Published online Jun 26, 2023. doi: 10.12998/wjcc.v11.i18.4295
Peer-review started: January 4, 2023
First decision: April 19, 2023
Revised: April 25, 2023
Accepted: May 9, 2023
Article in press: May 9, 2023
Published online: June 26, 2023
Processing time: 173 Days and 6.1 Hours
The Coexistence of myeloid and lymphoid malignancies is rare. Myeloid leukemia occurs more frequently as a secondary event in patients receiving chemotherapy agents for lymphoid malignancies. Synchronous diagnoses of diffuse large B-cell lymphoma (DLBCL), acute myeloid leukemia (AML), and untreated lymphoplasmacytic lymphoma/Waldenström macroglobulinemia (LPL/WM) in the same patient have not been reported. Here we report one such case.
An 89-year-old man had a chest wall mass histopathologically diagnosed as DLBCL. The bone marrow and peripheral blood contained two groups of cells. One group of cells fulfilled the criteria of AML, and the other revealed the features of small B lymphocytic proliferative disorder, which we considered LPL/WM. Multiple chromosomal or genetic changes were detected in bone marrow mononuclear cells, including ATM deletion, CCND1 amplification, mutations of MYD88 (L265P) and TP53, WT1 overexpression, and fusion gene of BIRC2-ARAP1, as well as complex chromosomal abnormalities. The patient refused chemotherapy because of old age and died of pneumonia 1 mo after the final diagnosis.
The coexistence of DLBCL, AML, and untreated LPL/WM in the same patient is extremely rare, which probably results from multiple steps of genetic abnor
Core Tip: The coexistence of myeloid and lymphoid malignancies is rare. Synchronous diagnosis of diffuse large B-cell lymphoma (DLBCL), acute myeloid leukemia (AML), and untreated lymphoplasmacytic lymphoma/Waldenström macroglobulinemia (LPL/WM) in the same patient has not yet been reported. Herein, we report one such case. We assumed that the three diseases have been occurred in a stepwise manner. Asymptomatic indolent LPL/WM might have occurred earlier, then insidious myelodysplastic syndrome developed into AML, while chest wall DLBCL grew separately.
- Citation: Zhang LB, Zhang L, Xin HL, Wang Y, Bao HY, Meng QQ, Jiang SY, Han X, Chen WR, Wang JN, Shi XF. Coexistence of diffuse large B-cell lymphoma, acute myeloid leukemia, and untreated lymphoplasmacytic lymphoma/waldenström macroglobulinemia in a same patient: A case report. World J Clin Cases 2023; 11(18): 4295-4305
- URL: https://www.wjgnet.com/2307-8960/full/v11/i18/4295.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i18.4295
Diffuse large B-cell lymphoma (DLBCL) and acute myeloid leukemia (AML) rarely occur concurrently. Only a few cases of DLBCL after AML chemotherapy[1,2] have been reported. Chemotherapy-induced immunosuppression predisposes patients to develop secondary malignancies, particularly virus-related ones[1,2]. Only two cases with a synchronous diagnoses of DLBCL and AML have been reported[3,4]. The coexistence of AML and small B lymphocytic proliferative disorder (LPD) is also rare. Most cases of AML develop after small B LPD (B-LPD), probably due to immunodeficiency over the long course of LPD[5,6] or after treatment[7]. Only a few cases of newly diagnosed synchronous AML and small B-LPD have been reported[8-10]. Lymphoplasmacytic lymphoma/Waldenström macroglobulinemia (LPL/WM) is a specific type of B-LPD. To the best of our knowledge, synchronous diagnosis of DLBCL, AML, and untreated LPL/WM in a same patient has not been reported yet. Herein, we report one such case.
An 89-year-old man was admitted to our hospital complaining of cough, expectoration, and shortness of breath for half a month.
The patient complained of cough and expectoration for half a month. His blood test results were abnormal: Leukocyte count, 12.6 (4.0-10.0 × 109/L); neutrophil count, 1.44 (2.00-7.50 × 109/L); lymphocyte count, 4.21 (0.80-4.00 × 109/L); monocyte count, 6.9 (0-0.8 × 109/L); hemoglobin level, 73 (120-160g/L); and platelet count 55 (100-300 × 109/L). The patient was therefore admitted to the Department of Hematology.
The patient had chronic bronchitis for 10 years, which often deteriorated in winter.
Physical examination revealed skin pallor, enlarged lymph nodes in the armpit, and moist rales in both lungs, but no hepatosplenomegaly.
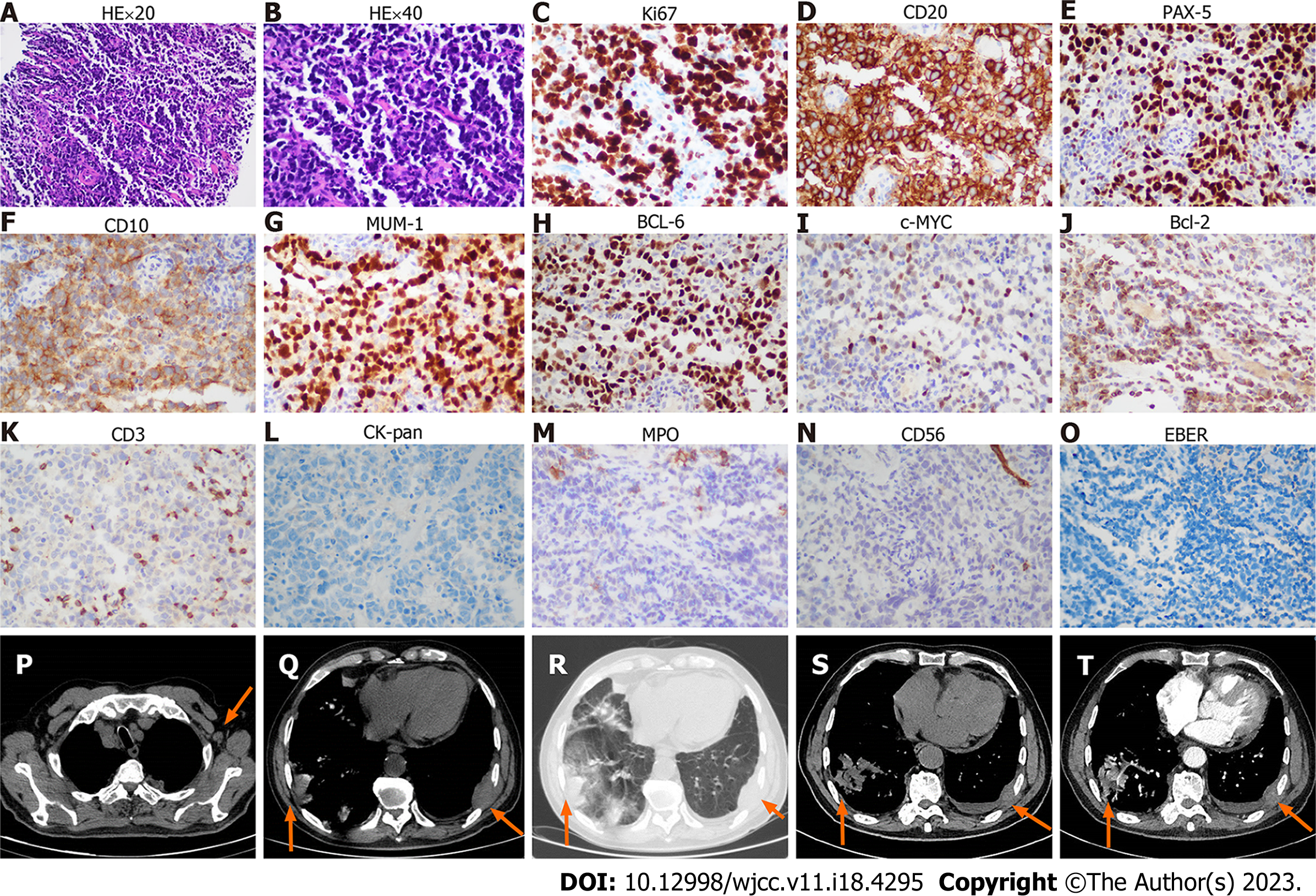
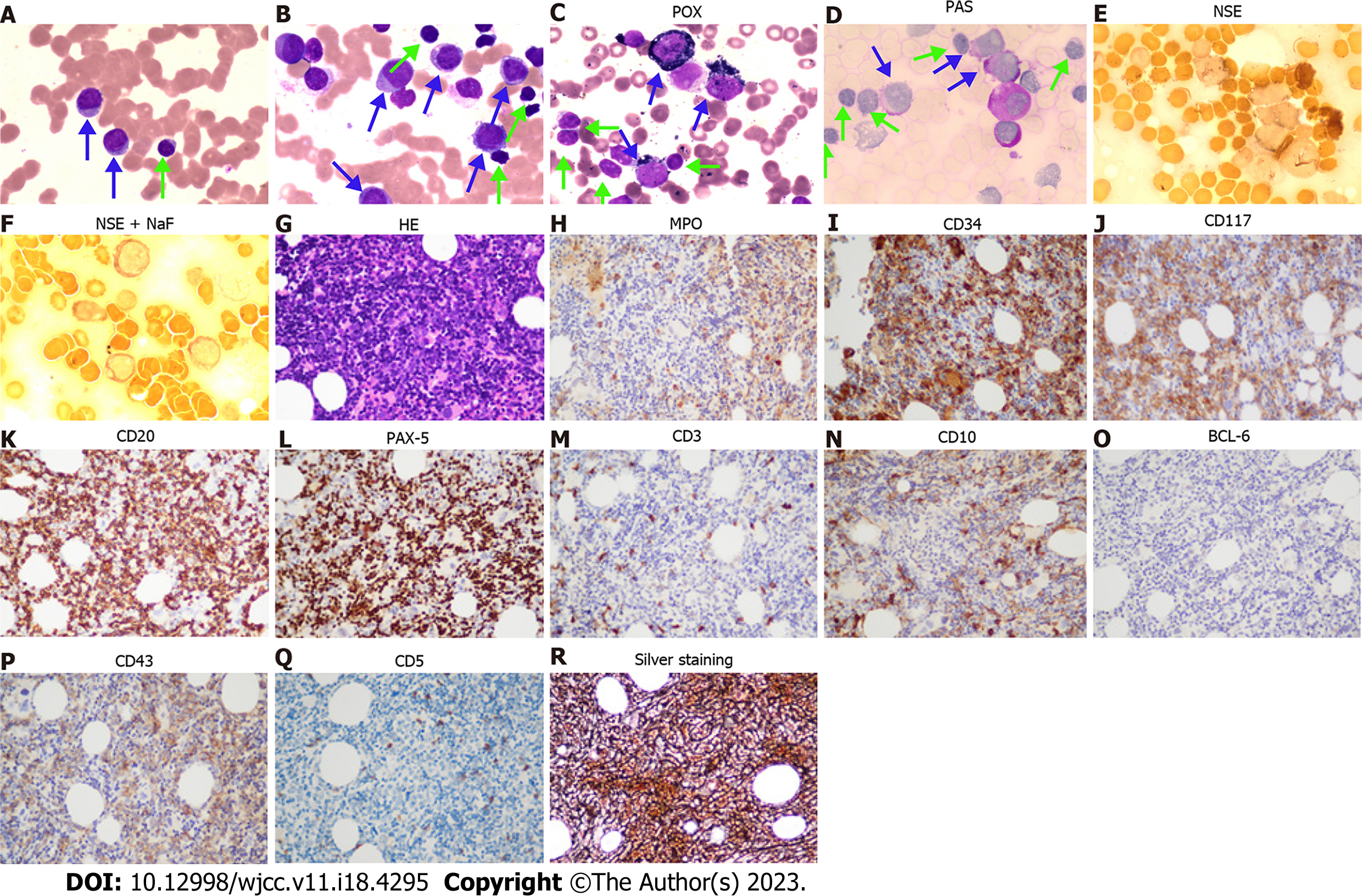
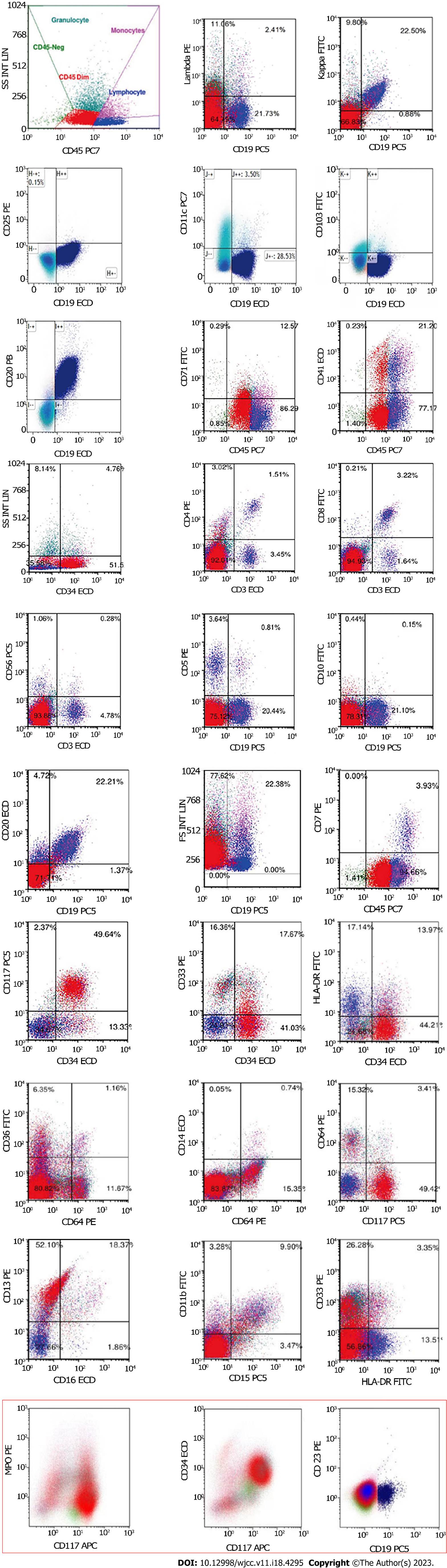
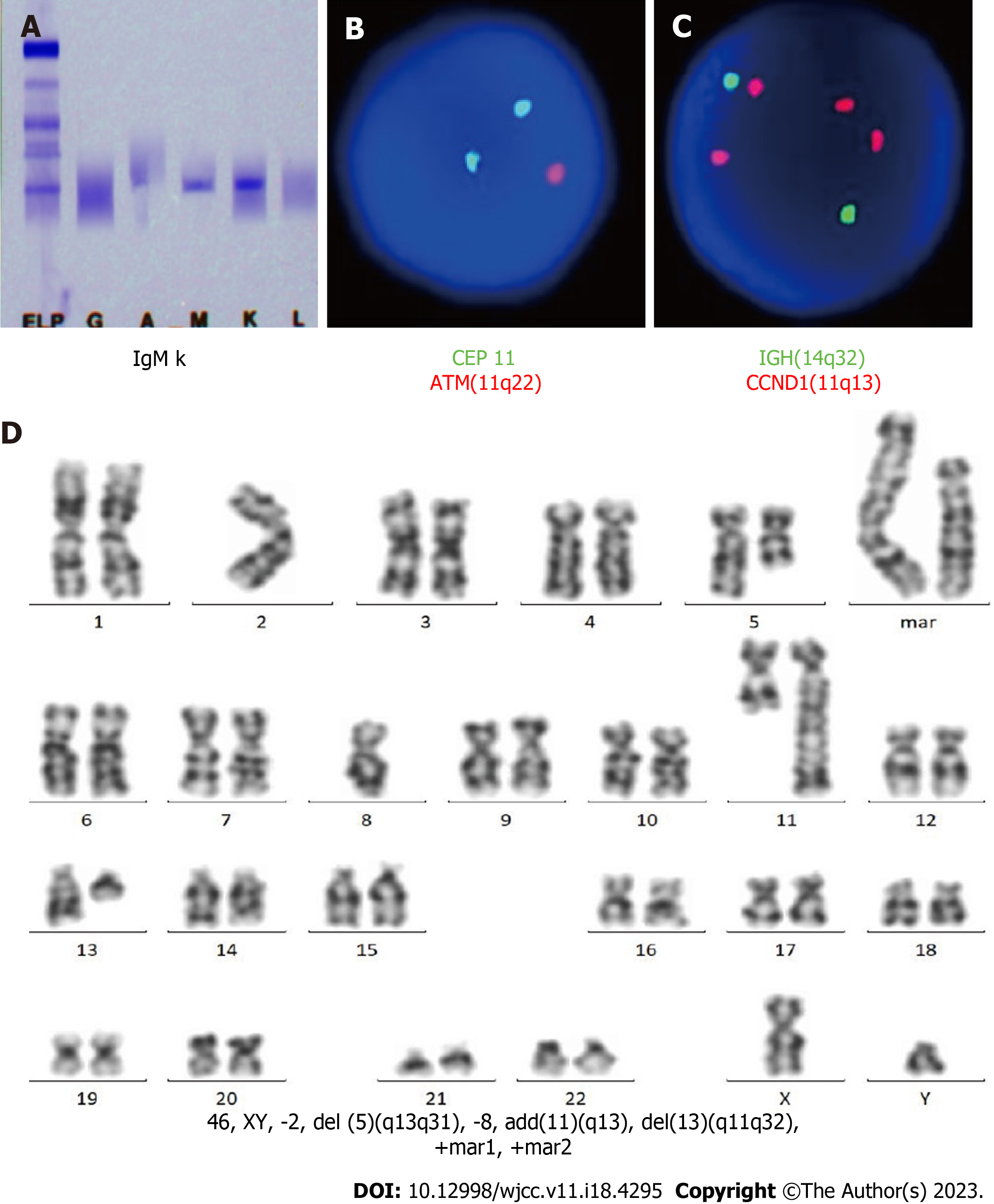
CT-guided puncture of the mass in the chest wall was performed for biopsy. The histopathological examination showed a hyperproliferation of large cells with Ki-67 (70%), CD20 (+++), PAX-5 (++), CD10 (+), MUM-1 (+), BCL-6 (+), c-MYC (< 40%), BCL-2 (< 50%), CD3 (-), CK-pan (-), TTF (-), NapsinA (-), CK7 (-), P40 (-), CK5/6 (-), Syn (-), CgA (-), CD56 (-), and EBER (-), suggesting a diagnosis of DLBCL (Figure 1A-O, Table 1). Blood tests were performed, and many abnormalities were found: Procalcitonin levels, 1.42 (< 0.05 ng/mL); erythrocyte sedimentation rate, 97 (0-20 mm/h); C-reactive protein levels, 370 (0-10 mg/L); lactate dehydrogenase levels, 460 (120-250 U/L); serum albumin levels, 34.3 (40.0-55.0 g/L); serum fibrinogen levels, 7.24 (2.00-4.00 g/L); CA 125, 48.2 (0-35.0 U/mL); pro-B-type natriuretic peptide, 1918 (< 300 pg/mL); serum immunoglobulin (Ig) M levels, 6.55 (0.40-2.30 g/L); urine Kappa light chain, 318 (0-50 mg/L); free triiodothyronine levels, 2.61 (3.10-6.80 pmol/L); and triiodothyronine levels, 0.77 (1.30-3.10 nmol/L). Peripheral blood and bone marrow smears were performed. Surprisingly, no large B lymphocytes were found, but there were 71.6% mature small lymphocytes, along with 23.2% myeloid blasts which were peroxidase (++), periodic acid-Schiff stain (+), and non-specific esterase (-/+, cannot be inhibited by NaF) (Figure 2A-F, Table 1). The patient’s peripheral blood had a high percentage of myeloblasts (50%) and a relatively low percentage of B lymphocytes (29%) (Figure 2A-F). Immunohistochemical analysis of the bone marrow biopsy showed mixed expression of myeloblast and mature B lymphocyte markers with CD10 (+), CD20 (+++), CD34 (++), CD43 (+), CD117 (++), PAX-5(+++), myeloperoxidase (MPO) (+), silver staining (++), BCL-6 (-), CD3 (-), and CD5 (-) (Figure 2G-R, Table 1). To identify the features of these mixed cells, flow cytometry was used to gate the two groups of cells from peripheral blood and bone marrow samples. The immunophenotypic results confirmed the morphological findings of the presence of two discrete abnormal cell populations: 49.64% large blasts with dim CD45 expression and 22.21% small lymphocytes with strong CD45 expression. The large blasts population expressed CD34, CD13, and CD117, and partially expressed CD33, HLA-DR, and MPO, but did not express CD3, CD4, CD5, CD7, CD8, CD10, CD11b, CD14, CD15, CD19, CD20, CD36, CD56, CD64, or CD23, compatible with myeloblasts. In contrast, the small lymphocytes expressed CD19, CD20, and Kappa light chain, but did not express CD103, CD25, CD11c, CD34, CD117, CD5, CD10, CD23 or Lambda light chain, which were considered LPD cells (Figure 3, Table 1). Therefore, AML-M2 along with a small B-LPD were suspected. Furthermore, cytogenetic and molecular biology tests were performed on bone marrow mononuclear cells (BMMNCs). Monoclonal IgM Kappa was identified by immunofixation electrophoresis (Figure 4A), which was consistent with the diagnosis of monoclonal B lymphocytosis or plasmacytosis. Fluorescence in situ hybridization (FISH) results showed deletion of 11q22 (ATM) (83%) and an increased copy number of 11q13 (CCND1) (80%) (Figure 4B and C), but no abnormalities of chromosomes or genes in 13q34, 13q14.3, 12, 17p13.1 (P53), or 13q14.2 (RB1). The IgH rearrangement was confirmed and t(11; 14) was not detected. Chromosomal karyotype analysis was performed and five metaphases were obtained using the routine method, whereas 20 metaphases were obtained after stimulation with CpG-oligodeoxynucleotides. All metaphases showed a complex abnormality with 46, XY, -2, del(5)(q13q31), -8, add(11)(q13), del(13)(q11q32), +mar1, and +mar2 (Figure 4D). A total of 281 genes associated with malignant lymphoid and myeloid diseases were tested by sequencing (Supplementary material). MYD88 (14.8%) mutation (p.L265P) and 27.3% of TP53 mutations (p.N29Kfs*14 and p.S215N) were detected (Table 2). Different kinds of copy number variations were found in chromosome 2p25.3p13.3 (2.56 copies), 5q14.3q34 (1.46 copies), 8p23.3p21.2 (4.30 copies), 8q21.3q24.3 (2.55 copies), 11q12.1q14.1 (3.05 copies), 11q14.1q22.1 (1.43 copies), 11q22.3q23.1 (1.44 copies), 11q23.1q25 (4.85 copies), 13q12.3q14.13 (1.43 copies), 13q21.1q22.1 (2.63 copies), and 13q31.1q31.3 (2.49 copies), which were consistent with the karyotype results (Table 2). AML-associated 56 fusion genes were tested using real-time PCR (Supplementary material) and WT1 overexpression was also detected (Table 2). Newly discovered fusion genes were also tested using RNA sequencing (Supplementary material), and a new fusion gene, BIRC2(11q22.2)-ARAP1(11q13.4), was detected (Table 2). Gene sequencing of the tissue from the chest wall mass was not available because of the inability to obtain sufficient tissue.
| Expression | Molecular marker |
| The immunophenotype of BMMNCs by flow cytometry | |
| Group A | 49.64% |
| Expression | CD34, CD13, CD117 |
| Partial expression | CD33, HLA-DR |
| No expression | CD3, CD4, CD5, CD7, CD8, CD10, CD11b, CD14, CD15, CD19, CD20, CD36, CD56, CD64, CD23 |
| Group B | 22.21% |
| Expression | CD19, CD20, Kappa |
| Partial expression | |
| No expression | CD103, CD25, CD11c, CD34, CD117, CD5, CD10, CD23, lambda |
| The immunohistochemical analysis of bone marrow biopsy | |
| Expression | CD10, CD20, CD34, CD43, CD117, PAX-5, MPO, silver dyeing |
| Partial expression | |
| No expression | BCL-6, CD3, CD5, MPO |
| The immunohistochemical analysis of chest wall tumor | |
| Expression | Ki-67 (70%), CD20, PAX-5, CD10, MUM-1, BCL-6, c-MYC (< 40%), BCL-2 (< 50%) |
| Partial expression | |
| No expression | CD3, CK-pan, TTF, NapsinA, CK7, P40, CK5/6, Syn, CgA, CD56, EBER |
| Expression | Abnormality |
| The mutation of genes | |
| MYD88 | p.L265P (14.8%) |
| TP53 | p.N29Kfs*14 (27.3%) |
| TP53 | p.S215N (27.2%) |
| The fusion of genes or gene expression | |
| WT1 | Positive |
| BIRC2-ARAP1 | Positive |
| The copy number variations | |
| chr2p25.3p13.3 | 2.56 |
| chr5q14.3q34 | 1.46 |
| chr8p23.3p21.2 | 4.30 |
| chr8q21.3q24.3 | 2.55 |
| chr11q12.1q14.1 | 3.05 |
| chr11q14.1q22.1 | 1.43 |
| chr11q22.3q23.1 | 1.44 |
| chr11q23.1q25 | 4.85 |
| chr13q12.3q14.13 | 1.43 |
| chr13q21.1q22.1 | 2.63 |
| chr13q31.1q31.3 | 2.49 |
The patient was diagnosed with the coexistence of DLBCL, AML, and LPL/WM.
The patient refused chemotherapy because of old age and performance status.
The patient died of pneumonia 1 mo later. The patient’s family refused an autopsy.
This patient was admitted to our hospital because of the chest wall mass, which was histopathologically identified as DLBCL (GCB subtype). The cells were large and strongly positive for CD20, PAX-5, BCL-6, and MUM-1, with high Ki-67expression, suggesting an aggressive feature. The positive expression of BCL-2, BCL-6, and c-MYC suggested that DLBCL was a “triple expressor” type. MPO-negative expression of these large cells ruled out the probability of myeloblast infiltration. Blood cell abnormalities were occasionally observed during regular examinations. The two groups of abnormal cells were found in peripheral blood and bone marrow. Morphology and immunohistochemistry suggested a diagnosis of AML and small B-LPD. The two groups of cells were identified by flow cytometry. The cells expressing CD13, CD34, and CD117 were from myeloid blasts, whereas the others expressing CD19, CD20, and Kappa were from B lymphoid mature cells. Based on the morphology and flow cytometry results, the B-cells were found to be small, consistent with a diagnosis of small B-LPD, which is a chronic monoclonal neoplasm, including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), follicular lymphoma (FL), marginal zone lymphoma (MZL), hairy cell leukemia (HCL), and LPL/WM. Negative expression of CD103, CD25, and CD11b ruled out the diagnosis of HCL or HCLv. The panel of CD5 (-), CD23 (-), and CD10 (-) ruled out the diagnosis of CLL/SLL and FL[11]. Additionally, the lack of t (11;14) ruled out the diagnosis of MCL[11]. Subsequently, LPL/WM and MZL were considered. The patient had monoclonal IgM Kappa and MYD88 L265P mutations, and LPL/WM was diagnosed, whereas, MZL with plasmacytoid differentiation could not be completely ruled out. Both LPL/WM and MZL seldom transform to DLBCL, although MZL often infiltrates extranodal tissues, including the lungs. Whether LPL/WM and DLBCL are from the same clone is still unknown since the genes from chest wall masses were not tested because of the limited tissue obtained through CT-guided puncture. Therefore, we considered that in the bone marrow LPL/WM occurred and the chest wall tumor was DLBCL.
Multiple chromosomal and gene changes were detected in the BMMNCs (Guangzhou KingMed Diagnostics Group Co., Ltd.). The routine karyotype analysis method, by which more metaphases were from myeloblasts, or CPG-oligodeoxynucleotide stimulating method, by which metaphases were from lymphocytes, detected the homogeneous chromosomal changes of 46, XY, -2, del(5)(q13q31), -8, add(11)(q13), del(13)(q11q32), +mar1, and +mar2. Myeloblasts (49.64%) and lymphocytes (22.21%) shared the same chromosomal abnormalities. Moreover, FISH detected 83% of 11q22 deletions, and 80% of 11q13 multiple copies. Based on this homogeneity, we suspected that myeloblasts and lymphocytes might be derived from the same progenitor, although different clones were not separated for molecular analysis due to the limited specimens.
Often, 5q- is found in AML or myelodysplastic syndrome (MDS), 11q- in small B-LPD, and 13q- in MDS, AML, or CLL. Although the copy number of 11q13 (CCND1) increases, no chromosomal change of t (11;14) is found; therefore, MCL should be ruled out. The deletion of 11q22.3 (ATM) often causes a defect in apoptosis, similar to deletion or mutation of 17p13 (TP53), suggesting a poor prognosis[12,13]. The frameshift mutation of TP53 (p.N29Kfs*14) leads to a change in amino acid sequence or early termination of protein translation, causing a loss of TP53 function. Missense mutation of TP53 (p.S215N) can be found in lymphoid or myeloid malignant diseases, such as CLL, DLBCL, MCL, FL, LPL/WM, AML, and MDS, and are associated with poor prognosis. WT1 is overexpressed in newly diagnosed or relapsed AML, CML in the accelerated and blastic phases, and high-risk MDS. BIRC2(11q22.2)-ARAP1(11q13.4) is a novel fusion gene discovered in this patient. Whether it is related with AML or LPD remains unknown. The patient’s complex chromosomal abnormality with 5q- and old age led us to speculate that AML may originate from MDS, although dysplasia in erythroblasts or megakaryocytes was not found.
Multiple theories behind the simultaneous development of several malignancies in an untreated patient have been proposed, including immunosuppression mediated by chronic small B-LPDs[14], a common stem cell defect[15], or just a chance. Therefore, we hypothesized that these diseases occurred in a stepwise manner. Asymptomatic indolent LPL/WM might have occurred earlier, then insidious MDS developed into AML, while chest wall DLBCL grew separately.
The coexistence of DLBCL, AML, and untreated LPL/WM in a same patient is extremely rare. Herein, we reported one such case that might have resulted from multiple steps of gene mutations. In the bone marrow, asymptomatic indolent LPL/WM might have occurred earlier, then insidious MDS developed into AML. Finally, aggressive DLBCL in the chest wall grew. Hematologists should pay close attention to this extremely rare case to avoid misdiagnoses. However, this report has some limitations. Ideally, different clones from BMMNCs should have been separated for molecular analysis, genes from chest wall masses should have been tested, and an autopsy should have been performed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saito M, Japan S-Editor: Chen YL L-Editor: A P-Editor: Ji MX
| 1. | Higuchi M, Sasaki S, Kawadoko S, Uchiyama H, Yasui T, Kamihira T, Aoki K, Sasaguri T, Nakano R, Uchiyama A, Muta T, Ohshima K. Epstein-Barr virus-positive diffuse large B-cell lymphoma following acute myeloid leukemia: a common clonal origin indicated by chromosomal translocation t(3;4)(p25;q21). Int J Hematol. 2015;102:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Ririe MR, Florell SR, Miles RR, Duffy KL. Secondary diffuse large B-cell lymphoma after chemotherapy for acute myeloid leukemia: looking for the unexpected diagnosis. Am J Dermatopathol. 2014;36:e125-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kunitomi A, Kotani S, Ukyo N, Ono K, Nakamine H, Nohgawa M. Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly complicated by the onset of acute myeloid leukemia. Intern Med. 2014;53:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Bumbea H, Popov VM, Tomuleasa C, Omer M, Dobrea C, Manea I, Zurac S, Popp C, Dumitru I, Simoiu M, Mastalier B. Coexistence of Trisomy 8 and 13 in a Newly Diagnosed Patient With Diffuse Large B Cell Non-Hodgkin Lymphoma and Acute Myeloid Leukemia Secondary to Primary Myelofibrosis. Cureus. 2022;14:e22217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Ito S, Fujiwara SI, Mashima K, Umino K, Minakata D, Nakano H, Yamasaki R, Kawasaki Y, Sugimoto M, Ashizawa M, Yamamoto C, Hatano K, Okazuka K, Sato K, Oh I, Ohmine K, Suzuki T, Muroi K, Kanda Y. Development of acute myeloid leukemia in patients with untreated chronic lymphocytic leukemia. Ann Hematol. 2017;96:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Stern N, Shemesh J, Ramot B. Chronic lymphatic leukemia terminating in acute myeloid leukemia: review of the literature. Cancer. 1981;47:1849-1851. [PubMed] [DOI] [Full Text] |
| 7. | Milosevic I. Coexistence of Chronic Lymphocytic Leukemia and Acute Myeloid Leukemia. Turk J Haematol. 2016;33:353-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Al Mussaed E, Osman H, Elyamany G. Simultaneous existence of acute myeloid leukemia and chronic lymphocytic leukemia: a case report. BMC Cancer. 2016;16:739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Chen RR, Zhu LX, Wang LL, Li XY, Sun JN, Xie MX, Zhu JJ, Zhou D, Li JH, Huang X, Xie WZ, Ye XJ. Synchronous diagnosis and treatment of acute myeloid leukemia and chronic lymphocytic leukemia: Two case reports. World J Clin Cases. 2021;9:9144-9150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 10. | Shoyele O, Gupta G. Synchronous Diagnosis of De Novo Acute Myeloid Leukemia with inv(16)(p13q22) and Chronic Lymphocytic Leukemia: A Case Report and Review of the Literature. Ann Clin Lab Sci. 2018;48:790-796. [PubMed] [DOI] [Full Text] |
| 11. | Lynch RC, Gratzinger D, Advani RH. Clinical Impact of the 2016 Update to the WHO Lymphoma Classification. Curr Treat Options Oncol. 2017;18:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | te Raa GD, Malcikova J, Pospisilova S, Trbusek M, Mraz M, Garff-Tavernier ML, Merle-Béral H, Lin K, Pettitt AR, Merkel O, Stankovic T, van Oers MH, Eldering E, Stilgenbauer S, Zenz T, Kater AP; European Research Initiative on CLL (ERIC). Overview of available p53 function tests in relation to TP53 and ATM gene alterations and chemoresistance in chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54:1849-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Skowronska A, Parker A, Ahmed G, Oldreive C, Davis Z, Richards S, Dyer M, Matutes E, Gonzalez D, Taylor AM, Moss P, Thomas P, Oscier D, Stankovic T. Biallelic ATM inactivation significantly reduces survival in patients treated on the United Kingdom Leukemia Research Fund Chronic Lymphocytic Leukemia 4 trial. J Clin Oncol. 2012;30:4524-4532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Gómez-Arbonés J, Gallart MA, Mellado A, Marco V, Panadés MJ, Macià JM. Concomitant diagnosis of acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL). Importance of flow cytometry in the diagnosis of CLL without lymphocytosis accompanying AML. Eur J Haematol. 1997;59:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Lima M, Porto B, Rodrigues M, Teixeira MA, Coutinho J, Ribeiro AC, Malheiro MI, Justiça B. Cytogenetic findings in a patient presenting simultaneously with chronic lymphocytic leukemia and acute myeloid leukemia. Cancer Genet Cytogenet. 1996;87:38-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |