Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4202
Peer-review started: April 22, 2023
First decision: April 26, 2023
Revised: May 4, 2023
Accepted: May 12, 2023
Article in press: May 12, 2023
Published online: June 16, 2023
Processing time: 50 Days and 13.3 Hours
Inflammatory bowel disease (IBD) is an autoimmune condition treated with immunosuppressive drugs. However, the need for immune system suppression becomes questionable when infection with the human immunodeficiency virus (HIV) occurs simultaneously and impacts the course of IBD. Our reported case represents the clinical course, prescribed treatment and its effect, as well as clinical challenges faced by physicians in a combination of such diseases. We also present a comprehensive literature review of similar cases.
A 49-year-old woman suffering from a newly diagnosed Crohn’s disease was hospitalized due to exacerbated symptoms (abdominal pain, fever, and weight loss). During her hospital stay, she tested positive for HIV. With conservative treatment, the patient improved and was discharged. In the outpatient clinic, her HIV infection was confirmed as stage C3, and antiretroviral treatment was initiated immediately. That notwithstanding, soon the patient was rehospitalized with pulmonary embolism and developed a series of complications because of the subsequent coexistence of IBD and HIV. After intensive and meticulous treatment, the patient’s condition has improved and she remains in remission.
The paucity of studies and data on the coexistence of HIV and IBD leaves clinicians doubting the optimal treatment options.
Core Tip: We present the case of a middle-aged female with Crohn’s disease and concomitant human immunodeficiency virus (HIV) infection to improve knowledge of the rare and understudied interrelation of these two conditions. The paucity of studies and data on HIV and inflammatory bowel disease coinfection leaves clinicians doubting the optimal treatment options. This report reminds experts of the importance of early diagnostics and discusses the specific treatment and prophylactic guidelines when both diseases are present to prevent a variety of complications.
- Citation: Vinikaite A, Kurlinkus B, Jasinskaite D, Strainiene S, Buineviciute A, Sadauskaite G, Kiudelis V, Kazenaite E. Crohn’s disease in human immunodeficiency virus-infected patient: A case report. World J Clin Cases 2023; 11(17): 4202-4209
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4202.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4202
Human immunodeficiency virus (HIV) is a double-strand RNA retrovirus transmitted through direct blood contact that puts the body in a state of immunodeficiency. Meanwhile, autoimmune inflammatory bowel disease (IBD) is an idiopathic chronic relapsing disease of the gastrointestinal tract (GI) that is often treated with immunosuppressive drugs. It is classified as either ulcerative colitis (UC) or Crohn’s disease (CD). Both IBD and HIV infection can coexist in the same person, although this coexistence is rarely observed; therefore, the relationship between one and the other and the impact of this relationship on the immune system are not fully understood yet[1,2]. However, there are speculations that the HIV-modified CD4+ lymphocyte count plays its role in altering the course of IBD alongside increasing the likelihood of opportunistic infections occurring[2,3].
The middle-aged patient was admitted to the Hepatology and Gastroenterology Department of Vilnius University Hospital Santaros Clinics in August 2022 with the following presenting complaints: Abdominal pain, diarrhea, high-grade fever (temperatures of up to 39 °C), and severe weight loss (25 kg over six months).
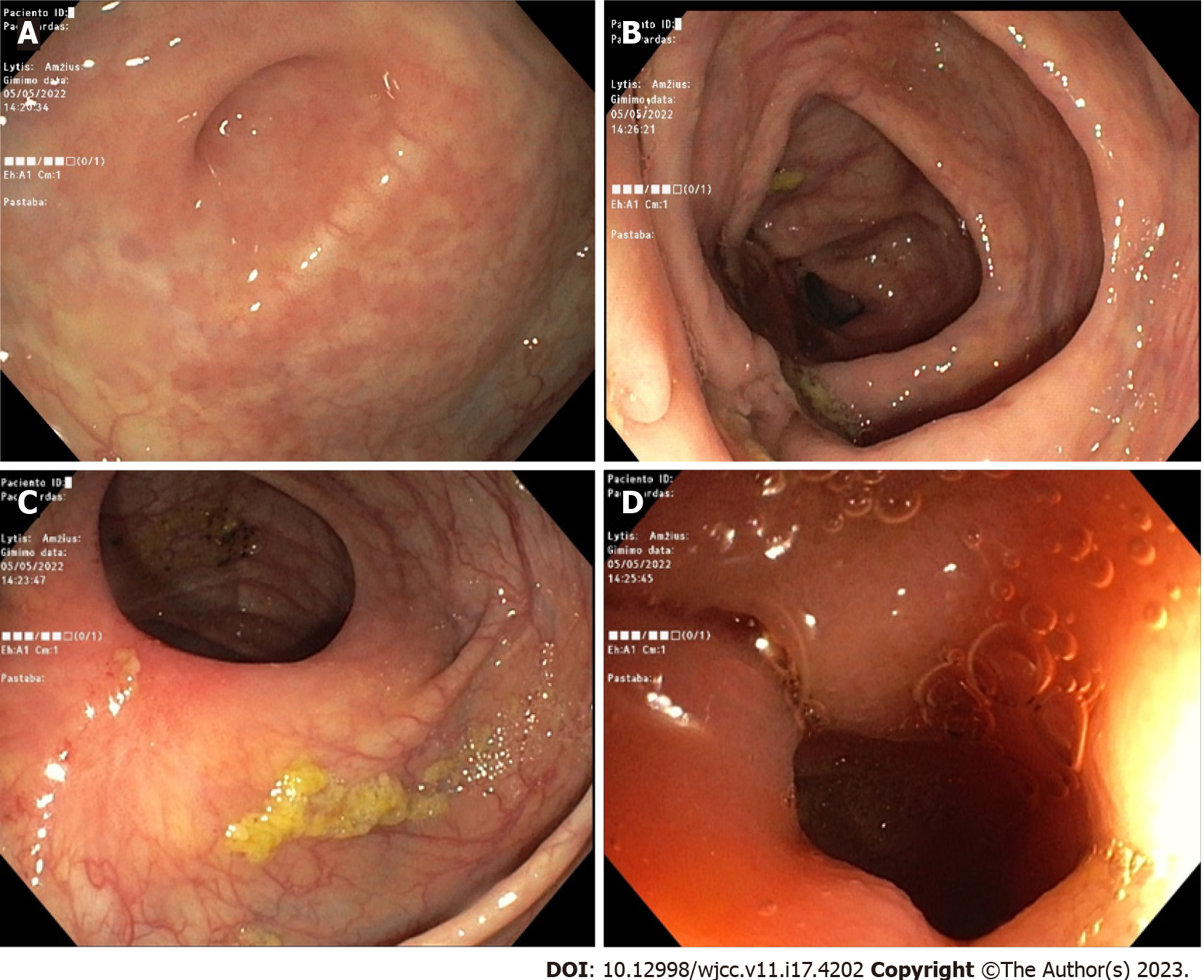
The mentioned abdominal pain, fever, and diarrhea started back in October 2021. However, the woman only sought medical attention in April of the following year when she consulted a gastroenterologist in an outpatient clinic. After running multiple diagnostic tests (such as blood analysis, upper GI endoscopy, colonoscopy, histology, and magnetic resonance imaging enterography) the diagnosis was finally confirmed by the end of May of that year, and it was CD with an inflamed transverse and descending colon (Figure 1). Subsequently, she was prescribed the following daily medication: Mesalazine 1000 mg × 2, azathioprine 50 mg × 2, prednisolone 15 mg × 2 with a steady dosage decrease until it was substituted with budesonide 3 mg × 3. Additionally, the patient was given two courses of ciprofloxacin 500 mg × 2 for 10 d overall. Although diarrhea subsided to watery defecation 1-2 times per day, the abdominal pain, fever, and persistent weight loss persisted. The patient was referred to the inpatient clinic for the preparation of the expected biological therapy.
In 2018, the woman was diagnosed with a hyperplastic endometrial polyp. Also, the patient was chronically anemic. She had no other illnesses, drug allergies, or addictive habits.
The patient works as a cook and is a mother of an adult son and daughter, both of whom are currently healthy. The woman had not had a sexual partner for about a year. However, since her husband’s death in 2011, she has had about 5-7 partners, with whom barrier contraceptives had not always been used.
The patient’s condition at admission was moderate. Her vital signs were as follows: Body temperature, 38 °C; blood pressure, 105/80 mmHg; heart rate, 110 beats/min. Her cardiopulmonary examination was normal, her abdomen was bloated and tender, and she had active bowel peristalsis. She had neither liver nor spleen enlargement, and no skin discoloration, rashes, peripheral edema, or lymphadenopathy was observed.
Before inducing CD remission with more aggressive treatment, several tests were performed to assess her condition and make sure she had not been infected with hepatitis B, hepatitis C, or HIV, and she tested positive for HIV. Inflammatory markers were also taken into account. Some of them are presented in Table 1. Testing for intestinal infections, such as Clostridioides difficile, Shigella, Salmonella, Campylobacter, and Yersinia, were negative.
| Timeline | Analyte | Results |
| August 22, 2022 | White blood count (× 109/L) | 3.36 |
| Neutrophils (× 109/L) | 2.20 | |
| Lymphocytes (× 109/L) | 0.40 | |
| Red blood count (× 1012/L) | 2.54 | |
| Hemoglobin (g/L) | 80 | |
| Platelet count (× 109/L) | 506 | |
| Reactive protein C-reactive protein (mg/L) | 5.07 | |
| HIV 1/2 Ag/Ab combo (s/co) | 472.53 | |
| HIV 1/2 Ag/Ab combo | Positive | |
| September 26, 2022 | Lymphocyte count (%) | 13.9 |
| Lymphocyte count (mm3) | 391 | |
| CD3+ CD4+ (T helper/inducers) (%) | 48 | |
| CD3+ CD4+ (T helper/inducers) (mm3) | 188 |
This patient with active CD (Montreal classification: A3 L2 B3) under glucocorticoid treatment was newly diagnosed with stage C3 HIV infection.
This patient with active CD (Montreal classification: A3 L2 B3) under glucocorticoid treatment was newly diagnosed with stage C3 HIV infection.
Antiretroviral therapy was started, and CD remission was induced by the daily oral medication prescribed while she was in the hospital and continued after she was discharged (Table 2). The patient was also prescribed 80 mg/d of ferrous sulfate for chronic anemia during her hospitalization and afterward.
| Inpatient treatment | |||||
| Crohn’s disease exacerbation treatment | HIV treatment | ||||
| Prednisolone | 30 mg × 1 | 5 mg/wk | Emtricitabine/tenofovir disoproxil | 200 mg/245 mg × 1 | |
| Metronidazole | 500 mg × 3 | Dolutegravir | 50 mg × 1 | ||
| Mesalazine | 2000 mg × 2 | ||||
| Azathioprine | 50 mg × 2 | ||||
| Outpatient treatment | |||||
| Crohn’s disease treatment | HIV treatment | ||||
| Prednisolone | Eventually discontinued | Emtricitabine/tenofovir disoproxil | 200 mg/245 mg × 1 | ||
| Mesalazine | 2000 mg × 2 | Dolutegravir | 50 mg × 1 | ||
| Azathioprine | 50 mg × 2 | ||||
A month after her first hospitalization, the patient started experiencing dyspnea; so, an ambulance was called and she was taken to the hospital where she was hospitalized again in the Department of Infectious Diseases where she complained of shortness of breath lasting for about 1 wk (initially, it only occurred during physical exertion; subsequently, it started occurring when resting), hoarseness of the voice, fever (temperatures of up to 38.5 °C), phalangeal paresthesia of the limb, and general fatigue.
During her prolonged stay in the hospital (from September 25 to October 31), the patient underwent multiple examinations. The patient underwent chest X-ray and computed tomography angiography and was diagnosed with pulmonary embolism (PE), which was later treated with anticoagulant and oxygen therapy. Second, signs of pulmonary infiltration on a chest X-ray raised the suspicion of concomitant infection. Therefore, a variety of tests were performed to differentiate the possible etiological pathogens. Apparently, the cause of her illness was not only Pneumocystis jirovecii (P. jirovecii) but also Cytomegalovirus (CMV) and Epstein Barr virus (EBV). Immediate treatment with antibiotics was initiated: Trimethoprim + sulfamethoxazole (TMP + SMX) for 18 d in addition to oral corticosteroids for hypoxemia (based on a consensus statement[4]) and antipyretics if necessary. At first, the patient seemed to be getting better. However, oral candidiasis was detected after a few days. Finally, after the antibiotic course was finished, the patient experienced a new fever (temperature of 38 °C) with chills. Her blood and urine cultures were positive for Escherichia coli (E. coli) and Enterococcus faecium (E. faecium); thus, she was also diagnosed with urinary tract infection and sepsis. The clinical pharmacologist adjusted her treatment accordingly; therefore, the patient’s condition improved, despite a minor episode of conjunctivitis.
During the hospitalization period, clinicians constantly questioned how to decipher the results of the blood tests. Chronic anemia, progressing leukocytopenia, lymphocytopenia, thrombocytopenia, as well as changes in her blood count were challenging to interpret while keeping in mind the context of autoimmune inflammatory disease (CD), HIV, and immunosuppressive treatment with azathioprine, not to mention that many other infections were also present and were being treated concurrently. Due to the progression of anemia and thrombocytopenia, blood transfusions were performed (erythrocyte mass - 9 units in total, thrombocyte mass - 5 units in total). Meanwhile, to combat neutropenia, a granulocyte colony-stimulating factor (Filgrastim) was added. Intermittent diarrhea also occurred; however, her stool sample analysis revealed no etiological findings; therefore, symptomatic treatment was prescribed.
We have summarized the most relevant follow-up information (Table 3) with chronological examination reports, health status fluctuations, and the additional treatment required to address new-onset health deteriorations. With time, her serum inflammation marker levels diminished steadily, her blood cell count returned to the normal range, and the patient expressed no significant complaints. Moreover, clinically, there were no signs of CD exacerbation, and it was recommended that she refrained from taking azathioprine due to the high associated risk of pancytopenia recurrence. With the significant improvement in the patient’s clinical situation, it was decided to discharge her with antiretroviral and anticoagulant treatment. The CD treatment continued with mesalazine, and azathioprine was suspended. Not long after, the patient was referred to an infectologist, gastroenterologist, and general practitioner.
| Timeline | Remarks | Specific treatment (daily dosage) |
| September 25, 2022 | 2 background illnesses: CD and HIV | Background medication: Azathioprine 50 mg × 2 + mesalazine 2000 mg × 2. Dolutegravire 50 mg × 1. Emtricitabine + Tenofovir disoproxil 445 mg × 1 |
| PE | Oxygen (99.50%) 3 L/min (if SpO2 is < 95%). Ketorolac trometamol 30 mg (once). Calcium nadroparin 0.6 mL × 2. Amoxicilline + clavulanic acid 1200 mg × 4 | |
| September 27, 2022 | CMV DNA detected: 420 copies/mL | Ibuprofen 400 mg (if > 38.5 °C). TMP + SMX 1920 mg × 3. Prednisolone 40 mg Ã-2 to 40 mg × 1 to 20 mg × 1 |
| EBV DNA detected: 13640 copies/mL P. jirovecii DNA detected Ct value: 25.79 | ||
| September 30, 2022 | Candida Ag (mannans): Positive | Fluconazol 150 mg × 1 to 300 mg × 1 |
| October 14, 2022 | New fever episode/sepsis | Azathioprine was suspended. Treatment of pneumocystosis (TMP + SMX and prednisolone) finished. Piperacillin + tazobactame 4500 mg × 4. Amikacine 250 mg × 2 |
| October 17, 2022 | E. coli detected in microbiological blood sample cultures | Piperacillin + tazobactame 4500 mg × 4. Amikacine 250 mg × 2. Paracetamol 1000 mg × 1 (if > 38.5 °C) |
| October 18, 2022 | E. faecium detected in microbiological urine sample cultures | |
| October 19, 2022 | No clinical effect in terms of sepsis | Linezolid 600 mg × 2 added to antibiotic therapy |
| October 26, 2022 | Acute conjunctivitis of both eyes | Hypromellose 1 mg × 4 |
| October 31, 2022 | Overall conditions stabilized |
Both CD and HIV have their own specific impact on the immune system. Their pathophysiological interaction is still to be unveiled; however, there are several reasons to think that the clinical symptoms of IBD could be attenuated by the presence of HIV[5]. The authors of a similar case report and the other authors of a comprehensive study mentioned that specifically, CD4+ cells are responsible for intestinal inflammation, while HIV reduces the CD4+ count. Moreover, CD relapse rates reduce in the presence of HIV[2,6]. In our case, the patient still suffered from CD symptoms; however, during rehospitalization, the clinical manifestations were not as severe. We hope she will experience fewer relapses in the future. Also, there is a thought-provoking option to combat HIV in patients with IBD using anti-TNF-α drugs as some authors had noticed improvements; unfortunately, others could not state any benefits[7]. Maybe a cure in development, which can potentially treat both diseases, might be of help in the future, as one study shows[8]. New treatment options are truly welcome, as current management approaches remain problematic for many physicians and patients.
Speaking of the observed complications, interestingly, a previous study reported that inflammatory diseases promote coronary heart diseases; however, for HIV and IBD, this only occurs when the illnesses are severe[1]. Our patient had PE, which could also have been caused by the corticosteroids prescribed after her first exacerbation. Based on recent literature, the adverse effects of corticosteroids are more likely in patients with IBD who experience exacerbations and go through the post-hospitalization period without any anti-embolic prophylaxis[9].
Continuing on the negative note, as HIV persists in the body, other infections have a greater chance of developing, including opportunistic ones such as P. jirovecii. New data shows that IBD patients with an additional risk factor, of which HIV is the most common one, are more likely to develop pneumocystic pneumonia. However, there are currently no clear recommendations on how to prevent it in the presence of IBD and a background of HIV[3]. The authors of the report of a similar clinical case of a patient who presented with CD without HIV but was infected with P. jirovecii, mention that pneumocystic pneumonia prophylaxis is still questionable for patients with IBD, while HIV-positive people can benefit from it if their CD4+ counts are < 200 cells/μL[10]. However, there are still no clear pneumocystosis chemoprophylaxis guidelines. Therefore, the risk factors and immune system criteria should be taken into account before its publication[10]. Additionally, since CD requires a person to take immu
Finally, we want to draw clinicians’ attention to possible threats and suggest preventive measures. Similarly, as another clinical case shows, the early detection of HIV risk factors in a patient who is newly diagnosed with IBD could help initiate appropriate treatment tactics and prepare for opportunistic infections ahead of time[17]. Even if the patient is HIV-negative, other sexually transmitted infections should be tested for because their symptoms and endoscopic findings can be similar to those of IBD[2]. As mentioned earlier, PE prophylaxis could also be taken into consideration whenever corticosteroid treatment is prescribed for IBD patients with a higher PE risk[9]. Moreover, in a state of immunodeficiency, the sexually transmitted human papillomavirus (HPV) infection is more likely to cause cervical cancer[16]. The latter has already been linked to acquired immune deficiency syndrome; however, a new meta-analysis has also revealed an association between IBD and immunosuppressive drugs; hence, ill women might need to attend the cervical cancer screening program more frequently[18]. Fur
The early diagnosis of HIV in patients with IBD is crucial; however, there is currently not enough information on the interaction between these diseases. As a result, clinicians find it difficult to choose an optimal treatment strategy to achieve and maintain remission in these patients who also require careful follow-up and proper prophylactic measures against many infections. In this paper, we present the case of an HIV-infected patient with CD to encourage other professionals to adopt early preventive measures against complications for immunocompromised patients or to diagnose the illnesses early enough. We believe that some of the shared insights on the interaction between IBD and HIV could be of value.
We wish to thank the patient and Vilnius University Hospital Santaros Clinics for giving consent and providing the data to report this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Duan SL, China; Wen XL, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Sinha A, Rivera AS, Chadha SA, Prasada S, Pawlowski AE, Thorp E, DeBerge M, Ramsey-Goldman R, Lee YC, Achenbach CJ, Lloyd-Jones DM, Feinstein MJ. Comparative Risk of Incident Coronary Heart Disease Across Chronic Inflammatory Diseases. Front Cardiovasc Med. 2021;8:757738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Siwak E, Suchacz MM, Cielniak I, Kubicka J, Firląg-Burkacka E, Wiercińska-Drapało A. Inflammatory Bowel Disease in Adult HIV-Infected Patients-Is Sexually Transmitted Infections Misdiagnosis Possible? J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Schwartz J, Stein DJ, Feuerstein JD. Comprehensive National Inpatient Sample data reveals low but rising Pneumocystis jiroveci pneumonia risk in inflammatory bowel disease patients. Ann Gastroenterol. 2022;35:260-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | National Institutes of Health-University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia. Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. N Engl J Med. 1990;323:1500-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 246] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Ho TH, Cohen BL, Colombel JF, Mehandru S. Review article: the intersection of mucosal pathophysiology in HIV and inflammatory bowel disease, and its implications for therapy. Aliment Pharmacol Ther. 2014;40:1171-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Casiraghi S, Baggi P, Lanza P, Bozzola A, Vinco A, Villanacci V, Castelli F, Ronconi M. Simultaneous Diagnosis of Acute Crohn's Disease and Endometriosis in a Patient Affects HIV. Case Rep Gastrointest Med. 2018;2018:1509167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Chebli JM, Gaburri PD, Chebli LA, da Rocha Ribeiro TC, Pinto AL, Ambrogini Júnior O, Damião AO. A guide to prepare patients with inflammatory bowel diseases for anti-TNF-α therapy. Med Sci Monit. 2014;20:487-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Vautrin A, Manchon L, Garcel A, Campos N, Lapasset L, Laaref AM, Bruno R, Gislard M, Dubois E, Scherrer D, Ehrlich JH, Tazi J. Both anti-inflammatory and antiviral properties of novel drug candidate ABX464 are mediated by modulation of RNA splicing. Sci Rep. 2019;9:792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Cheng K, Faye AS. Venous thromboembolism in inflammatory bowel disease. World J Gastroenterol. 2020;26:1231-1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Omer O, Cohen P, Neong SF, Smith GV. Pneumocystis pneumonia complicating immunosuppressive therapy in Crohns disease: A preventable problem? Frontline Gastroenterol. 2016;7:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Wisniewski A, Kirchgesner J, Seksik P, Landman C, Bourrier A, Nion-Larmurier I, Marteau P, Cosnes J, Sokol H, Beaugerie L; the Saint-Antoine IBD network. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United European Gastroenterol J. 2020;8:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Neurath M. Thiopurines in IBD: What Is Their Mechanism of Action? Gastroenterol Hepatol (N Y). 2010;6:435-436. [PubMed] |
| 13. | Mushtaq K, Khan Z, Aziz M, Alyousif ZA, Siddiqui N, Khan MA, Nawras A. Trends and outcomes of fungal infections in hospitalized patients of inflammatory bowel disease: a nationwide analysis. Transl Gastroenterol Hepatol. 2020;5:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Stamatiades GA, Ioannou P, Petrikkos G, Tsioutis C. Fungal infections in patients with inflammatory bowel disease: A systematic review. Mycoses. 2018;61:366-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Sharif M, Saddique MU, Zahid M, Khan D, Bashir T, Murshed K. Oropharyngeal Candidiasis as a Presenting Symptom of Crohn's Disease. Case Rep Gastroenterol. 2020;14:178-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Santiago-Rodriguez TM, Hollister EB. Human Virome and Disease: High-Throughput Sequencing for Virus Discovery, Identification of Phage-Bacteria Dysbiosis and Development of Therapeutic Approaches with Emphasis on the Human Gut. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 17. | Davis W, Vavilin I, Malhotra N. Biologic Therapy in HIV: To Screen or Not to Screen. Cureus. 2021;13:e15941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 18. | Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflamm Bowel Dis. 2015;21:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Zizza A, Banchelli F, Guido M, Marotta C, Di Gennaro F, Mazzucco W, Pistotti V, D'Amico R. Efficacy and safety of human papillomavirus vaccination in HIV-infected patients: a systematic review and meta-analysis. Sci Rep. 2021;11:4954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Jordan A, Mills K, Sobukonla T, Kelly A, Flood M. Influenza, PCV13, and PPSV23 Vaccination Rates Among Inflammatory Bowel Disease Patients With Additional Co-Morbidities as per CDC Recommendations. Cureus. 2021;13:e18387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Besombes J, Souala F, Bouguen G, Guyader D, Grolhier C, Thibault V, Pronier C. Acute hepatitis B virus infection despite vaccination in a patient treated by infliximab: a case report. BMC Gastroenterol. 2022;22:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |