Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4360
Peer-review started: March 4, 2023
First decision: April 13, 2023
Revised: May 7, 2023
Accepted: May 30, 2023
Article in press: May 30, 2023
Published online: June 26, 2023
Processing time: 114 Days and 12.7 Hours
Metastatic carcinoma of the thyroid gland is a rare encounter in clinical practice, but autopsy series showed that it is not so rare. Thyroid metastasis from colorectal cancer (CRC) is rare and has a poor prognosis. We herein report a rare case of solitary thyroid metastasis from rectal cancer combined with needle tract implantation after fine-needle aspiration (FNA) of the thyroid nodule and review the relevant literature.
A 54-year-old woman with a history of TNM stage III CRC presented a 1.3 cm × 1.0 cm mass in the left thyroid gland. FNA and histological examination of the left thyroid lobe surgical specimen confirmed the diagnosis of isolated metastatic adenocarcinoma from the rectum. Needle tract implantation was observed in the neck 11 mo after the FNA examination. The 2.5-cm seeding lesion was success
For a patient with a thyroid mass and a history of CRC, metastatic thyroid carcinoma should be considered even if the patient has no evidence of other organ metastasis from CRC. FNA cytological examination of the thyroid mass is useful in the differential diagnosis between primary thyroid disease and metastatic thyroid carcinoma. Thyroid lobectomy of the gland containing the metastatic tumor is suggested in patients with metastatic carcinoma of the thyroid.
Core Tip: A rare case of solitary thyroid metastasis from rectal cancer combined with needle tract implantation after fine-needle aspiration (FNA) of the thyroid nodule is reported. The literature relevant to this clinical condition, the diagnostic workup, spread pathway, and surgical management of these rare lesions is reviewed. For a patient with a thyroid mass and a history of colorectal cancer, metastatic thyroid carcinoma should be considered. FNA cytological examination is useful in the differential diagnosis between primary thyroid disease and metastatic thyroid carcinoma. Thyroid lobectomy of the gland containing the metastatic tumor is suggested in patients with metastatic carcinoma of the thyroid.
- Citation: Chen Y, Kang QS, Zheng Y, Li FB. Solitary thyroid gland metastasis from rectal cancer: A case report and review of the literature. World J Clin Cases 2023; 11(18): 4360-4367
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4360.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4360
Clinically evident metastases of cancers to the thyroid gland are rarely seen. The rate is around 1.4%-3.0% of all patients who underwent surgery for suspected thyroid cancer[1-4]. However, the prevalence appears to be up to 24% in autopsy series of patients who died of non-thyroid malignancies[5], suggesting that thyroid metastasis might be more common than what is clinically apparent.
The most common colorectal cancer (CRC) metastasis sites are the liver, lung, and peritoneum[6]. CRC metastasis to the thyroid gland is rare, found in only 0.1% of CRC cases[7]. Clinically, only 24 cases of thyroid gland metastases of CRC have been reported in the literature[8-26]. CRC with the thyroid gland as the only distant metastatic site is extremely rare. Herein we report a rare case of solitary thyroid metastasis from rectal cancer combined with needle tract implantation after fine-needle aspiration (FNA) of the thyroid nodule and summarize the main points of diagnosis and treatment for this rare case and review the relevant literature.
A 54-year-old woman presented to our hospital with a left thyroid mass incidentally discovered during a routine health examination.
The patient had no history of neck irradiation.
One year previously, the patient had undergone rectectomy and lymph node dissection for moderately differentiated rectal tubular adenocarcinoma (AC). The rectal tumor was 2.5 cm and penetrated the serosa, with lymph node metastasis but without distant metastases. The disease was classified as T3N1M0 (i.e., stage III). The patient did not receive chemotherapy after surgery for her own reason.
The patient had no history of neck irradiation.
No abnormal lymph nodes were detected during the clinical examination. An approximately 1.3 cm × 1.0 cm mass was detected in the left thyroid lobe, without abnormal findings on the right side of the neck.
Thyroid function markers, calcitonin, carbohydrate antigen 199, and carcinoembryonic antigen (CEA) levels were normal.
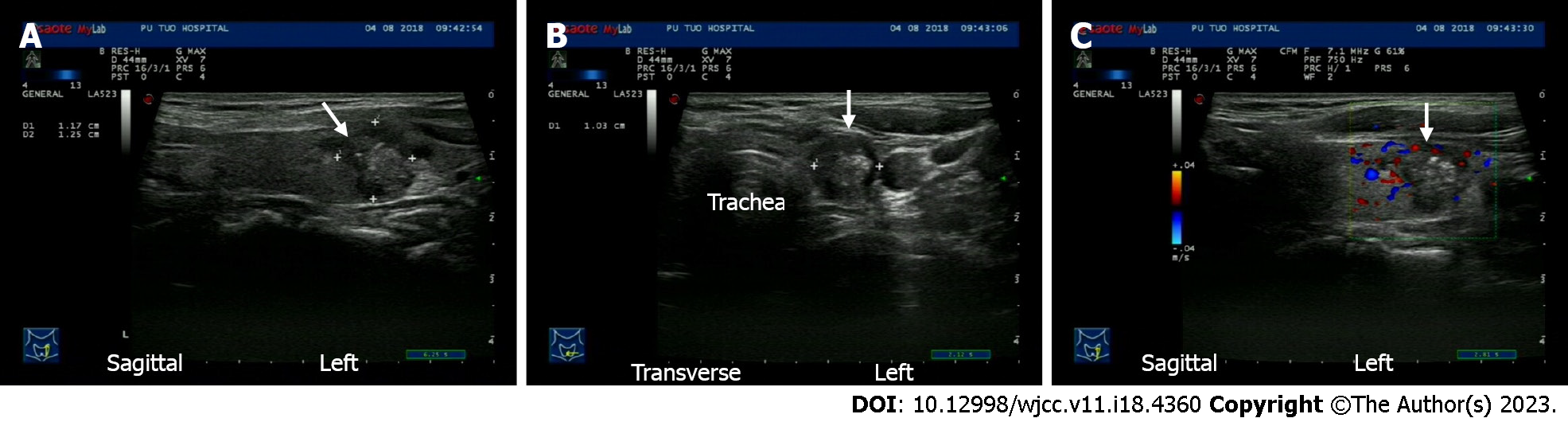
Ultrasonography revealed a 1.17 cm × 1.25 cm × 1.03 cm irregular hypoechoic nodule with rich peripheral blood supply in the left thyroid lower pole (Figure 1). Ultrasonography did not show enlarged lymph nodes in the bilateral neck.
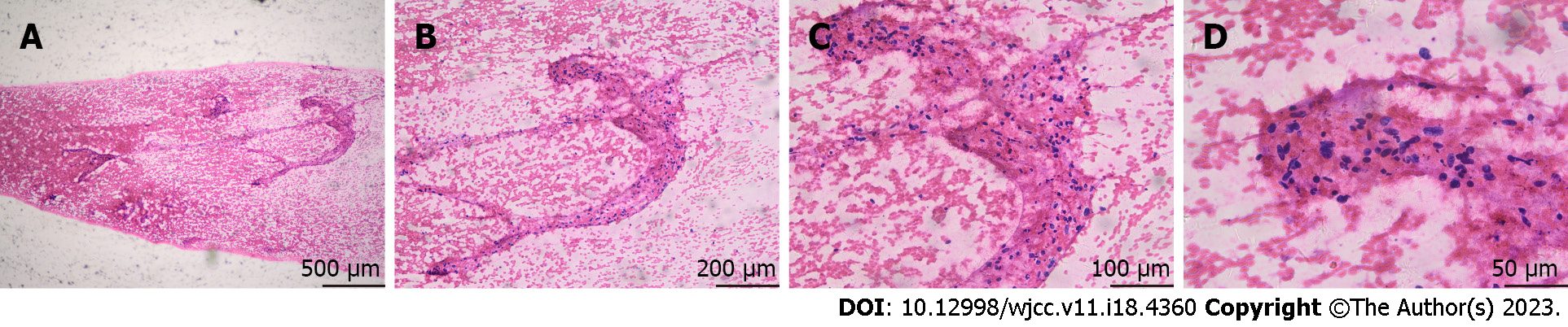
The patient underwent FNA of the hypoechoic nodule in the left thyroid. The procedure was performed under ultrasound guidance, with three sampling passes using a 23-gauge needle. The cytological diagnosis was “suspicious for malignancy” (Figure 2).
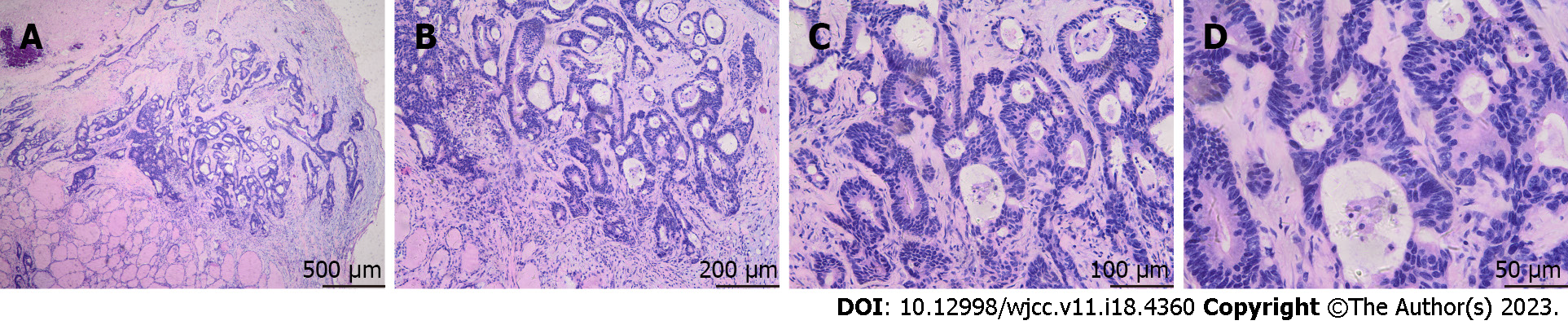
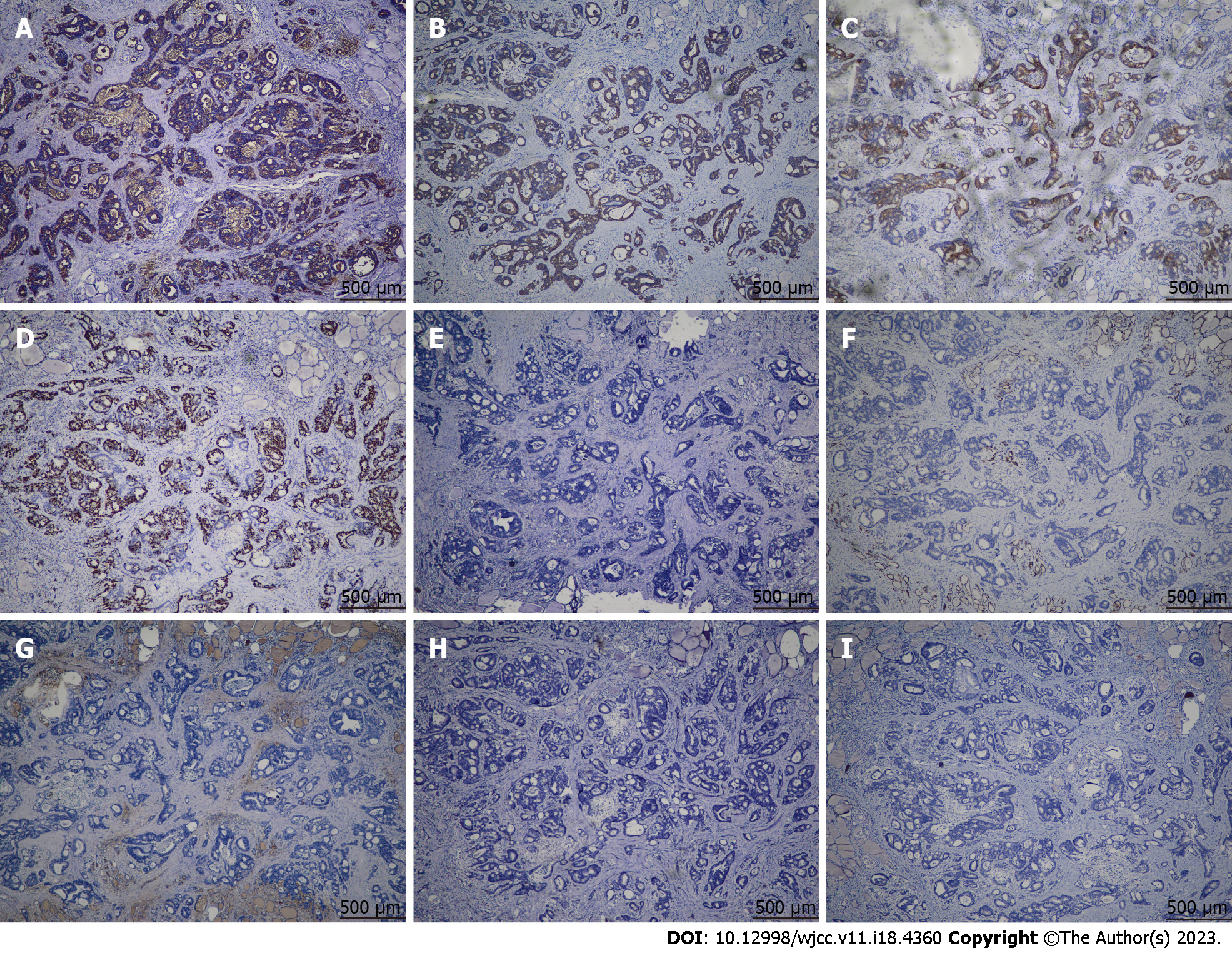
Histological examination of the resected tissue revealed invasive moderately differentiated AC (Figure 3). Eight lymph nodes from the central compartment were all negative. Immunohistochemistry showed CEA, cytokeratin 20 (CK20), EMA, and Ki67 expression in the thyroid specimen. HBME1, cytokeratin 7 (CK7), thyroglobulin, synaptophysin, and calcitonin were not expressed (Figure 4). Therefore, the nodule in the left thyroid was considered a metastatic AC from the rectal tubular AC. The patient had no surgical complications. Positron emission tomography-computed tomography (CT) was performed after thyroid surgery to detect distant metastasis in other organs, and the results were negative. She received six cycles of single-agent chemotherapy with capecitabine.
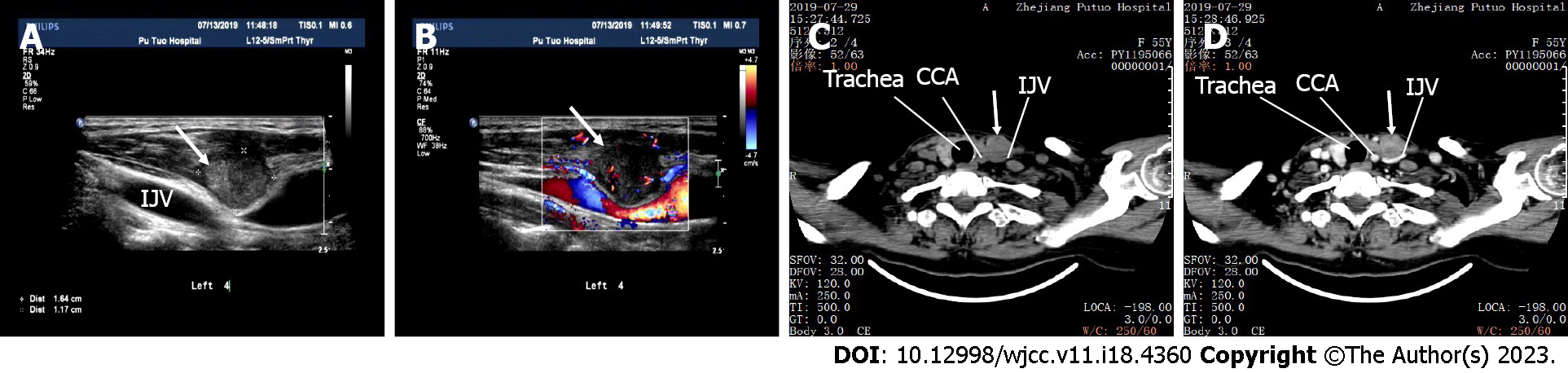
A 2.5-cm mass was detected by ultrasonography in the neck within the left strap muscles and sternocleidomastoid muscle along the needle track 11 mo after FNA (Figure 5A and B). A CT scan also showed a 2.5-cm mass within the muscles above the internal jugular vein (IJV), with the IJV being depressed (Figure 5C and D). Needle tract implantation was suspected. The mass was excised, and the final histology examination confirmed the diagnosis of metastatic AC. Local recurrence of thyroid metastasis from needle track seeding was confirmed.
A unilateral lobectomy with central neck dissection on the left side was performed.
A mass in the right lung was detected by a CT scan 5 mo after thyroidectomy. The mass was excised successfully, and the histology examination confirmed the diagnosis of metastatic AC. In the 4 years of follow-up after thyroidectomy, the patient was alive and had no neck recurrence. There were no surgical complications. The timeline of the case is shown in Table 1.
| Information | Timeline |
| Underwent rectectomy for moderately differentiated rectal tubular adenocarcinoma | One year ago |
| A left thyroid mass discovered, underwent thyroidectomy, diagnosed with metastatic adenocarcinoma | Now |
| A mass in the lung detected, excised, diagnosed with metastatic adenocarcinoma | 5 mo later |
| A mass in the left neck detected, excised, diagnosed with metastatic adenocarcinoma, confirmed with needle track seeding | 11 mo later |
| Alive and no recurrence in the neck | 4 yr later |
By searching the literature, 24 previous cases[8-26] were identified, of which there were 16 females and eight males. The median age was 57.5 (range: 28-82) years. The primary CRC was in the sigmoid in seven patients, the rectum in ten, the ascending colon in five, the descending colon in one, and unspecified in one. All patients had adenocarcinoma. CRC treatment included surgery (n = 23), chemotherapy (n = 9), and chemoradiotherapy (n = 5). The thyroid metastases were bilateral (n = 9) or in the right (n = 4) or left (n = 11) lobe. FNA was performed on 21 patients. The median time from the primary lesion to metastasis was 36 (range: 0-96) mo. The finding was incidental in eight patients; the other signs and symptoms included neck mass (n = 11), hoarseness (n = 4), dyspnea (n = 4), dysphagia (n = 3), and dry cough (n = 1). Treatments for thyroid metastases included surgery (n = 17), chemotherapy (n = 15), radiotherapy (n = 3), chemoradiotherapy (n = 1), and bevacizumab (n = 1). The median follow-up was 7 (range: 1-42) mo. At the last follow-up, 12 patients were alive with the disease, and eight were dead from the disease.
Metastasis in the thyroid gland is rare in clinical practice because of it is an richly arterialised organ[27]. Next to the adrenal gland, the thyroid gland is the most richly arterialised tissue in the body. Taking the total weights of the liver and thyroid gland to be 1500 and 25 g, respectively, the thyroid actually receives approximately one-half the volume of arterial blood received by the entire liver. Yet, while the liver is very frequently the site of metastases from tumors of diverse kinds distributed in the systemic blood stream, metastatic growths in the thyroid gland are unusual[27]. Such metastases of other primary cancers represent about 1.3%-3% of malignant lesions of the thyroid[5,28]. In a series of 43 patients with metastatic thyroid tumors, Nakhjavani et al[29] reported that CRC cancer metastases were the least frequent. The primary cancers in their series included 14 cases of kidney carcinomas (33%), seven lung carcinomas (16%), seven breast carcinomas (16%), four esophageal carcinomas (9%), three uterine carcinomas (7%), and six other tumors[29]. CRC metastasis to the thyroid gland is rare, found in only about 0.1% of the patients with CRC, and most cases had concomitant metastases at other sites[7]. Keranmu et al[25] reported concomitant lung metastases in 81.0% of patients with thyroid metastases of CRC.
Hematogenous spread might be the most important pathway for CRC metastasis to the thyroid[19]. Indeed, in many cases, thyroid metastasis is accompanied by lung and liver metastases[8,9,12,14-21,23-25]. Furthermore, among 25 cases reported previously and the present case, 72.0% of all patients (18/25) showed concomitant lung metastasis. Therefore, CRC may metastasize to the thyroid via the portal vein, vena cava, and pulmonary vein[5,15,16,18,20,23]. The case reported here and cases reported by De Ridder et al[13] and Onorati et al[22] had isolated thyroid metastasis with no other organ metastases, which suggests that a circulatory pathway to the thyroid gland bypassing the portal vein, pulmonary vein, and vena cava, through the vertebral venous system, is present[30]. In the case reported here, the thyroid metastasis was the first sign of hematogenous spread 1 year after the primary diagnosis of CRC. In the previously reported 24 cases of metastatic CRC to the thyroid, only two presented thyroid metastases as the first sign of hematogenous spread (Supplementary Tables 1-8).
Despite the rare occurrence, the possibility of metastatic carcinoma should be considered in the differential diagnosis for any patient with thyroid nodules and a history of cancer. FNA is recommended and has received great attention in the current literature. The diagnosis of a primary thyroid tumor can often be made by this technique. A thyroid nodule in a patient with a history of cancer is a diagnostic challenge. Such a lesion can be benign, metastatic, or a new primary malignancy of the thyroid gland. In the previously reported 24 cases with metastatic CRC to the thyroid (Supplementary Tables 1-8), together with the present case, 21 patients underwent an FNA examination of the thyroid mass. The results of the FNA suggested metastases from CRC in 13 cases and malignancy in seven cases (one case was not diagnostic because of lack of material). These studies showed that CRC metastases to the thyroid could be diagnosed with great accuracy by FNA. Rosen et al[31] reported that eight of nine FNA procedures for metastatic thyroid mass yielded correct results, with a true positive rate of 90%. In such cases, immunohistochemistry for CK7 and CK20 can be used to differentiate primary thyroid cancers from metastatic CRC. Indeed, thyroid carcinomas are generally positive for CK7 and negative for CK20, while CRCs are generally CK7-negative and CK20-positive. The immunohistochemistry results in the case reported here also showed positive CK20 and negative CK7 expression in the thyroid specimen.
Elevated serum CEA levels can indicate the recurrence of CRC. Therefore, when a patient has a thyroid nodule and a history of CRC, the CEA levels should be checked. In the previously reported 24 cases with metastatic CRC to the thyroid (Supplementary Tables 1-8), together with the present case, 11 patients had available CEA data. The serum CEA levels in nine of 11 patients were elevated. In 10 of 25 patients reported previously and the present case, a thyroid mass was detected > 5 years after the primary CRC. Therefore, patients with a known history of previous CRC presenting a new thyroid mass should be regarded as potentially metastatic. Such mass should be treated as a metastatic lesion until proven otherwise.
Needle tract implantation can occur after thyroid FNA. Hayashi et al[32] reported that the cumulative incidence of needle tract implantation was 0.37% and 0.58% at 5 and 10 years after FNA, respectively. Needle track seeding of metastatic CRC after thyroid FNA has not been reported previously. This study reports the first case of solitary thyroid metastasis from CRC with seeding along the needle tract. Salvage surgery is also adequate for locally controlling needle tract seeding lesions from metastatic CRC. The patient reported here had no recurrence in the neck 3 years after resection of the seeding lesion.
In the management of the case reported here, the NCCN guidelines for metastatic rectal cancer were followed[33]. A thyroid gland metastasis often indicates poor prognosis, and aggressive surgery could help avoid crises such as dyspnea and dysphagia, resulting in better prognosis and quality of life, especially for patients with isolated thyroid metastasis[16,18,29]. Nakhjavani et al[29] reported that in patients with thyroid metastases from malignant disease, the mean survival of all patients who underwent thyroidectomy alone or with adjuvant therapy was 34 mo, compared with 25 mo for patients who were treated non-surgically. Surgical treatment should include a thyroid lobectomy of the gland containing the metastatic tumor. Postoperative radiotherapy is strongly suggested if the tumor has extrathyroidal extension. The patient reported here had no recurrence in the neck and was alive 4 years after the thyroidectomy.
Therefore, based on the literature review and the present case, most patients found the neck mass by themselves, or it was found during a physical examination. Very few patients went to the hospital due to hoarseness or difficulty breathing or swallowing. Ultrasound-guided FNA of the thyroid nodules, combined with a rectal cancer history, CEA, and other indicators, can provide a preliminary indication for a diagnosis. A final diagnosis requires the surgical resection of the thyroid gland and the mass and histopathological examination of the lesion. If the patient can tolerate general anesthesia, surgery to remove one side of the thyroid and the metastatic tumor is preferred. If a patient cannot tolerate general anesthesia or if the thyroid metastases cannot be surgically removed, chemotherapy, radiation, and targeted drugs can be considered. If the thyroid metastases can be removed completely, death from thyroid metastases is rare. For example, in the case reported in this study, the thyroid tumor did not recur. If the metastatic thyroid tumor is not completely removed, the patient can die from the pressure of the thyroid tumor on the trachea. As cases of rectal cancer metastasis to the thyroid gland are very rare, a literature review performed in this study provides some clinical guidance since there are no specific guidelines for diagnosing and treating thyroid metastasis of CRC.
This study is only a single case report, and the recommendations for diagnosis and treatment of CRC thyroid metastases summarized in this study are only empirical summaries that lack the support of stronger evidence-based medical evidence. Future research should summarize more cases of thyroid metastasis of CRC, conduct a controlled study on the treatment plan, provide the best treatment plan for thyroid metastasis in CRC, improve the prognosis of patients, and develop relevant guidelines.
The present report illustrates two important points that deserve to be emphasized. On one hand, in a patient with a thyroid mass and a history of CRC, metastatic thyroid carcinoma should be considered even if the patient has no evidence of other organ metastasis. On the other hand, an FNA cytological examination of the thyroid mass is suggested to assist in the differential diagnosis between primary thyroid disease and metastatic thyroid carcinoma. If the histological examination confirms the diagnosis of metastatic thyroid carcinoma, the possibility of a needle tract implantation should be kept in mind.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia; Sano W, Japan S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Cichoń S, Anielski R, Konturek A, Barczyński M, Cichoń W. Metastases to the thyroid gland: seventeen cases operated on in a single clinical center. Langenbecks Arch Surg. 2006;391:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Mirallié E, Rigaud J, Mathonnet M, Gibelin H, Regenet N, Hamy A, Bretagnol F, de Calan L, Le Néel JC, Kraimps JL. Management and prognosis of metastases to the thyroid gland. J Am Coll Surg. 2005;200:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012;22:258-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the Royal Marsden experience. Eur J Surg Oncol. 2004;30:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Calzolari F, Sartori PV, Talarico C, Parmeggiani D, Beretta E, Pezzullo L, Bovo G, Sperlongano P, Monacelli M, Lucchini R, Misso C, Gurrado A, D'Ajello M, Uggeri F, Puxeddu E, Nasi P, Testini M, Rosato L, Barbarisio A, Avenia N. Surgical treatment of intrathyroid metastases: preliminary results of a multicentric study. Anticancer Res. 2008;28:2885-2888. [PubMed] |
| 6. | Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 680] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 7. | Lièvre A, Leboulleux S, Boige V, Travagli JP, Dromain C, Elias D, Ducreux M, Malka D. Thyroid metastases from colorectal cancer: the Institut Gustave Roussy experience. Eur J Cancer. 2006;42:1756-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Lester JW Jr, Carter MP, Berens SV, Long RF, Caplan GE. Colon carcinoma metastatic to the thyroid gland. Clin Nucl Med. 1986;11:634-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Nachtigal D, Dharan M, Luboshitzky R, Honigman J, Rosen G. Bilateral secondary mucinous adenocarcinoma of thyroid: case report. Otolaryngol Head Neck Surg. 1992;107:466-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Mesko TW, Friedman J, Sendzischew H, Nixon DD. Rectal carcinoma metastatic to the thyroid gland. J Laryngol Otol. 1996;110:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Takashima S, Takayama F, Wang Q, Kobayashi S, Sone S. Thyroid metastasis from rectal carcinoma coexisting with Hashimoto's thyroiditis: gray-scale and power Doppler sonographic findings. J Clin Ultrasound. 1998;26:361-365. [PubMed] [DOI] [Full Text] |
| 12. | Kim CH, Park YW, Ayala AG, Ro JY. Colonic adenocarcinoma metastatic to the thyroid gland: a case report with immunohistochemical investigation. J Korean Med Sci. 1999;14:455-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | De Ridder M, Sermeus AB, Van de Steene J, Storme GA. Two unusual sites of colon cancer metastases and a rare thyroid lymphoma. Case 1. Metastatic colon cancer to a multinodular goiter. J Clin Oncol. 2001;19:3572-3574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Akimaru K, Onda M, Tajiri T, Shimanuki K, Iwama H, Furukawa K, Sugiyama Y. Colonic adenocarcinoma metastatic to the thyroid: report of a case. Surg Today. 2002;32:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Fujita T, Ogasawara Y, Doihara H, Shimizu N. Rectal adenocarcinoma metastatic to the thyroid gland. Int J Clin Oncol. 2004;9:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Poon D, Toh HC, Sim CS. Two case reports of metastases from colon carcinoma to the thyroid. Ann Acad Med Singap. 2004;33:100-102. [PubMed] |
| 17. | Kumamoto K, Utsumi Y, Sugano K, Hoshino M, Suzuki S, Takenoshita S. Colon carcinoma metastasis to the thyroid gland: report of a case with a review of the literature. Tumori. 2006;92:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Cheung WY, Brierley J, Mackay HJ. Treatment of rectal cancer metastases to the thyroid gland: report of two cases. Clin Colorectal Cancer. 2008;7:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Nakamura K, Nozawa K, Aoyagi Y, Ishihara S, Matsuda K, Fukushima J, Watanabe T. A case report of thyroid gland metastasis associated with lung metastasis from colon cancer. Tumori. 2011;97:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Goatman C, Goldsmith PJ, Antonopoulos V, Ali B. Metastasis of colorectal adenocarcinoma to the thyroid: a case report and review of the literature. Case Rep Surg. 2012;2012:179407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Froylich D, Shiloni E, Hazzan D. Metachronous colon metastasis to the thyroid: a case report and literature review. Case Rep Surg. 2013;2013:241678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Onorati M, Uboldi P, Bianchi CL, Nicola M, Corradini GM, Veronese S, Fascì AI, Di Nuovo F. Solitary thyroid metastasis from colon cancer: fine-needle aspiration cytology and molecular biology approach. Pathologica. 2015;107:192-196. [PubMed] |
| 23. | Payandeh M, Sadeghi M, Sadeghi E. The Report of KRAS Mutation and NRAS Wild Type in a Patient with Thyroid Metastasis from Colon Cancer: a Rare Case Report. Iran J Pathol. 2016;11:71-75. [PubMed] |
| 24. | Minami S, Inoue K, Irie J, Mine T, Tada N, Hirabaru M, Noda K, Ito S, Haraguchi M. Metastasis of colon cancer to the thyroid and cervical lymph nodes: a case report. Surg Case Rep. 2016;2:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Keranmu A, Zheng H, Wu Y, Zhao J, Xu X, Liu F, Cai S, Wang Y, Xu Y. Comparative study of single-center patients with thyroid metastases from colorectal cancer and previously reported cases in the literature. World J Surg Oncol. 2017;15:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Sakaleshpura Mallikarjunappa S, Valluru N, Park JW, Gattuso P, Cheng L. A case of metastatic rectal adenocarcinoma diagnosed by thyroid cytology. Diagn Cytopathol. 2020;48:778-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Willis RA. Metastatic Tumours in the Thyreoid Gland. Am J Pathol. 1931;7:187-208.3. [PubMed] |
| 28. | Papi G, Fadda G, Corsello SM, Corrado S, Rossi ED, Radighieri E, Miraglia A, Carani C, Pontecorvi A. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol (Oxf). 2007;66:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Nakhjavani MK, Gharib H, Goellner JR, van Heerden JA. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574-578. [PubMed] [DOI] [Full Text] |
| 30. | Batson OV. The function of the vertebral veins and their role in the spread of metastases. 1940. Clin Orthop Relat Res. 1995;4-9. [PubMed] |
| 31. | Rosen IB, Walfish PG, Bain J, Bedard YC. Secondary malignancy of the thyroid gland and its management. Ann Surg Oncol. 1995;2:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Hayashi T, Hirokawa M, Higuchi M, Kudo T, Ito Y, Miyauchi A. Needle Tract Implantation Following Fine-Needle Aspiration of Thyroid Cancer. World J Surg. 2020;44:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Rectal Cancer. Version 3.2022. Fort Washington: National Comprehensive Cancer Network, 2022. |