and fibrin degradation products (D-Di+FDP) measurement + coagulation screening (2022-05-26) showed: Fibrinogen: 4.68 g/L ↑, D-dimer: 0.82 mg/L ↑, the remaining examinations were normal. Thyroid function was normal. Blood gas analysis + lactate measurement (2022-05-30) showed total CO2: 52.6 mmol/L↑, lactate: 2.6 mmol/L ↑, and all other measurements were normal.
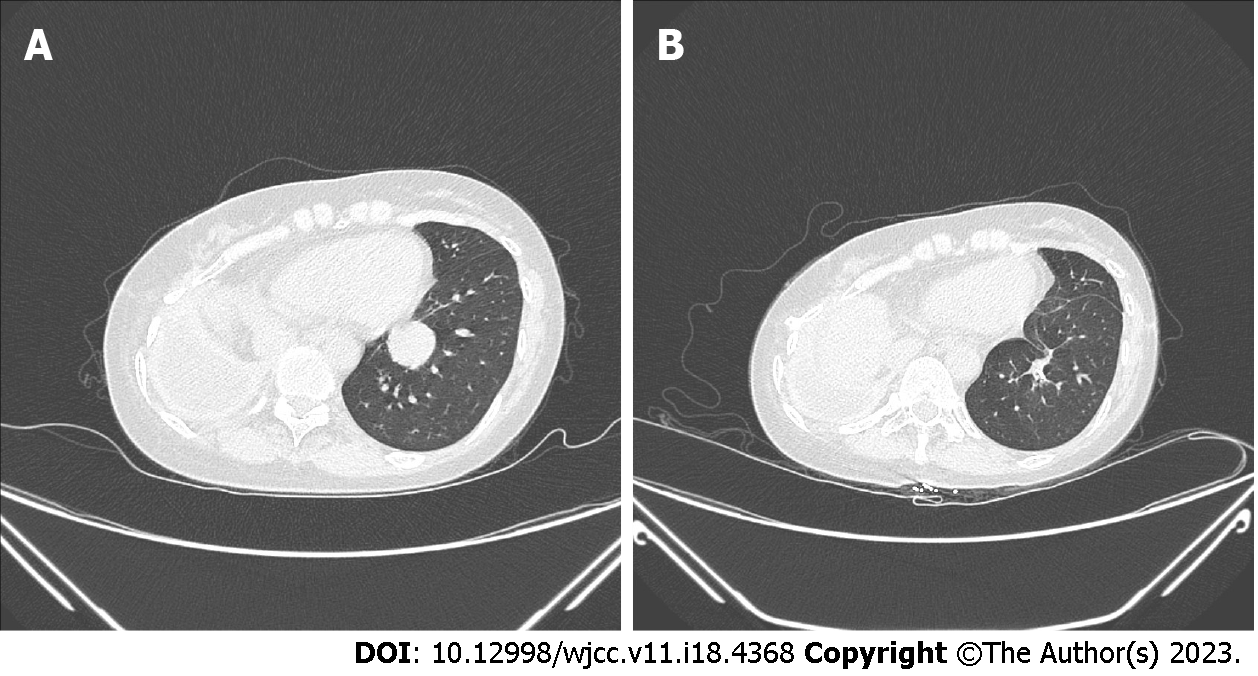
Lung CT (2022-05-09) showed the following: (1) Right chest wall tumor + right lung lesion with total pneumonectomy changes; and (2) Nodular shadow in the lower lobe of the left lung, suggesting lung metastasis. The patient had right pleural encapsulated effusion (for details, see Figure 1). Positron emission tomography (PET-CT) indicated no metastasis in the rest of the body except the left lung nodule.
Cardiac ultrasound (2022-05-10) showed a left ventricular ejection fraction of 63%, stroke volume (SV) 48 mL, small left atrium, and tricuspid regurgitation (mild). An ambulatory electrocardiogram showed the following: (1) Sinus rhythm; (2) Occasional premature atrial contractions; (3) Occasional premature ventricular contractions; and (4) Reduced heart rate variability.
Pulmonary function tests yielded the following results: Forced expiratory volume in 1 second (FEV1) 0.72 L (27.8%), forced vital capacity 1.0 L (33%), maximal voluntary ventilation 33.9 L (35.5%), severe restrictive ventilatory disorder, and mixed ventilatory disorder.
Cardiopulmonary exercise test showed peak oxygen uptake VO2 15.0 mL/min/kg (intermediate risk), anaerobic threshold oxygen uptake 10.9 mL/min/kg (high risk). Static lung function showed: Severe restrictive ventilation impairment, mixed ventilation impairment; no chest pain present with exercise, chest tightness, heart rate reserve not exhausted; moderate decrease in exercise tolerance, exercise intolerance, and restricted respiratory function. The patient had an exercise cardiac function class C (Weber KT criteria); her surgical risk was high (AT VO2 10.9 < 11) (for details, see Table 1).
| Summary | Resting | Reference | VT2 Man | Max VO2 | Max Watts | Pred | Max/ pred | Recov 60 s | Recov 240 s | |
| Time averaging 10 s | ||||||||||
| Time | min | 1:50 | 3:50 | 5:30 | 7:40 | 7:40 | 09:30 | 12:00 | ||
| MET | 1.2 | 2.6 | 3.1 | 4.3 | 4.3 | 0 | 0 | |||
| RER | 0.81 | 0.93 | 0.94 | 1.15 | 1.15 | 0 | 0 | |||
| Wat | W | 0 | 0 | 18 | 44 | 44 | 95 | 46 | 0 | 0 |
| HR | /min | 88 | 97 | 103 | 129 | 129 | 170 | 76 | 118 | 100 |
| HRR | /min | 82 | 73 | 67 | 41 | 41 | 52 | 70 | ||
| 02/HR | mL | 2.5 | 4.9 | 5.5 | 6 | 6 | 8 | 76 | 0 | 0 |
| SVc | mL | 28 | 40 | 41 | 37 | 37 | 0 | 0 | ||
| Psys | mmHg | 153 | 133 | 133 | 131 | 131 | 131 | 153 | ||
| Pdia | mmHg | 69 | 74 | 74 | 79 | 79 | 79 | 86 | ||
| SpO2 | % | 99 | 99 | 98 | 98 | 98 | 97 | 99 | ||
| V'O2 | mL/min | 216 | 473 | 566 | 779 | 779 | 1436 | 54 | 0 | 0 |
| VO2/kg | mL/min/kg | 4.2 | 9.1 | 10.9 | 15 | 15 | 27.6 | 54 | 0 | 0 |
| V'CO2 | mL/min | 176 | 440 | 535 | 894 | 894 | 0 | 0 | ||
| dO2/dW | mL/min/Watt | 0 | 0 | 19.46 | 12.8 | 12.8 | 0 | 0 | ||
| BF | /min | 28 | 44 | 49 | 60 | 60 | 42 | 145 | 4 | 4 |
| V'E | L/min | 8 | 16 | 18 | 29 | 29 | 75 | 39 | 0 | 0 |
| BR | % | 92 | 82 | 80 | 68 | 68 | 28 | 242 | 100 | 100 |
| EqO2 | 21 | 23.6 | 23.1 | 28.9 | 28.9 | 0 | 0 | |||
| EqCO2 | 25.8 | 25.3 | 24.5 | 25.2 | 25.2 | 0 | 0 | |||
| PETO2 | mmHg | 110.34 | 111.55 | 109.74 | 115.31 | 115.31 | 148.2 | 148.2 | ||
| PETCO2 | mmHg | 33.95 | 35.66 | 37.67 | 37.81 | 37.81 | 1.83 | 1.83 | ||
| VDc/VT | % | 0 | 0 | 1 | 4 | 4 | 19 | 21 | 0 | 0 |
| VTex | L | 0.271 | 0.364 | 0.379 | 0.484 | 0.484 | 0 | 0 | ||
| Marker chart | ||||||||||
| VECO2s | OUESs | |||||||||
| L/L | mL/logL | |||||||||
| 30.22 | 0 | |||||||||
Medical imaging department: A nodular soft tissue density shadow approximately 2.5 cm in diameter was seen in the lower lobe of the left lung, and enhancement changes were visible after the enhancement scan. Combined with the patient's medical history and examination, the tumor in the lower lobe of the left lung was considered to be metastasis, and combined with the patient's PET-CT examination, no metastasis was seen in the remaining systemic sites.
Respiratory medicine: The patient's lung function was poor, and the perioperative risk was high. Intraoperative ECMO could be considered to ensure the safety of surgery.
Medical oncology: The patient had metastasis in the lower lobe of the left lung after chemotherapy and started oral pazopanib and camrelizumab immunotherapy after right lung synovial sarcoma surgery. The patient had no mutated targets in previous genetic testing and continued to be treated with programmed death ligand 1 +apatinib to assess clinical efficacy and decide further treatment options. Postoperative genetic testing could be considered again to develop a plan for postoperative adjuvant therapy for the patient or to consider postoperative chemotherapy with anthracyclines.
Rehabilitation department: The patient's examination after admission showed blood gas analysis + lactate measurement (2022-05-30): Total carbon dioxide: 52.6 mmol/L↑, lactate: 2.6 mmol/L↑, and the remaining examinations were normal. Pulmonary function: Severe restrictive ventilation disorder and mixed ventilation disorder. The patient's lung function improved after pulmonary rehabilitation exercise following admission compared with before, and she could climb 4 floors. Her oxygen saturation was 94% after climbing 4 floors, which was considered exercise tolerance ≥ 4 metabolic equivalents (METs) and could tolerate the surgery.
Department of anesthesiology: Combined with the patient's examination, the patient then had a clear diagnosis of left lung metastasis after right lung synovial sarcoma surgery. The patient had metastasis in the left lung, with no obvious metastasis in the rest of the body. The following were indications for surgery: The patient was in good general condition; she could tolerate climbing 4 flights of stairs; and preoperative blood gas analysis was good, but preoperative lung function was poor. The patient demonstrated the following: FEV1 0.72 L (accounting for 27.8%), predicted after lung subsegmental resection FEV1 0.696 L (26.9%); inadequate respiratory reserve, high surgical risk, surgical anesthesia risk score American Society of Anesthesiologists class IV, predicted risk of postoperative respiratory failure, and a greater incidence of intraoperative and postoperative pulmonary complications. The patient and her family were fully informed of the surgical risks. It was recommended that surgery be planned well in advance to minimize the operating time and reduce the occurrence of pulmonary complications, and intraoperative and postoperative support and transition with ECMO was required.
Intensive care unit (ICU): The patient had poor lung function, and after right total pneumonectomy, the risk of intraoperative and postoperative pulmonary complications was higher. Veno-venous (VV)-ECMO could be prepositioned intraoperatively to ensure the safety of surgery, but the cost was higher. The patient and her family were informed, and it was possible that the tracheal tube might not be able to be removed after surgery. There was a possibility of needing a long-term tube, and the patient's family was fully informed of the related surgical risks.
Thoracic surgery summary: The patient was unsuitable for interventional treatment and was at high risk of intraoperative and postoperative respiratory failure due to poor lung function; subsegmental lung resection should be performed under general anesthesia, and VV-ECMO assistance could be given after adequate preoperative preparation and postoperative ICU support treatment.
Pulmonary nodule (occupying nature of the lower lobe of the left lung to be investigated): Combined with the patient's medical history and examination, a metastatic tumor was considered, pending postoperative pathology to confirm the diagnosis.
Right lung malignancy (right lung synovial sarcoma after comprehensive treatment): Combined with the patient's medical history and previous examination, the diagnosis was made.
Hypothyroidism: Combined with the patient's previous medical history, the diagnosis was made.
Anesthesia protocol: General anesthesia (all intravenous) and postoperative multimodal analgesia.
After the patient was admitted, her vital signs were monitored: 85 bpm, 20 breaths/min, blood pressure 140/92 mmHg, and SpO2 98%. The peripheral veins were opened (18 G indwelling needle), the right radial artery was punctured and the needle placed (22 G indwelling needle), bedside hemodynamic monitoring (Mostcare) was connected, GDFT was started, bispectral index (BIS) electrode monitoring was connected, and muscle relaxation monitoring was connected. Intubation and maintenance of anesthesia were induced with propofol-remifentanil-rocuronium bromide, the electroencephalogram BIS was 40-60, the surgical stress index was 20-50, and four string stimulation was ≤ 2.
ECMO support: After general anesthesia, the patient was placed in the supine position with the right lower extremity in an abducted and externally rotated position. The area was routinely disinfected; sterile towels were placed; an ultrasound-guided puncture needle was maintained at negative pressure into the right femoral vein; a guidewire was placed; a 21F ECMO catheter was placed along the guidewire; the guidewire was withdrawn; and the blocking forceps were temporarily clamped close to a depth of 39.0 cm in a flat position. Ultrasound of the right neck was used to locate the internal jugular vein puncture site. A 15F ECMO catheter was inserted to a depth of 14.0 cm, sutured and fixed, and the ECMO was connected. The area was covered with a sterile patch.
The intraoperative anesthetic treatment included the following: Performing a lung protective ventilation strategy, with the respiratory mode as volume-controlled ventilation and with a tidal volume of 50-100 mL, a respiratory rate of 6-8 breaths/min, an inhaled oxygen concentration of 40%-50%, a positive end expiratory pressure (PEEP) of 5 cmH2O, a peak airway pressure of 10-15 cmH2O, a plateau pressure of 8-13 cmH2O, and a driving pressure of 3-8 cmH2O, and there was intermittent pulmonary resuscitation with a low tidal volume. The surgical field was increased. End-expiratory carbon dioxide monitoring was performed with ECMO assistance to increase the amount of oxygen inhaled and to help expel carbon dioxide. The SpO2 was kept at 92%-100%, and the PetCO2 was 36-45 mmHg.
Intraoperative hemodynamic monitoring of arterial blood pressure (IBP) was maintained at 130-100/80-60 mmHg, and the heart rate was maintained at 60-80 bpm. GDFT was performed according to the variability index per beat volume (SVV). When SVV > 13%, the infusion rate was appropriately accelerated to maintain 10% ≤ SVV ≤ 13%, the infusion rate was then controlled, and the infusion volume was adjusted in combination with IBP, per beat volume, central venous oxygen saturation
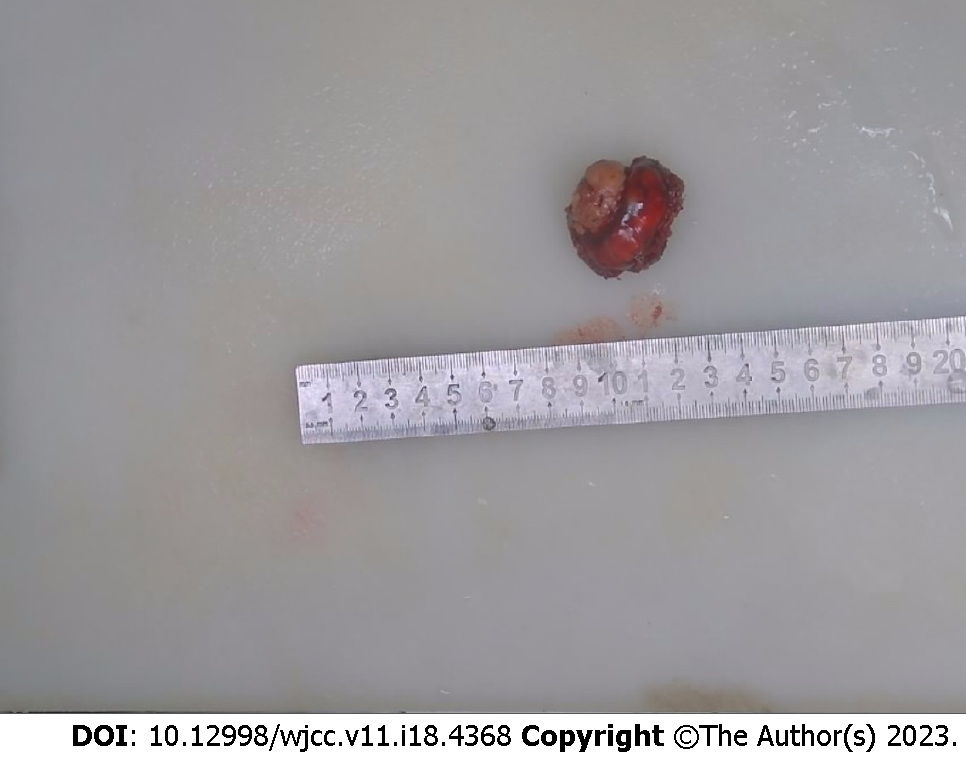
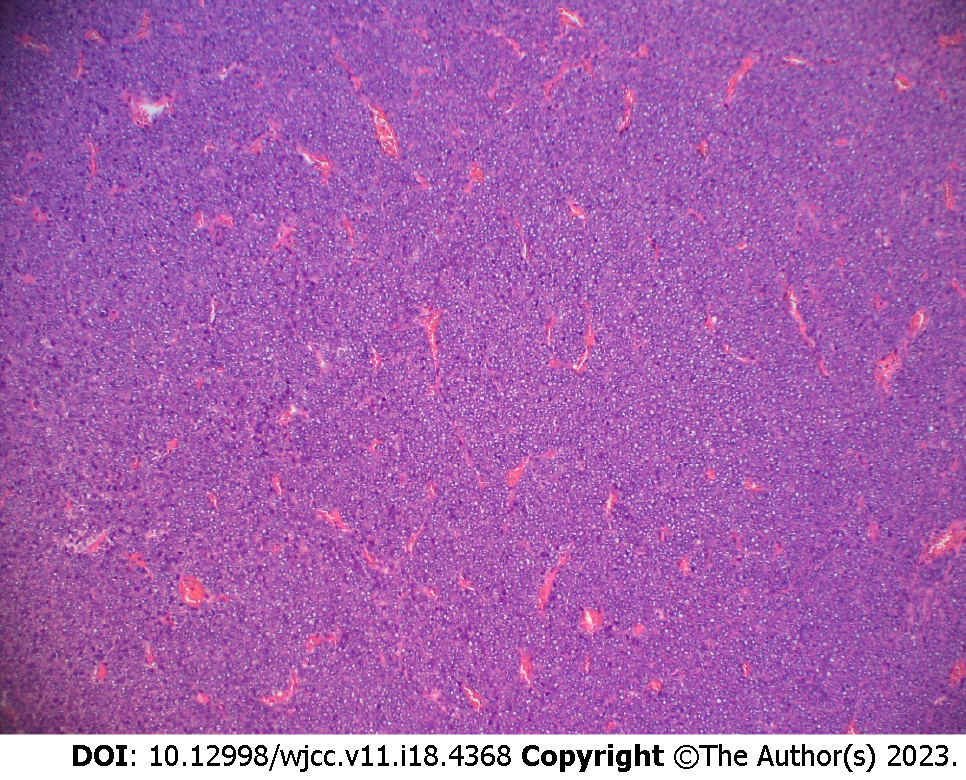
Surgical treatment included combined thoracoscopic left lower lung lobe S9bii+S10bii subsegmental resection. The operation was completed smoothly, the operation time was 5 h, and the blood loss was 50 mL. The postoperative pathologic findings were as follows: Tumor size 3.0 cm × 2.5 cm × 2.3 cm and synovial sarcoma (monomorphism) (for details, see Figures 2 and 3).
The patient tried to go offline after surgery, but her PetCO2 was elevated, and her arterial blood pH was decreased. Considering that it was temporarily impossible to stop the machine, the patient continued VV-ECMO support and was sent to the ICU for treatment. The postoperative multimodal analgesia included 0.5% ropivacaine intercostal nerve block + sufentanil intravenous self-administered analgesia. On postoperative Day 1, ECMO was withdrawn, and pulmonary rehabilitation was started. On postoperative Day 4, the tracheal tube was removed, and the patient returned to the general ward of thoracic surgery. On postoperative Day 15, she recovered and was discharged. A CT scan at three months postoperatively showed changes after resection of the left lung tumor (for details, see Figure 1).
The ECMO technique is widely used in conventional lung transplantation and complex tracheobronchial surgery[1], but there are few reports of pneumonectomy in which one-lung ventilation cannot be maintained[2].
The characteristics of the present patient's condition were as follows: the patient had a history of multiple lung operations, shortness of breath was felt up to the 3rd floor on the ladder climbing test, the change in range of SpO2 was less than 4%, and the physical tolerance was ≥ 4 METs. After total resection of the right lung, the patient underwent left subsegmental pneumonectomy to predict a postoperative residual lung of 0.696 L (26.9%), which may make it difficult to tolerate normal life activities in the future. A cardiopulmonary exercise test showed an anaerobic threshold oxygen uptake of 10.9 mL/min/kg, and the patient’s surgical risk was high. There was possible difficulty in deconditioning after intraoperative use of ECMO assistance, and there was a high risk of postoperative respiratory failure. Precise resection of the combined sub-segment of lung S9bii+S10bii was performed to try to protect the healthy lung segment and was beneficial in reducing the risk of postoperative respiratory failure.
This type of operation can use single-point intubation and low-flow VV-ECMO, which provides good respiratory support and hemodynamic support, ensures the safety of perioperative patients and is easy to perform[3].
The patient's right lung was resected, and only left one-lung ventilation and left thoracoscopic lung subsegmental surgery were performed. A lung protective ventilation strategy was implemented to reduce the tidal volume, reduce the inhaled oxygen concentration, maintain PEEP, and ensure intermittent lung dilatation[4]. The use of VV-ECMO during the operation can ensure oxygen and effectively remove CO2 from the patient's blood to avoid acidosis. However, long-term low tidal volume ventilation will increase the incidence of postoperative atelectasis. During the use of ECMO, air inhalation and maintenance of PEEP 5-10 cmH2O greatly reduce the possibility of postoperative pulmonary complications[5]. The limitations and shortcomings of this operation are as follows: The conventional operation requires pulmonary collapse, and a protective pulmonary ventilation strategy is implemented during this operation to maintain a positive end-expiratory pressure of 5-10 cmH2O. Additionally, after the change in pulmonary blood flow, the boundary between pulmonary subseg
ECMO provides the opportunity for many complex thoracic surgeries, but its perioperative management difficulties should not be underestimated, such as bleeding, infection, thrombosis, hypoxemia and other complications[6]. Factors that may affect the outcome of treatment include poor surgical skills, which can lead to complications such as bleeding and pneumothorax; a large scope pneumonectomy, which can cause respiratory failure; poor respiratory management during surgery, which can lead to postoperative pneumonia; excessive infusion, which leads to pulmonary edema; and improper management of ECMO, which can lead to infection, hypoxia, thrombosis and so on[7].
The operation under ECMO technology is complex, and there may be risks such as surgical failure, hemodynamic instability and tracheal prolapse; thus, teamwork is necessary. Therefore, the multidisciplinary treatment (MDT) group is composed of thoracic surgeons, anesthesiologists, operating room nurses and ICU doctors who conduct multidisciplinary consultation discussions before the operation, formulate a detailed operation plan, predict the problems that may occur during the operation, and formulate medical measures.
GDFT can effectively evaluate blood volume, optimize cardiac function, ensure microcirculation perfusion, maintain the balance of oxygen supply and demand and improve prognosis[8]. In this patient, stroke volume variability (stroke volume variation, SVV) was used as the main monitoring index to guide infusion, setting 10% ≤ SVV ≤ 13% to avoid pulmonary edema caused by excessive infusion; additionally, according to the peripheral circulation resistance, systemic vascular resistance was the main monitoring index to guide the use of norepinephrine, and the intraoperative blood pressure was controlled at ± 20% of the basic value.
Postoperative treatment: The patient continued to receive pulmonary protective ventilation after the operation, and the blood gas was reexamined after ECMO stopped breathing and decreased the flow rate. On postoperative Day 1, ECMO was removed, and pulmonary rehabilitation began without carbon dioxide retention and hypoxemia. On postoperative Day 4, the endotracheal tube was removed, and the patient returned to the general ward of thoracic surgery. She recovered and was discharged from the hospital on postoperative Day 15.
Although ECMO can provide temporary support for lung function, we should also take into account that if patients cannot withdraw from ECMO after surgery, lung transplantation may be the only choice for their survival. For example, palliative treatment for life-conscious patients without conditional transplantation is also faced with complex ethical issues[9].
The MDT provided maximum medical optimization for surgical anesthesia in this case; the combined protocol of total intravenous anesthesia, VV-ECMO mode, GDFT, perioperative pulmonary protection and rehabilitation, and multimodal analgesia provided adequate protection for the patient's perioperative treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand; Rabie AA, Saudi Arabia S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Redwan B, Ziegeler S, Freermann S, Nique L, Semik M, Lavae-Mokhtari M, Meemann T, Dickgreber N, Fischer S. Intraoperative veno-venous extracorporeal lung support in thoracic surgery: a single-centre experience. Interact Cardiovasc Thorac Surg. 2015;21:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Reeb J, Olland A, Massard G, Falcoz PE. Extracorporeal life support in thoracic surgery. Eur J Cardiothorac Surg. 2018;53:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Heward E, Hayes T, Evison M, Booton R, Barnard J, Shah R. Extracorporeal Membrane Oxygenation Assisted Segmentectomy for Metachronous Lung Cancer After Pneumonectomy. Ann Thorac Surg. 2016;102:e187-e189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Wang TL, Wang DX, Ouyang W, Yan M, Li M, Xiao W, Mu DL, He ST, Liang XQ. Expert consensus on anesthesia and perioperative management of nonpulmonary surgery in patients with chronic obstructive pulmonary disease. Chinese Anesthesiology Guidelines and Expert Consensus. 2017. Available from: https://csahq.cma.org.cn/guide/detail_400.html. |
| 5. | Min Su, Ao Hushan. Expert consensus on the clinical application of extracorporeal membrane pulmonary oxygenation in adults under different conditions. Zhongguo Xuanhuan Zazhi. 2020;11:1052-1062. [DOI] [Full Text] |
| 6. | Novellis P, Monaco F, Landoni G, Rossetti F, Carretta A, Gregorc V, Zangrillo A, Veronesi G. Venoarterial Extracorporeal Membrane Oxygenation Support in Lung Cancer Resection. Ann Thorac Surg. 2022;113:e191-e193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Petrella F, Zorzino L, Frassoni S, Bagnardi V, Casiraghi M, Bardoni C, Mohamed S, Musso V, Simonini E, Rossi F, Alamanni F, Venturino M, Spaggiari L. Intraoperative Extra Corporeal Membrane Oxygenator for Lung Cancer Resections Does Not Impact Circulating Tumor Cells. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Cavaleri M, Veroux M, Palermo F, Vasile F, Mineri M, Palumbo J, Salemi L, Astuto M, Murabito P. Perioperative Goal-Directed Therapy during Kidney Transplantation: An Impact Evaluation on the Major Postoperative Complications. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Gillon SA, Toufektzian L, Harrison-Phipps K, Puchakayala M, Daly K, Ioannou N, Meadows CI, Wyncoll DL, Barrett NA. Perioperative Extracorporeal Membrane Oxygenation to Facilitate Lung Resection After Contralateral Pneumonectomy. Ann Thorac Surg. 2016;101:e71-e73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |