Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4133
Peer-review started: March 12, 2023
First decision: April 20, 2023
Revised: April 27, 2023
Accepted: May 12, 2023
Article in press: May 12, 2023
Published online: June 16, 2023
Processing time: 91 Days and 17.1 Hours
Klippel-Trenaunay syndrome (KTS) is a rare congenital disorder characterized by a combination of capillary malformations, soft-tissue or bone hypertrophy, and varicose veins or venous malformations. The syndrome predisposes patients to hypercoagulable states, including venous thromboembolism and pulmonary embolism (PE).
A 12-year-old girl with KTS was scheduled excision of verrucous hyperkeratosis in the left foot and posterior aspect of the left leg and left thigh and excision of a cutaneous hemangioma in the right buttock. After induction, the surgeon elevated the patient’s leg for sterilization, whereupon she experienced a massive PE and refractory cardiac arrest. Extracorporeal membrane oxygenation (ECMO) was performed after prolonged resuscitation, and she had a return of spontaneous circulation. After this episode, the patient was discharged without any neurologic complications.
The mechanism of PE, a lethal disease, involves a preexisting deep vein throm
Core Tip: Klippel-Trenaunay syndrome (KTS) is a congenital vascular disorder. Patients with KTS are at high risk for pulmonary embolism. All patient having KTS should be evaluated of lower limb circulation and pre-existing deep vein thrombosis (DVT) pre-operatively. Also, all patients with KTS should receive DVT prophylaxis at least 8 hours prior to surgery irrespective of the age. Care should be taken to monitor for PE in patients with KTS while leg raising for sterilization. Anesthesiologists should consider potential difficulties in managing the airway and avoid neuraxial anesthesia.
- Citation: Lo CY, Chen KB, Chen LK, Chiou CS. Massive pulmonary embolism in Klippel-Trenaunay syndrome after leg raising: A case report. World J Clin Cases 2023; 11(17): 4133-4141
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4133.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4133
Klippel-Trenaunay syndrome (KTS) is a rare congenital disorder and diagnosis of KTS is established when two of the triad features, viz. capillary malformation (port wine stains), hypertrophy of soft tissue or bones and varicose veins, are present. Vascular malformations involve the lower limbs, gastro
A 12-year-old girl with KTS (height, 124 cm; weight, 20 kg) was referred to our hospital for recurrent left lower limb lymphangioma since the age of eight years.
She presented with clitoral hypertrophy, hemihypertrophy in the left lower limb along with varicose veins and multiple lipomas, and gastrointestinal bleeding. She was admitted for excision of verrucous hyperkeratosis on the left foot and posterior aspect of the left leg and thigh and excision of a cutaneous hemangioma on the right buttock.
Before induction, the patient was irritably crying in her father's arms at the time. The patient’s vital signs were as follows: Heart rate, 111 beats/min; blood pressure, 127/75 mmHg; saturation, 100%; respiratory rate, 18 breaths/min; and body temperature, 36.2°C. After preoxygenation, lidocaine (20 mg), propofol (50 mg), and cisatracurium (4 mg) were administered, and a 5.5 mm cuffed endotracheal tube was inserted. Monitoring was established using standard monitors and an intra-arterial line. Anesthesia was maintained with 45% oxygen and 2% sevoflurane.
After induction, the vital signs were as follows: Saturation, 100%; blood pressure, 97/61 mmHg; heart rate, 113 beats/min; respiratory rate, 17 breaths/min; tidal volume, 200 mL (controlled by ventilator); and body temperature, 36.2°C. Ten minutes after induction, her baseline blood gas test showed pCO2, 26 mmHg; pO2, 216.2 mmHg; HCO3-, 19.0 mmol/L; and Hb, 8.4. Sixty minutes after induction, the surgeon elevated the patient’s leg for sterilization. Her end-tidal CO2 dropped from 35 to 7 mmHg within a few minutes. She had hypotension and bradycardia; therefore, atropine and epinephrine were sequentially administered intravenously. The ABG analysis was repeated, and testing revealed acidosis (pH, 7.263; pCO2, 42.4 mmHg; pO2, 69.4 mmHg; HCO3-, 19.4 mmol/L; lactate, 1.2 mmol/L; and Ca2+, 1.04 mmol/L). Sixty-three minutes after induction, the patient have a cardiac arrest and cardiopulmonary cerebral resuscitation (CPCR) was started. Seventy-eight minutes after induction, a return of spontaneous circulation occurred, and transesophageal echocardiography (TEE) was performed. TEE revealed severe right ventricular distension and little blood flow to the right and left pulmonary arteries. Eighty-six minutes after induction, the patient had a second cardiac arrest and CPCR was started again. Ninety minutes after induction, a right femoral 5 French-size two-way central line was placed at 13 cm. A cardiac surgeon was consulted who conducted ECMO 106 min after induction. Thirty minutes after ECMO, a normal sinus rhythm returned. CPCR was performed for approximately 70 min. The cardiac surgeon started target temperature management (TTM) of 32°C to protect the patient’s brain. Due to the incident of severe PE, the surgery was abandoned. Before getting transferred to the pediatric intensive care unit (PICU), her vital signs were as follows: Heart rate, 112 beats/min; blood pressure, 119/100 mmHg; saturation, 100%; respiratory rate, 17 breaths/min; and body temperature, 31.2°C (Figure 1).
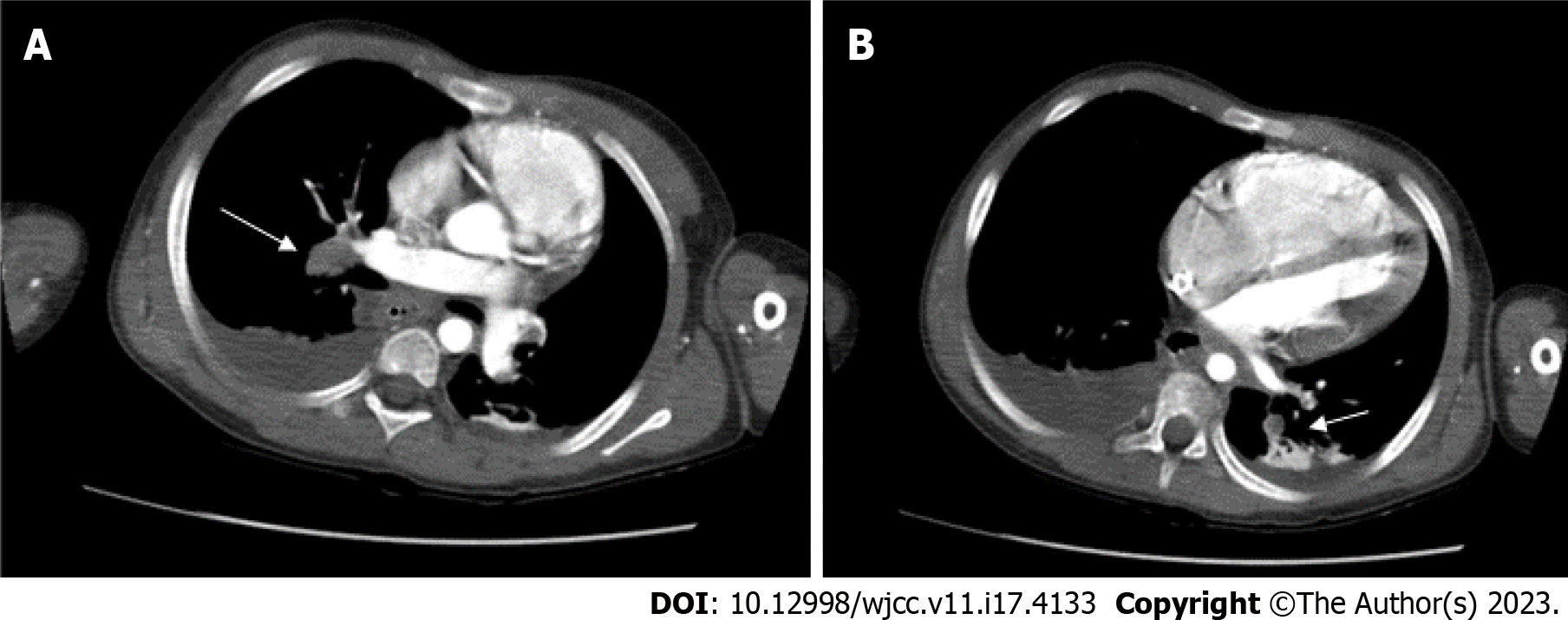
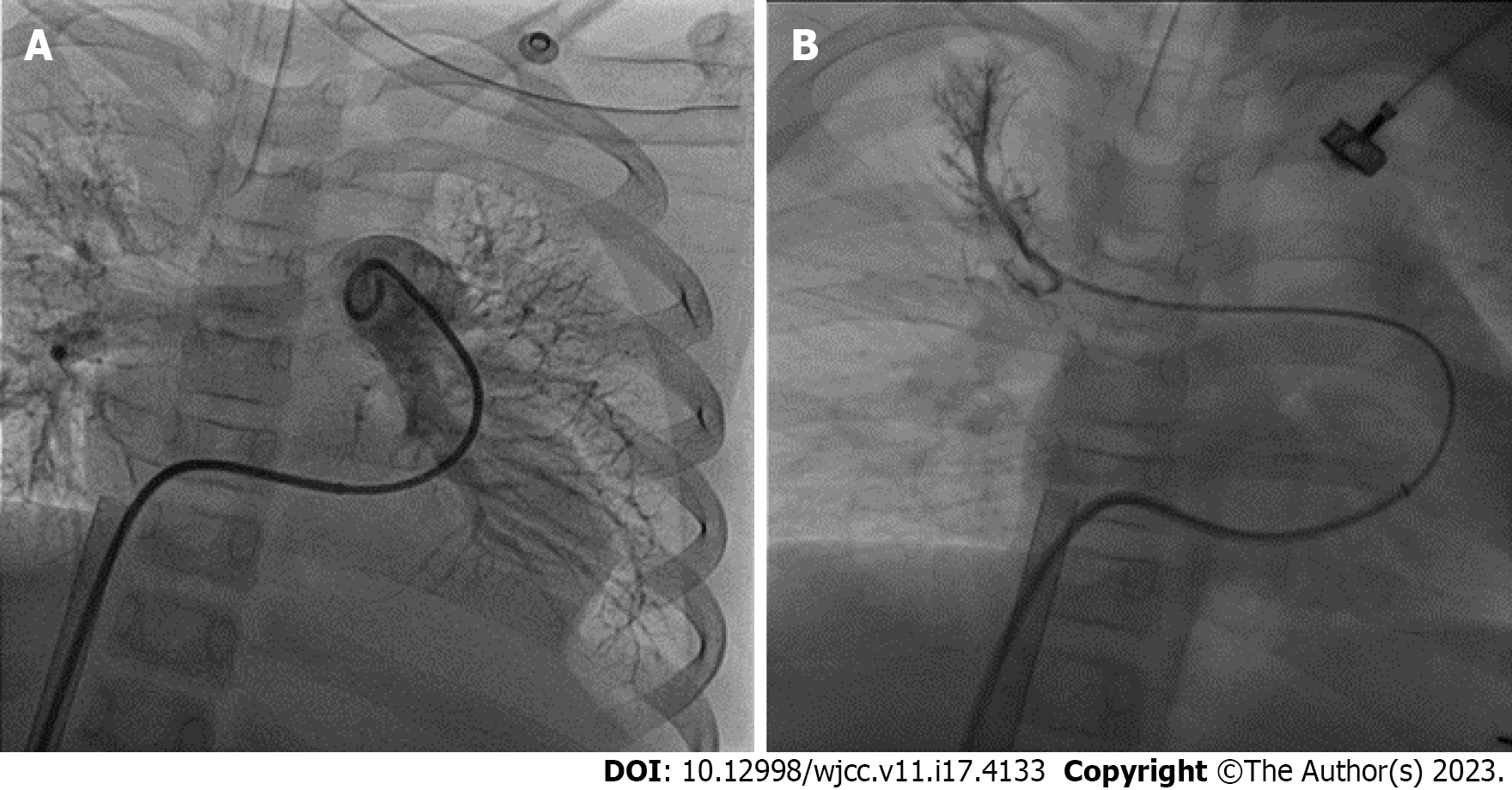
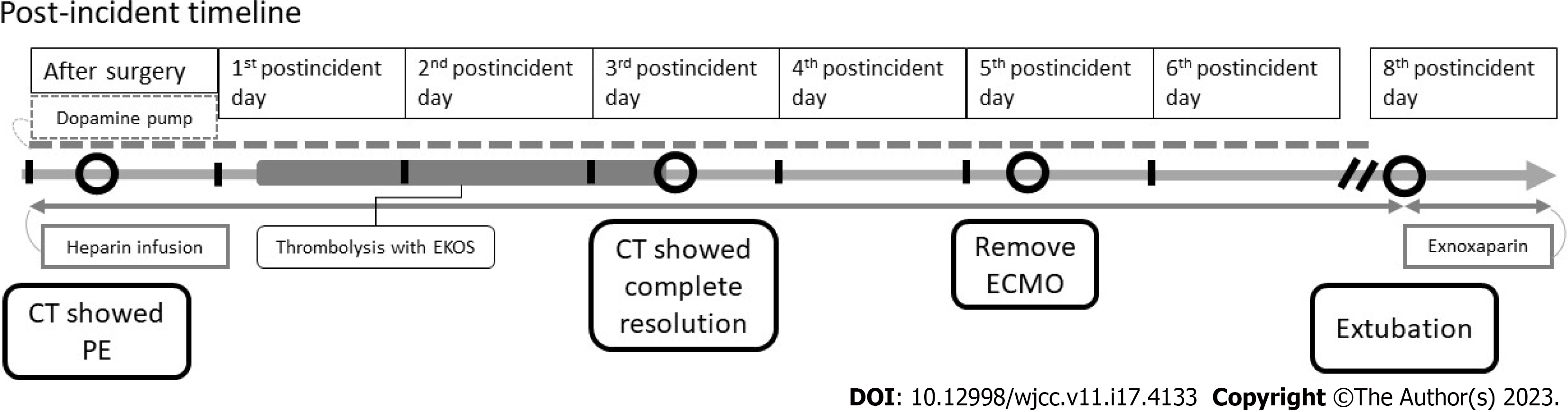
Initially, in the PICU, her bilateral pupil was dilated approximately 9 mm with no light reflex. She was under sedation with a midazolam pump, as well as a dopamine (10 mcg/kg/min) and heparin pump. Computed tomography (CT) performed three hours after the surgery revealed PEs in the left truncus anterior, right interlobar, bilateral lower lobar, and segmental pulmonary arteries (Figure 2). Laboratory data showed elevated D-dimer (7939.76 ng/mL) and decreased fibrinogen (203.3 mg/dL) levels. Six hours later, her body temperature had risen from 31.2°C to 35°C, and her bilateral pupil size was 5 mm with a light reflex. A cardiologist was consulted for thrombolysis six hours after the surgery. Ultrasound-facilitated catheter thrombolysis with the Ekosonic Endovascular System (EKOS) was performed via the right femoral vein to the right upper pulmonary arteryand left lower pulmonary artery for 48 h from the morning of the first post-incident day (Figure 3). On the third post-incident day, a post-procedure CT showed complete resolution of the PEs. The patient was weaned from ECMO on the fifth postoperative day, extubated on the eighth post-incident day, and prophylaxis treatment with enoxaparin was provided. Two months later, the patient was discharged without any neurologic complications (Figure 4). The patient had received the surgery 3 months later after the episode
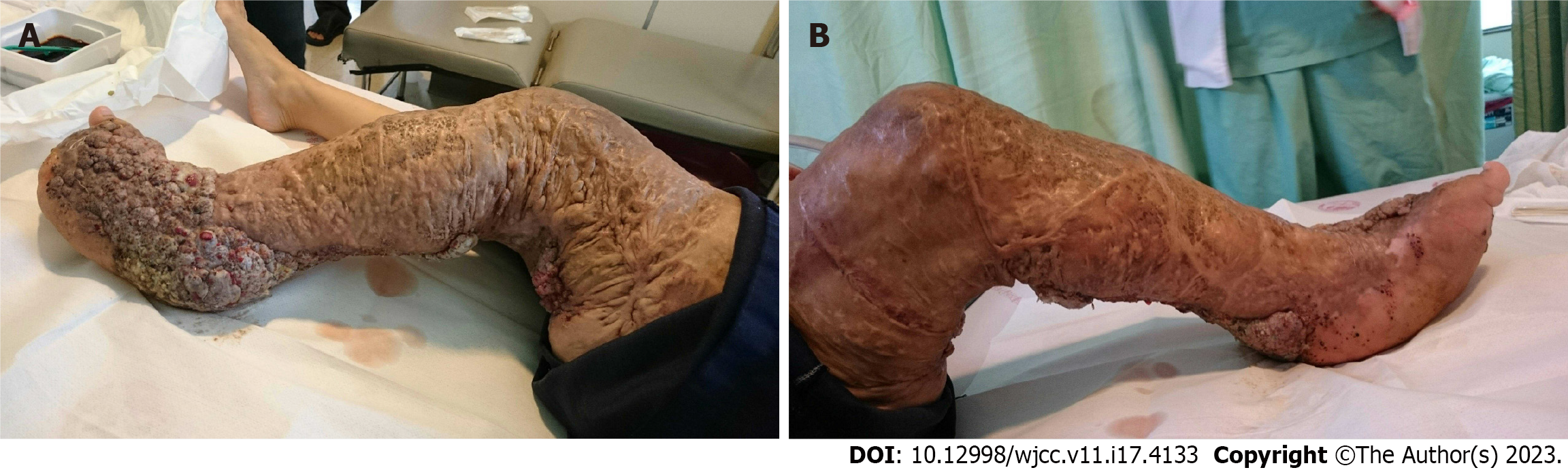
Four years ago, she had undergone extensive resection of the vascular malformations in the left lower limb, including amputation of the lateral fourth toe on her left foot (Figure 5). There was no record of difficulty in intubation.
There was no specific personal or family history.
Physical examination presented with clitoral hypertrophy, hemihypertrophy in the left lower limb.
Preoperative laboratory data showed anemia (hemoglobulin: 9.2 g/dL, mean corpuscular volume: 66.9 fL) no thrombocytopenia or coagulopathy (platelet count, 379000/μL; prothrombin time, 11.1 s; international normalized ratio, 1.07; and partial thromboplastin time, 27.9 s), liver and renal function were within normal limit, C-reactive protein: 7.43 mg/L.
The preoperative CT revealed angiomatosis extending from the left pelvis to the left foot, pelvic hemangiomas involving the rectum, a splenic lymphangioma, and engorged epidural vessels on the left side of the sacral canal. There is no obvious deep vein thrombosis (DVT) on the preoperative CT.
Klippel-Trenaunay syndrome complicated by refractory pulmonary embolism.
The patient underwent ECMO and received ultrasound-facilitated catheter thrombolysis with the EKOS.
The patient was discharged without any neurologic complications.
KTS was first described by two French doctors, Klippel and Trenaunay (1900). KTS is a congenital vascular disorder characterized by a triad of main symptoms affecting one or more limbs, namely cutaneous hemangiomas, varicose veins, and bone and soft tissue hypertrophy. Approximately 95% of patients have lower limb malformations[2]. If capillary malformations are sufficiently large, the cutaneous lesions may sequester platelets[3]. In addition, large varicose veins may cause localized intravascular coagulation, and many patients have hematologic evidence of coagulopathy, defined by elevated D-dimer and soluble fibrin complex levels, decreased fibrinogen levels, and elevated prothrombin times, with normal to moderately low platelet counts[4]. Therefore, patients with KTS have a known significantly increased risk for PE[5].
PE while leg raising for sterilization before surgery is rare. The mechanism of PE is that a preexisting deep vein thrombosis is mechanically dislodged by compression or changing positions and travels to the pulmonary artery. Patients can be stratified before surgery based on the clinical prediction scoring system, the Modified Well’s criteria (Table 1) recommended by the European Society of Cardiology and the American College of Chest Physicians (ACCP), which standardizes the diagnosis and management of acute PEs. Our patient’s score of the Modified Well’s criteria is 1.5. However, the Modified Wells' criteria may not be applicable to patients with KTS, as the score may not accurately reflect the patient's high risk for pulmonary embolism[6]. Patients with KTS are at high risk and require a series of examinations, such as ultrasonography, CT, or magnetic resonance imaging. In some case reports[7-9], the thrombi can dislodge from the vein by leg massage, probe compression, and application of sterile elastic exsanguination tourniquets. If thromboembolism is suspected, prevention using anticoagulants and inferior vena cava (IVC) filters might be indicated. However, prophylactic anticoagulants are not prescribed in some Asian regions, and studies have reported a lower incidence of VTE in the Asian population than in Caucasians[10,11]. Although data on pediatric thromboembolism prophylaxis are limited, a systemic review demonstrated that low-molecular-weight heparin is safe and effective in children[12]. The IVC filter is ineffective in KTS because of an anomalous venous connection between the lower extremities and the IVC, effectively bypassing the filter[13]. Considering the risk of lower limb thrombophlebitis associated with venous anomalies, femoral cannulation is better avoided[14].
| Modified well’s criteria | Points |
| Clinical symptoms of DVT | 3 |
| PE the most likely diagnosis | 3 |
| Heart rate > 100/min | 1.5 |
| Immobilization or surgery with 4 weeks | 1.5 |
| Previous DVT or PE | 1.5 |
| Hemoptysis | 1 |
| Malignancy | 1 |
If PE is suspected intraoperatively, supportive care should be started in unstable patients, including ventilation with 100% O2, use of positive end-expiratory pressure, resuscitation of the circulating fluid volume, and administration of inotropic drugs as early as possible. In addition, invasive monitors should be placed for diagnosis and management, including arterial and central venous lines, transthoracic echocardiography, and trans-esophageal echocardiogram, which is generally considered the primary diagnostic technique for identifying intraoperative PE because of its high safety, availability, and utility in the operating room and its lack of interference with resuscitation efforts[15]. If vital signs are profoundly unstable, cardiopulmonary cerebral resuscitation should be performed, and a cardiac surgeon should be consulted for cardiopulmonary bypass or venoarterial ECMO. Extracorporeal life support is an effective therapy for unstable patients, offering acceptable outcomes; however, these studies lacked pediatric patients. The American Heart Association guidelines, updated for pediatric advanced life support in 2019[16], recommend ECPR for pediatric patients with cardiac diagnoses and an in-hospital cardiac arrest in settings with existing ECMO protocols, expertise, and equipment. For infants and children who remain comatose after out-of-hospital or in-hospital cardiac arrest, either TTM from 32°C to 34°C followed by TTM of 36°C to 37.5°C or TTM of 36°C to 37.5°C can be used. There were no significant differences in the outcomes between the two TTM groups in the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials[17].
Postoperatively, the patient should be admitted to the intensive care unit, and CT pulmonary angiography should be performed. If there is no major bleeding, anticoagulant treatment should be started as soon as possible, and experts should be consulted for systemic or catheter-directed thrombolysis.
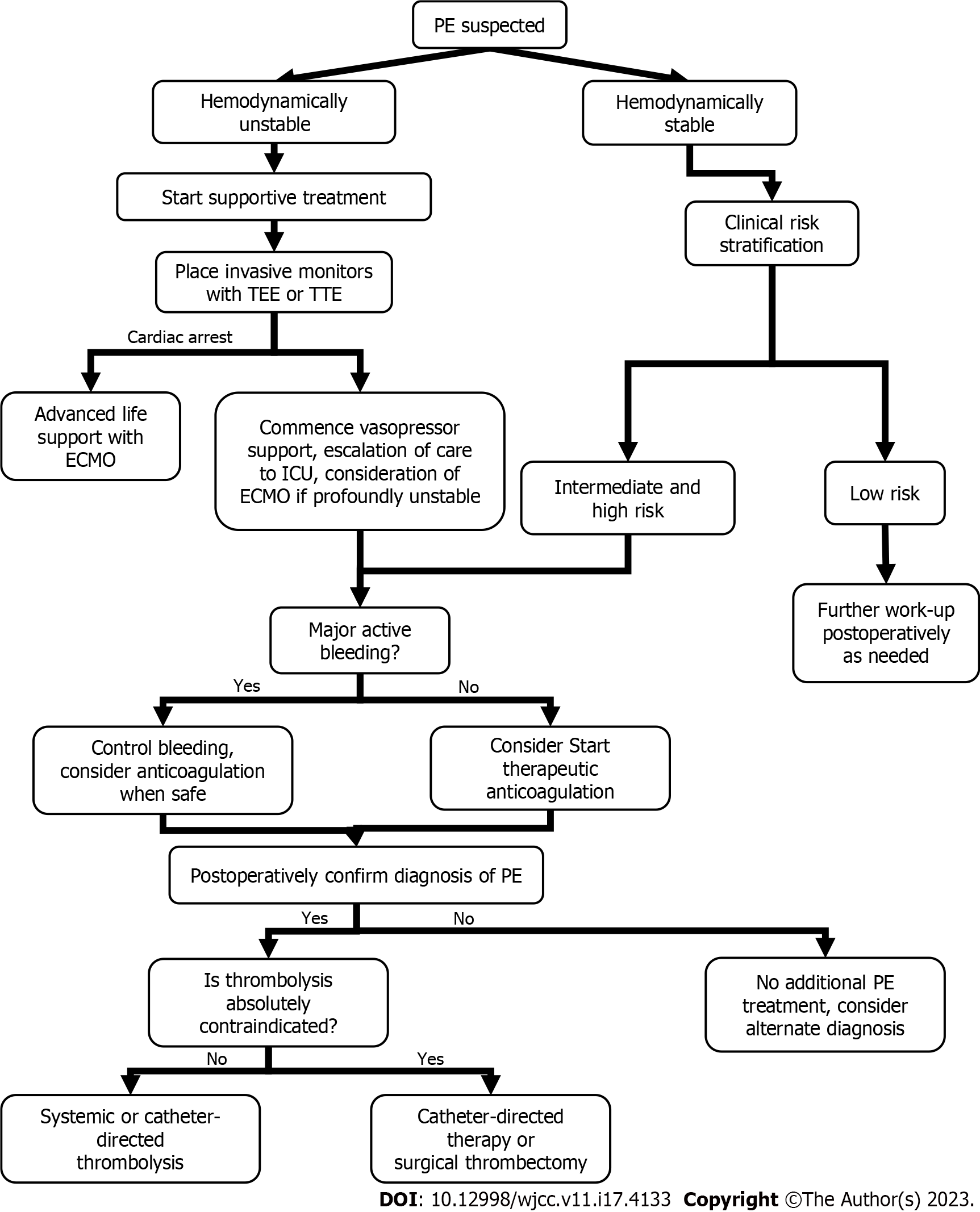
In current standard therapy, if the patient has no major active bleeding, the ACCP recommends immediate initiation of parenteral anticoagulation using low-molecular-weight heparin and fondaparinux, as they are superior to intravenous unfractionated heparin, owing to a lower risk of adverse bleeding events and the absence of need for serial laboratory monitoring. However, there are no guidelines or group consensus for therapies for intraoperative PE. Therefore, the authors organized a reference algorithm for intraoperative PE management (Figure 6).
Despite the lack of established strategies for anesthetic management of KTS patients undergoing surgery, some preoperative evaluations have been recommended. Firstly, anesthesiologists should consider the potential difficulties in managing the airway due to soft tissue hypertrophy in the mouth, hypopharynx, and facial anomalies commonly observed in KTS patients. Secondly, neuraxial anesthesia should be avoided as it is contraindicated due to the possibility of neurovascular malformations in the spinal cord and surrounding structures. It must be noted that central regional blockade can be performed safely in KTS patients, provided that vascular malformations in the central nervous system have been ruled out through computed tomography/magnetic resonance imaging and that there are no cutaneous lesions at the site of needle insertion. Thirdly, the risk of excessive intraoperative blood loss should always be taken into consideration, even in minor surgeries, due to the presence of widespread varicosities and venous malformations. Fourthly, KTS patients have a relatively high risk of developing venous thrombosis and pulmonary thromboembolism. Chronic coagulopathy such as disseminated intravascular coagulation can also occur in these patients. Finally, intracerebral aneurysm is a potential complication in KTS patients, which can rupture during the perioperative period[14,18,19].
KTS is a congenital vascular disorder that primarily affects the lower limbs and is characterized by cutaneous hemangiomas, varicose veins, and bone and soft tissue hypertrophy. Patients with KTS are at high risk for PE. All patient having KTS should be evaluated of lower limb circulation and pre-existing DVT pre-operatively. Also, all patients with KTS should receive DVT prophylaxis at least 8 h prior to surgery irrespective of the age. The Modified Wells' criteria may not be applicable to patients with KTS. Care should be taken to monitor for PE in patients with KTS while leg raising for sterilization. Intraoperative PE is lethal, and the management of hemodynamically unstable patients requires efficient CPR and early mechanical support. Anesthesiologists should consider potential difficulties in managing the airway and avoid neuraxial anesthesia. Central regional blockade can be performed safely in KTS patients if vascular malformations in the central nervous system have been ruled out.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: DeSousa K, India; Viswanathan VK, United States S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Alwalid O, Makamure J, Cheng QG, Wu WJ, Yang C, Samran E, Han P, Liang HM. Radiological Aspect of Klippel-Trénaunay Syndrome: A Case Series With Review of Literature. Curr Med Sci. 2018;38:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Jacob AG, Driscoll DJ, Shaughnessy WJ, Stanson AW, Clay RP, Gloviczki P. Klippel-Trénaunay syndrome: spectrum and management. Mayo Clin Proc. 1998;73:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 264] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Cha SH, Romeo MA, Neutze JA. Visceral manifestations of Klippel-Trénaunay syndrome. Radiographics. 2005;25:1694-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Mazoyer E, Enjolras O, Laurian C, Houdart E, Drouet L. Coagulation abnormalities associated with extensive venous malformations of the limbs: differentiation from Kasabach-Merritt syndrome. Clin Lab Haematol. 2002;24:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Samuel M, Spitz L. Klippel-Trenaunay syndrome: clinical features, complications and management in children. Br J Surg. 1995;82:757-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 99] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Ndzengue A, Rafal RB, Balmir S, Rai DB, Jaffe EA. Klippel-trenaunay syndrome: an often overlooked risk factor for venous thromboembolic disease. Int J Angiol. 2012;21:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Sutham K, Na-Nan S, Paiboonsithiwong S, Chaksuwat P, Tongsong T. Leg massage during pregnancy with unrecognized deep vein thrombosis could be life threatening: a case report. BMC Pregnancy Childbirth. 2020;20:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Sufian S, Arnez A, Lakhanpal S. Case of the disappearing heat-induced thrombus causing pulmonary embolism during ultrasound evaluation. J Vasc Surg. 2012;55:529-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Feldman V, Biadsi A, Slavin O, Kish B, Tauber I, Nyska M, Brin YS. Pulmonary Embolism After Application of a Sterile Elastic Exsanguination Tourniquet. Orthopedics. 2015;38:e1160-e1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Lee CH, Lin LJ, Cheng CL, Kao Yang YH, Chen JY, Tsai LM. Incidence and cumulative recurrence rates of venous thromboembolism in the Taiwanese population. J Thromb Haemost. 2010;8:1515-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Liew NC, Alemany GV, Angchaisuksiri P, Bang SM, Choi G, DE Silva DA, Hong JM, Lee L, Li YJ, Rajamoney GN, Suviraj J, Tan TC, Tse E, Teo LT, Visperas J, Wong RS, Lee LH. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol. 2017;36:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Klaassen ILM, Sol JJ, Suijker MH, Fijnvandraat K, van de Wetering MD, Heleen van Ommen C. Are low-molecular-weight heparins safe and effective in children? A systematic review. Blood Rev. 2019;33:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 13. | Huiras EE, Barnes CJ, Eichenfield LF, Pelech AN, Drolet BA. Pulmonary thromboembolism associated with Klippel-Trenaunay syndrome. Pediatrics. 2005;116:e596-e600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | George SE, Sreevidya A, Asokan A, Mahadevan V. Klippel Trenaunay syndrome and the anaesthesiologist. Indian J Anaesth. 2014;58:775-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Dudaryk R, Benitez Lopez J, Louro J. Diagnosis and Thrombolytic Management of Massive Intraoperative Pulmonary Embolism Guided by Point of Care Transthoracic Echocardiography. Case Rep Anesthesiol. 2018;2018:8709026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Duff JP, Topjian AA, Berg MD, Chan M, Haskell SE, Joyner BL Jr, Lasa JJ, Ley SJ, Raymond TT, Sutton RM, Hazinski MF, Atkins DL. 2019 American Heart Association Focused Update on Pediatric Advanced Life Support: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2019;140:e904-e914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Browning B, Pemberton VL, Page K, Gildea MR, Scholefield BR, Shankaran S, Hutchison JS, Berger JT, Ofori-Amanfo G, Newth CJ, Topjian A, Bennett KS, Koch JD, Pham N, Chanani NK, Pineda JA, Harrison R, Dalton HJ, Alten J, Schleien CL, Goodman DM, Zimmerman JJ, Bhalala US, Schwarz AJ, Porter MB, Shah S, Fink EL, McQuillen P, Wu T, Skellett S, Thomas NJ, Nowak JE, Baines PB, Pappachan J, Mathur M, Lloyd E, van der Jagt EW, Dobyns EL, Meyer MT, Sanders RC Jr, Clark AE, Dean JM; THAPCA Trial Investigators. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N Engl J Med. 2017;376:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 18. | Lee JH, Chung HU, Lee MS. An anesthetic management of a patient with Klippel-Trenaunay syndrome. Korean J Anesthesiol. 2012;63:90-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Barbara DW, Wilson JL. Anesthesia for surgery related to Klippel-Trenaunay syndrome: a review of 136 anesthetics. Anesth Analg. 2011;113:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |