Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4326
Peer-review started: March 14, 2023
First decision: April 19, 2023
Revised: May 5, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: June 26, 2023
Processing time: 104 Days and 6 Hours
Lung endometriosis is an extremely rare gynecological disease. Current literature reports suggest that the majority of patients will present with only generic symptoms, such as hemoptysis, pneumothorax, and hemopneumothorax, which often leads to misdiagnosis. To date, there are 18 case reports of lung endometriosis that describe the clinical manifestation, imaging changes, treatment, and prognosis of the disease. To provide further insights into this rare disease, we present a new case report and a brief review of pulmonary endometriosis.
We report here about a 19-year-old woman who was admitted to the hospital for repeated catamenial hemoptysis over a 3-mo period. computed tomography (CT) imaging during menstruation revealed patchy high-density shadows, approximately 0.5 cm3 in size, in the right middle lobe of the lung. The patient’s hemoptysis and changes in the CT scans resolved after menstruation. Thoracoscopic right middle lobectomy, right lower lung repair, and closed thoracic drainage were performed. Postoperative histopathology confirmed lung endometriosis. There was no recurrence of symptoms at the 6 mo follow-up.
We propose diagnosing lung endometriosis by thoroughly taking reproductive history, clinical details, imaging, and histopathology followed by treatment with surgical resection.
Core Tip: Lung endometriosis, a form of extra-endometrial growth of the endometrial glands or stroma, is an extremely rare disease with complex etiology and can easily be misdiagnosed. No comprehensive treatment guidelines exist for lung endometriosis. Understanding the relationship between medical history and clinical manifestations will help in the diagnosis and timely treatment of endometriosis. We propose an integrated approach for diagnosing, combining reproductive history taking, clinical details, imaging, and histopathology followed by treatment with surgical resection.
- Citation: Yao J, Zheng H, Nie H, Li CF, Zhang W, Wang JJ. Endometriosis of the lung: A case report and review of literature. World J Clin Cases 2023; 11(18): 4326-4333
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4326.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4326
Endometriosis is a common gynecological disease wherein the endometrial glands or stroma with normal endometrial function appear outside the endometrium. The incidence of endometriosis has increased significantly in recent years. The endometrial glands typically grow locally and bleed repeatedly, which can cause pain, infertility, and a series of other clinical manifestations[1]. Extra-endometrial growth of the endometrial glands or stroma most commonly occurs in the pelvic cavity (mainly in the ovaries) or in adjacent tissues (e.g., the broad ligament of the uterus, rectovaginal lacunae, pelvic peritoneum, abdominal surgical scar, umbilical region, vagina, vulva, and vermiform appendix)[2,3]. However, endometriosis can also occur in other organs, such as the lung, pleura, kidney, ureter, bladder, cranial cavity, and mammary glands[4]. Lung endometriosis is very rare and is usually accompanied by a history of miscarriage or uterine cavity surgery. Here, we describe a case report of a 19-year-old woman diagnosed with pulmonary endometriosis and further review the literature to describe current knowledge on this disorder.
The patient was a 19-year-old woman who was admitted for hemoptysis during her menstrual period for 3 consecutive months.
The patient reported experiencing hemoptysis during the first menstrual period following an abortion 1 year prior. In the 3 mo leading up to admission, the patient had experienced hemoptysis again, along with the expulsion of approximately 200 mL of blood.
The symptoms appeared on the third day of menstruation and disappeared after menstruation. There were no accompanying symptoms such as chest pain, respiratory distress, coughing, fever, night sweats, or weight loss.
The patient did not have a medical history of infectious diseases such as hepatitis, typhoid fever and tuberculosis; chronic diseases such as diabetes, hypertension, and coronary heart disease; and no history of major trauma or blood transfusion. Moreover, she did not have a history of an allergy to food or drugs.
Physical examination at admission showed the following: Body temperature 36.7 °C, pulse 73 beats/min, respirations 20/min, blood pressure 114/80 mmHg, clear cooperation, and normal development. The skin and mucosa of the whole body had no yellow pigmentation and hemorrhagic spots, and the superficial lymph nodes of the whole body were not enlarged. Cranial features indicated no deformity and bilateral pupils were large and round; additional findings included a symmetrical thoracic corridor without deformity, symmetrical tactile chatter of the two lungs, silent bilateral lung percussion, clear bilateral breath sounds, absence of dry and wet rales, and absence of pathological murmurs. The abdomen was soft without rebound tenderness; the liver and spleen were unaffected; there was no percussion pain in both kidney areas and the mobile dullness was negative. Physiological reflex was present but pathological reflex was not elicited.
Laboratory test findings were as follows: Routine red blood cell count 3.75 × 1012/L (normal value: 3.8-5.1 × 1012/L); liver function indicator, serum albumin 36.4 g/L (normal value: 40-55 g/L); kidney function indicator, serum potassium 3.42 mmol/L (normal value: 3.5-5.5 mmol/L); coagulation function indicator, serum fibrinogen 1.9 g/L (normal value: 2-4 g/L).
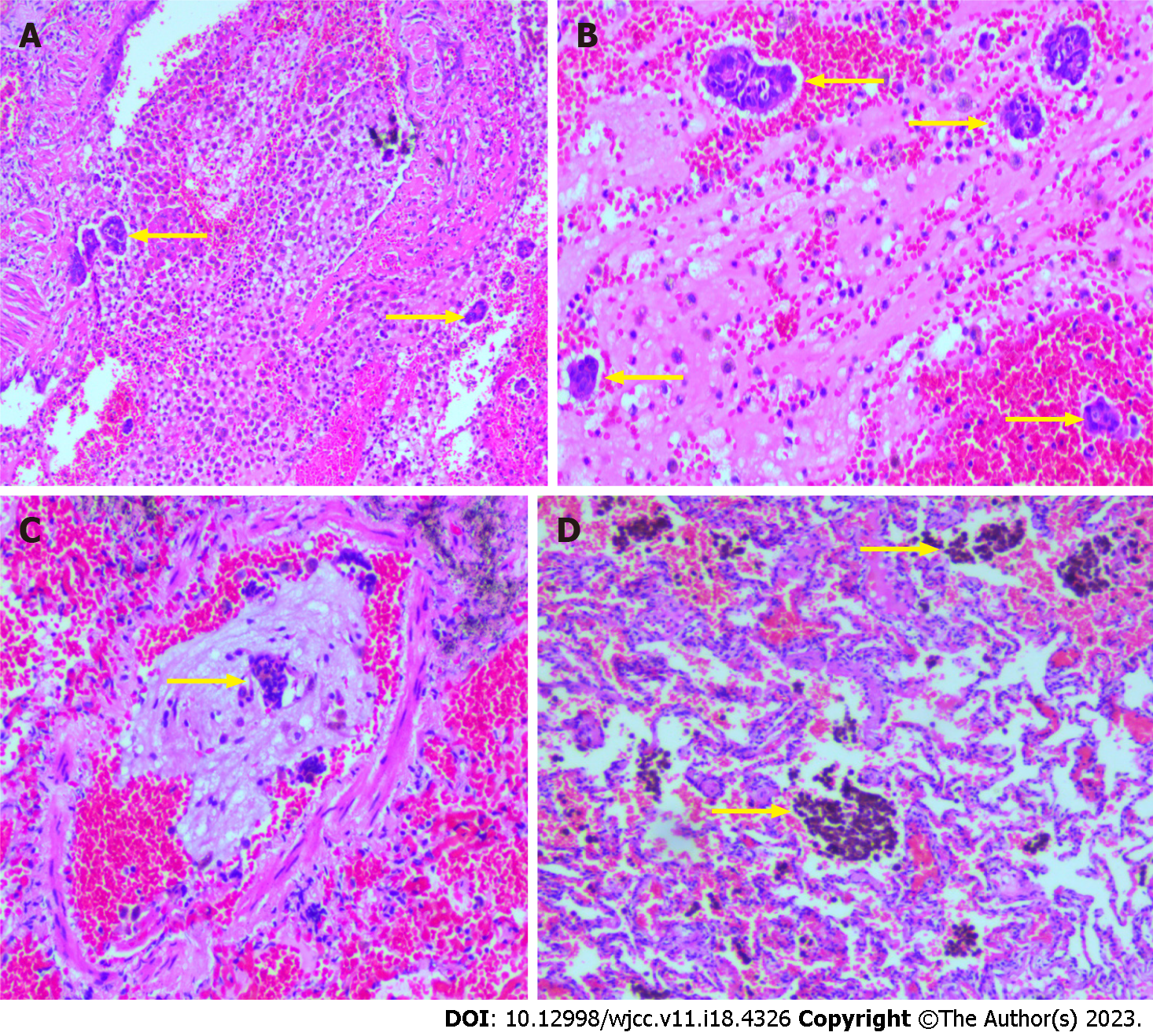
Computed tomography (CT) imaging revealed several patchy high-density shadows in the middle lobe of the right lung of approximately 0.5 cm3 in size (Figure 1). Cytopathological analysis following right middle lobe bronchial brushing revealed many ciliated columnar epithelial cells and a few lymphocytes; no malignant cells were observed. Assessment of the tissue specimen revealed that the lung tissue in the right middle lobe was 9.0 cm × 5.0 cm × 1.0 cm in size. The section was grey red and soft. A hemorrhagic area (1.5 cm × 0.8 cm × 0.5 cm) was observed locally (Figure 2). Microscopic assessment of the right middle lobe lung tissue revealed hemorrhagic foci in the alveolar cavity, with scattered glandular epithelial cells and inflammatory cells within the bleeding foci (Figure 3A and B). Glandular epithelial cells were found in some vascular cavities (Figure 3C), whereas hemosiderin deposits were observed in alveolar cavities (Figure 3D). The immunohistochemical staining showed staining for cytokeratin (CK) (Figure 4A) but no staining for estrogen receptor (ER), progesterone receptor (PR), and CD10. Special Prussian blue staining for hemosiderin was positive (Figure 4B).
The pathological diagnosis was lung endometriosis with a prior hemorrhage of the right middle lobe of the lung.
(1) According to the routine nursing of thoracic surgery, secondary nursing, pay attention to rest, general food, disclose the condition; and (2) Actively improve the auxiliary examination, observe the condition, judge conservative treatment or hand treatment.
At 3 years postoperatively, the patient was still alive.
Patients with endometriosis often present with recurrent hemoptysis, chest pain, dyspnea, spontaneous pneumothorax, and hemopneumothorax. Chest CT findings often show patchy high-density opacity and reduced lung texture at the pneumothorax site. These patients usually have a history of abortion or intrauterine surgery, and the above symptoms and imaging changes are related to the menstrual cycle.
We assessed the new case report of lung endometriosis described in the present study in combination with 18 previously reported cases (the clinical details of which are summarized in Table 1). The age at onset was typically between 19 and 54 years. The majority of patients had a history of miscarriage or uterine cavity surgery and typically presented with repeated catamenial hemoptysis. Hemoptysis coincided with and subsided after the menstrual cycle; consistent with this, most patients exhibited imaging changes during, but not after, the menstrual cycle. Almost all such patients reported thus far underwent local lobectomy; histopathology confirmed the presence of endometrial glands, interstitial cells, hemosiderin deposits, fibrous tissue hyperplasia, or similar pathological findings in the lung tissue. Some patients underwent additional immunohistochemical detection of ER, PR, CD10, or CD68, which suggested the presence of endometrial tissue. Prussian blue staining of the tissues of some patients showed hemosiderin in the alveolar cavity (Figure 4B). In all patients, hemoptysis, pneumothorax, and imaging changes disappeared postoperatively, and no recurrences were reported.
| Case | Gender (age) | Symptom | Chest CT findings | Treatment | Follow up |
| Haruki et al[5] | F (29) | Repeated hemoptysis | Exudation of right lower lung | Lobectomy | NED (4 mo) |
| Tong et al[6] | F (22) | Repeated hemoptysis | Right middle lobe shadow | Lobectomy | NED (5 mo) |
| Ghigna et al[13] | F (54) | Exercise dyspnea | Right lung shadow | Lobectomy | Not provided |
| Vercellini et al[14] | F (39) | Hemoptysis | Left lower lobe fibrosis | Medical treatment | NED (30 mo) |
| Kawaguchi et al[15] | F (20) | Repeated hemoptysis | Right anterior basal ganglia shadow | Lobectomy | No recurrence |
| Kawaguchi et al[15] | F (40) | Repeated hemoptysis | Not provided | Lobectomy | Not provided |
| Rousset-Jablonski et al[16] | F (32) | Repeated hemoptysis | Right lung nodules | Treatment | Not provided |
| L'Huillier[20] | F (31) | Repeated hemoptysis | Right lung high-density shadow | Lobectomy | None |
| Alzayer[21] | F (30) | Pneumothorax | Right lung pneumothorax | Lobectomy | No recurrence |
| Gupta and Gupta[22] | F (39) | Repeated hemoptysis | Fibrosis of left lower lobe | medical treatment | Not provided |
| Matsushima et al[23] | F (37) | Repeated hemoptysis | Right lung cystic shadow | Lobectomy | NED (30 mo) |
| Huang et al[24] | F (29) | Repeated hemoptysis | Middle and lower lobe of left lung | Lobectomy | NED (24 mo) |
| Lawrence[25] | F (34) | Repeated hemoptysis | Lower left lung shadow | Lobectomy | Not provided |
| Zanetti et al[26] | F (29) | Hemoptysis | Hole in the lower right leaf | Lobectomy | Not provided |
| Poh et al[27] | F (41) | Chest pain | Pneumothorax | Lobectomy | Not provided |
| Marques et al[28] | F (28) | Repeated hemoptysis | Lower right leaf shadow | Lobectomy | No recurrence |
| Furuya et al[29] | F (21) | Repeated hemoptysis | Right middle lobe shadow | Lobectomy | NED (36 mo) |
| Tulandi et al[30] | F (42) | Spontaneous pneumothorax | Not provided | Lobectomy | Not provided |
Endometriosis is characterized by the growth of the endometrium outside the uterine cavity. It is a common gynecological, hormone-dependent disease[5] that affects fertility, with an incidence of approximately 15% in women of childbearing age[6]. Endometriosis mostly occurs in the pelvic cavity, but it can also occur outside the pelvic cavity. A rare form of endometriosis outside the pelvic cavity is lung endometriosis, which accounts for approximately 20% of cases of pleural endometriosis[7,8]. Pulmonary endometriosis was described in 1938[9]. It is an extremely rare disease with complex etiology and unclear pathogenesis. As it often has non-specific clinical and imaging presentations, it can easily be misdiagnosed.
Thus far, several common clinical features of lung endometriosis have been reported, including repeated hemoptysis, coughing, pneumothorax, hemothorax, and pulmonary nodules, coinciding with the menstrual cycles; however, not all clinical features are present in all patients. Approximately 82% of patients with lung endometriosis experience catamenial hemoptysis as their main symptom[10], whereas the remaining patients present with catamenial pneumothorax, catamenial hemothorax, or other symptoms. Radiography or CT scans can assist in the diagnosis of lung endometriosis; however, this disease is frequently misdiagnosed due to its non-specific symptoms and rarity. Other diseases must generally be ruled out before a clear diagnosis of lung endometriosis can be made[6].
In the present case, the young patient resided in an area with a high incidence of tuberculosis. She exhibited hemoptysis but did not have symptoms such as low-grade fever and night sweats. CT images revealed no nodules, and because the sputum smear test tested negative for Mycobacterium tuberculosis, tuberculosis was excluded as a potential diagnosis.
The typical pathological features of endometriosis are extra-endometrial endometrial glands, interstitium, and hemosiderin depositions, which can be confirmed histologically[11]. However, compared with pelvic endometriosis, these microscopic features might not all be observed concurrently in lung endometriosis. Ghigna et al[13] reported that these three characteristics appeared concurrently only in 44% cases of lung endometriosis, whereas in the remaining cases, only endometrial stroma was found. When only a small amount of endometrial stroma is found in the lung tissue, the diagnosis is rendered even more difficult[13,14]. In addition, it is sometimes difficult to distinguish between endometrial stroma and inflammatory cells by hematoxylin and eosin staining alone. Therefore, immunohistochemical detection is needed to assist in the diagnosis of lung endometriosis[14]. In the present case, the ectopic endometrium in the lung tissue led to alveolar hemorrhage, hemosiderin deposition, chronic inflammation, and fibrosis due to long-term incomplete absorption or excretion. Only a few glandular epithelial cells were observed in the alveolar and vascular cavities. Immunohistological CK staining and Prussian blue staining for hemosiderin showed positive results; ER, PR, and CD10 staining results were negative. Combining the above histopathological and immunohistochemical findings with the history of miscarriage, clinical symptoms, imaging changes, and the exclusion of other lung diseases, the diagnosis of lung endometriosis was established[15].
The pathogenesis of endometriosis is still unclear, but seven theories exist:
Endometrial implant theory: This is the most common view. Sampson proposed that when the menstrual blood flows backward, the shed endometrium can enter the pelvic cavity through the fallopian tube and implant outside the endometrium[11,16]. Local injuries to the cervix, vagina, and vulva can therefore easily cause endometriosis[17,18];
Body cavity epithelial metaplasia theory: There are researchers proposed that the epithelium lining body cavities had the potential to differentiate into endometrial tissue[16] and that it may be transformed into endometrial-like tissue by certain stimulators;
Iatrogenic endometrium implantation: This theory suggests a type of artificial endometrial transplantation to certain body parts, such as to the abdominal wall scar after a cesarean delivery or perineal side incision during childbirth. A history of multiple uterine cavity operations, repeated abortions, tubal drainage, or similar, may therefore lead to endometriosis[19,20];
Embryo theory: In the embryo, the accessory mesonephros, ovary germinal epithelium, pelvic peritoneum, rectovaginal septum, umbilical region, and other tissues evolve from the epithelium lining the body cavities, and they are able to differentiate into the endometrial tissue when stimulated by inflammation, which is common on the surface of the ovary;
Induction theory: Under the induction of endogenous biochemical substances, undifferentiated peritoneal tissue can be transformed into endometrial tissue;
Genetic factors: Endometriosis has a certain genetic predisposition and family aggregation, and people with a family history of endometriosis are more likely to experience this disease;
Immune factors: Studies have speculated that diseased stem cells play an important role in the spread of epithelium through the blood and long-distance implantation[17,18].
At present, there are no comprehensive treatment guidelines for lung endometriosis. It is generally believed that the best therapeutic options are conservative drug treatment or surgery. The most appropriate treatment method is selected based on the patient’s clinical symptoms and their severity. The first choice for conservative treatment is gonadotrophin releasing hormone (GnrH) agonists, which are now widely used in the treatment of endometriosis[4]. GnrH agonists inhibit the release of estrogen and progesterone from the ovaries and result in a decrease in plasma estradiol levels, mimicking a state of pseudomenopause in order to control the growth of extraendometrial lesions. However, GnrH agonist treatment is costly, can elicit many adverse reactions, needs to be carried out long-term, and is symptomatic rather than curative[7]. In addition, the recurrence rate with GnrH agonists is high and long-term treatment may harm patients’ ovulation and fertility[19]. Hence, most patients choose surgical treatment, in particular pulmonary wedge resection. This has a lower recurrence rate and a better prognosis compared with pharmaceutical therapy.
Endometriosis is a chronic condition that affects 5%-10% of women of reproductive age worldwide. Despite its prevalence, little is known about how the disease manifests outside the female reproductive tract. In addition, because of occurrence at various sites and clinical manifestations, diagnosis is usually delayed for several years, which increases the difficulty of diagnosis and misdiagnosis, and prolongs the implementation time of effective treatment[4]. In particular, functional effects (pain, infertility) or organ dysfunction can lead to a significant decline in patients' quality of life (in terms of pain and fertility). Therefore, diagnosis and timely treatment of endometriosis are very important[1], and it is necessary to further understand the relationship between medical history (abortion history, uterine surgery history) and clinical manifestations. Doing so would help to provide a reference for the promotion and prevention of the disease in the future, to avoid and reduce disease occurrence, so as to provide patients with appropriate treatment before the disease progresses and/or symptoms worsen.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ampollini L, Italy; Parra RS, Brazil S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Yoldemir T. Evaluation and management of endometriosis. Climacteric. 2023;26:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Ashkenazi MS, Huseby OL, Kroken G, Trocha M, Henriksson A, Jasiak H, Cuartas K, Loschiavo A, Kuhn I, Støve D, Grindahl H, Latour E, Melbø M, Holstad K, Kwiatkowski S. The Clinical Presentation of Endometriosis and Its Association to Current Surgical Staging. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Parra RS, Feitosa MR, Camargo HP, Valério FP, Zanardi JVC, Rocha JJRD, Féres O. The impact of laparoscopic surgery on the symptoms and wellbeing of patients with deep infiltrating endometriosis and bowel involvement. J Psychosom Obstet Gynaecol. 2021;42:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397:839-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 632] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 5. | Haruki T, Fujioka S, Adachi Y, Miwa K, Taniguchi Y, Nakamura H. Successful video-assisted thoracic surgery for pulmonary endometriosis: Report of a case. Surg Today. 2007;37:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Tong SS, Yin XY, Hu SS, Cui Y, Li HT. Case report of pulmonary endometriosis and review of the literature. J Int Med Res. 2019;47:1766-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11-22; discussion 34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 309] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Andres MP, Arcoverde FVL, Souza CCC, Fernandes LFC, Abrão MS, Kho RM. Extrapelvic Endometriosis: A Systematic Review. J Minim Invasive Gynecol. 2020;27:373-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 9. | de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 539] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 10. | Alifano M, Legras A, Rousset-Jablonski C, Bobbio A, Magdeleinat P, Damotte D, Roche N, Regnard JF. Pneumothorax recurrence after surgery in women: clinicopathologic characteristics and management. Ann Thorac Surg. 2011;92:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Fujimoto K, Kasai H, Suga M, Sugiura T, Terada J, Suzuki H, Oota M, Yoshino I, Nakatani Y, Tatsumi K. Pulmonary Endometriosis which Probably Occurred through Hematogenous Metastasis after Artificial Abortion. Intern Med. 2017;56:1405-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Alwadhi S, Kohli S, Chaudhary B, Gehlot K. Thoracic Endometriosis-A Rare Cause of Haemoptysis. J Clin Diagn Res. 2016;10:TD01-TD02. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ghigna MR, Mercier O, Mussot S, Fabre D, Fadel E, Dorfmuller P, de Montpreville VT. Thoracic endometriosis: clinicopathologic updates and issues about 18 cases from a tertiary referring center. Ann Diagn Pathol. 2015;19:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1246] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 15. | Kawaguchi Y, Hanaoka J, Ohshio Y, Igarashi T, Okamoto K, Kaku R, Hayashi K, Ishida M. Diagnosis of thoracic endometriosis with immunohistochemistry. J Thorac Dis. 2018;10:3468-3472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Rousset-Jablonski C, Alifano M, Plu-Bureau G, Camilleri-Broet S, Rousset P, Regnard JF, Gompel A. Catamenial pneumothorax and endometriosis-related pneumothorax: clinical features and risk factors. Hum Reprod. 2011;26:2322-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Augoulea A, Lambrinoudaki I, Christodoulakos G. Thoracic endometriosis syndrome. Respiration. 2008;75:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Li F, Alderman MH 3rd, Tal A, Mamillapalli R, Coolidge A, Hufnagel D, Wang Z, Neisani E, Gidicsin S, Krikun G, Taylor HS. Hematogenous Dissemination of Mesenchymal Stem Cells from Endometriosis. Stem Cells. 2018;36:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 787] [Article Influence: 112.4] [Reference Citation Analysis (0)] |
| 20. | L'Huillier JP. Endobronchial endometriosis Nd-YAG therapy vs drug therapy. Chest. 2005;127:684-5; author reply 685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Alzayer H. Pulmonary endometriosis: a rare cause of hydropneumothorax. Respirol Case Rep. 2019;7:e432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Gupta A, Gupta S. Pulmonary parenchymal endometriosis: a diagnostic dilemma. J Obstet Gynaecol India. 2014;64:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 23. | Matsushima K, Ono M, Hayashi S, Sonoda D, Matsui Y, Shiomi K, Satoh Y, Ohbu M. Resection of intra-pulmonary endometriosis by video-assisted thoracoscopic surgery under pre-operative CT-guided marking synchronized with menstrual cycle. Gen Thorac Cardiovasc Surg. 2020;68:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Huang H, Li C, Zarogoulidis P, Darwiche K, Machairiotis N, Yang L, Simoff M, Celis E, Zhao T, Zarogoulidis K, Katsikogiannis N, Hohenforst-Schmidt W, Li Q. Endometriosis of the lung: report of a case and literature review. Eur J Med Res. 2013;18:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Lawrence HC 3rd. Pulmonary endometriosis in pregnancy. Am J Obstet Gynecol. 1988;159:733-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Zanetti G, Hochhegger B, Marchiori E. Pulmonary endometriosis: an unusual cause of hemoptysis. J Bras Pneumol. 2020;46:e20190335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 27. | Poh CL, Yan TD, Vallely MP, Bannon PG, McCaughan BC. Pulmonary parenchymal endometriosis presenting as bilateral pneumothoraces. J Obstet Gynaecol. 2011;31:452-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Marques VD, de Mattos LA, Pimenta AM, Pelloso SM, Bandeira COP, Lemos MM, Moraes WAS, Carvalho MDB. Resection of Pulmonary Endometriosis by Video-Assisted Thoracoscopic Surgery Using Bronchoscopy as a Preoperative Strategy. Ann Thorac Surg. 2020;110:e391-e393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Furuya K, Otsuka H, Koezuka S, Makino T, Hata Y, Wakayama M, Shibuya K, Iyoda A. Resection of pulmonary endometriosis using video-assisted thoracoscopic surgery under preoperative CT-guided marking. Gen Thorac Cardiovasc Surg. 2017;65:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Tulandi T, Sirois C, Sabban H, Cohen A, Murji A, Singh SS, Chen I, Belland L. Relationship between Catamenial Pneumothorax or Non-catamenial Pneumothorax and Endometriosis. J Minim Invasive Gynecol. 2018;25:480-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |