Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4098
Peer-review started: March 10, 2023
First decision: April 26, 2023
Revised: April 30, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: June 16, 2023
Processing time: 93 Days and 16.1 Hours
Massive pulmonary embolism (PE) results in extremely high mortality rates. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) can provide circulatory and oxygenation support and rescue patients with massive PE. However, there are relatively few studies of extracorporeal cardiopulmonary resuscitation (ECPR) in patients with cardiac arrest (CA) secondary to PE. The aim of the present study is to investigate the clinical use of ECPR in conjunction with heparin anticoagulation in patients with CA secondary to PE.
We report the cases of six patients with CA secondary to PE treated with ECPR in the intensive care unit of our hospital between June 2020 and June 2022. All six patients experienced witnessed CA whilst in hospital. They had acute onset of severe respiratory distress, hypoxia, and shock rapidly followed by CA and were immediately given cardiopulmonary resuscitation and adjunctive VA-ECMO therapy. During hospitalization, pulmonary artery computed tomography angiography was performed to confirm the diagnosis of PE. Through anticoagulation management, mechanical ventilation, fluid management, and antibiotic treatment, five patients were successfully weaned from ECMO (83.33%), four patients survived for 30 d after discharge (66.67%), and two patients had good neurological outcomes (33.33%).
For patients with CA secondary to massive PE, ECPR in conjunction with heparin anticoagulation may improve outcomes.
Core Tip: Massive pulmonary embolism (PE) remains the leading clinical cause of death. Unfortunately, relatively few new technologies are available to reduce the morbidity and mortality of massive PE. Anticoagulation therapy is still the gold standard treatment for PE. In this study, we report the clinical details of six patients with cardiac arrest secondary to massive PE treated with extracorporeal cardiopulmonary resuscitation (ECPR) in conjunction with heparin anticoagulation. Our findings suggest that the use of ECPR is feasible in this cohort of patients and may improve resuscitation success rate and neurologically intact survival.
- Citation: Qiu MS, Deng YJ, Yang X, Shao HQ. Cardiac arrest secondary to pulmonary embolism treated with extracorporeal cardiopulmonary resuscitation: Six case reports. World J Clin Cases 2023; 11(17): 4098-4104
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4098.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4098
Massive pulmonary embolism (PE) is an obstructive shock that can lead to right ventricular afterload and hemodynamic instability. The overall mortality rate of PE can be as high as 50% and can even reach 52%–84% for patients with cardiac arrest (CA) secondary to PE. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) can provide necessary circulatory and oxygenation support and is an effective means of rescuing patients with massive PE[1,2]. A multifactorial regression analysis by Hobohm et al[3] showed that patients with PE who developed CA and were treated with VA-ECMO alone had a lower risk of in-hospital death [odds risk (OR) = 0.68, 95%CI: 0.57–0.82, P < 0.001) than patients who were treated with thrombolysis alone (OR = 1.04, 95%CI: 0.99–1.01, P = 0.116). However, most current studies of patients with CA secondary to PE who underwent VA-ECMO were conducted in patients with return of spontaneous circulation prior to VA-ECMO, and there are relatively few studies examining the use of extracorporeal cardiopulmonary resuscitation (ECPR) in patients with CA secondary to PE[4]. Here, we review the clinical data of six patients admitted to our hospital between June 2020 and June 2022 who underwent ECPR for CA secondary to PE, as well as the experience of other researchers in the existing literature.
Patients presented with acute onset severe respiratory distress, hypoxia, and shock rapidly followed by cardiac arrest.
We included a total of six patients admitted to the intensive care unit (ICU) of Dongguan People's Hospital between June 2020 and June 2022 who received ECPR for CA secondary to massive PE in the study. The demographic characteristics, laboratory test results, imaging findings, ECMO data, and clinical outcomes of patients were collected. Data are presented in Tables 1–3.
| Case | Sex | Age (yr) | BMI (kg/m2) | Underlying disease | Predisposing factors | ||||||
| Hypertension | Diabetes | Hyperlipidemia | Recent hospitalization | Cancer | Trauma | Travel | Thrombosis | ||||
| 1 | F | 63 | 19.48 | N | N | N | Y | N | Y | N | Y |
| 2 | F | 68 | 31.25 | N | Y | Y | N | N | N | N | Y |
| 3 | F | 73 | 17.58 | N | N | N | N | N | N | N | Y |
| 4 | F | 51 | 23.43 | Y | Y | N | Y | N | Y | N | N |
| 5 | F | 40 | 20.54 | N | N | N | N | N | N | Y | Y |
| 6 | M | 47 | 24.8 | N | N | N | N | Y | N | N | Y |
Two patients (33.33%) had at least one underlying disease (including hypertension, diabetes, hyperlipidemia), and all patients had at least one pre-disposing factor for thrombosis (including recent hospitalization, cancer, trauma, travel, or thrombosis) (Table 1).
The personal and family history was determined to be noncontributory.
All patients were experienced witnessed CA in hospital. They lost consciousness, no heartbeat was apparent, and blood pressure was undetectable.
The patients had significantly elevated D-dimer and lactate levels at the time of transfer to the ICU. Four patients (66.67%) had acute kidney injury requiring continuous renal replacement therapy (Table 2).
| Case | Laboratory indexes | RV/LV | CT | CRRT | APACHE II score | SOFA score | ||||
| D-dimer (μg/mL) | CK-MB (U/L) | Troponin (ng/ml) | BNP (pg/mL) | Lactate (mmol/L) | ||||||
| 1 | > 20 | 2.7 | 0.04 | 778 | 12 | 25/43 | Emboli in multiple pulmonary artery branches in the distal left main pulmonary artery and multiple emboli in the bilateral pulmonary artery branches | Y | 24 | 14 |
| 2 | 1.97 | 9.7 | 0.182 | 275 | 13.1 | 35/34 | Emboli in the bilateral main pulmonary arteries | N | 38 | 13 |
| 3 | > 20 | 41.6 | 5.119 | 42 | > 15 | 26/34 | Emboli in the distal right main pulmonary artery trunk and multiple emboli in the bilateral pulmonary artery branches | N | 47 | 16 |
| 4 | 3.55 | 81.9 | 0.193 | 119 | 11.8 | 23/51 | Emboli in the bilateral lower lobar arteries | Y | 46 | 16 |
| 5 | > 20 | 263.6 | 0.31 | 643 | 14.22 | 36/26 | Emboli in multiple pulmonary artery branches | Y | 33 | 13 |
| 6 | 7.42 | 151.6 | 0.05 | 225 | 9 | 42/39 | Emboli in the distal pulmonary trunk, left and right main pulmonary arteries, and bilateral pulmonary artery branches | Y | 30 | 15 |
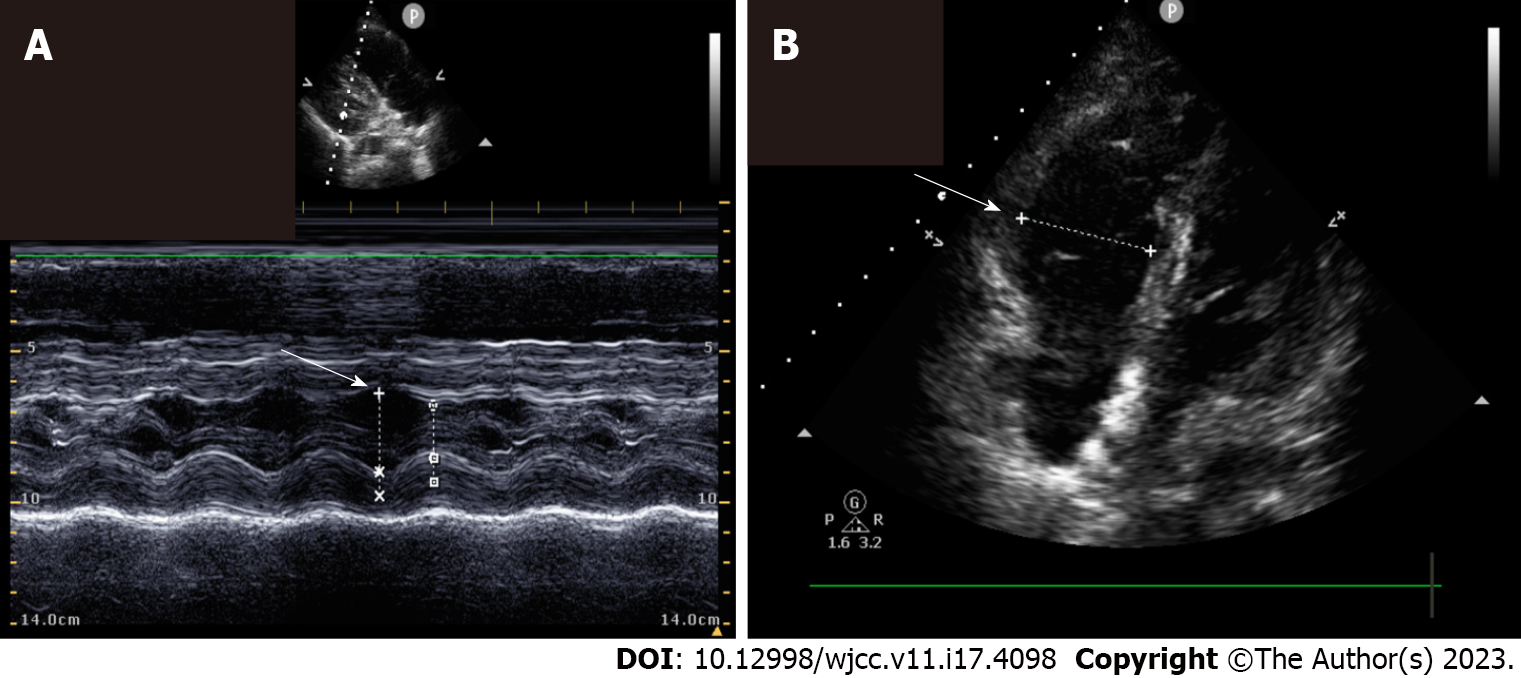
Diagnosis of PE was confirmed in all patients by computed tomography (CT)-pulmonary angiography. Echocardiographic findings revealed a right ventricle/Left ventricle ratio > 1 in three patients (50%) (Table 2). Example echocardiographic images are presented in Figure 1.
Considering the patients’ history and CT-pulmonary angiography results, all six patients were diagnosed with CA secondary to PE.
With reference to an expert consensus[5], patient selection and intervention timing were as follows: (1) Patients aged 18–75 years; (2) CA witnessed by medical personnel, with effective cardiopulmonary resuscitation (CPR) implemented and a no-flow time < 5 min; (3) Standard CPR > 10 min without return of spontaneous circulation > 1 min; and (4) able to initiate ECPR within 60 min. Exclusion criteria were as follows: (1) Concomitant severe irreversible or advanced disease, such as cancer and advanced cirrhosis; and (2) Uncontrolled bleeding from trauma. This study was approved by the Clinical Ethics Committee of Dongguan People’s Hospital, No. KYKT2021-028.
When the criteria were met, all patients were immediately given CPR and VA-ECMO therapy. None of the patients underwent thrombolysis, surgical embolization, or percutaneous catheter-directed therapy. After all cases in the present study were successfully initiated onto ECMO, their coagulation status was evaluated. Cerebral hemorrhage was ruled out by cranial CT examination, and heparin therapy was initially administered by continuous intravenous infusion at 4–10 U/kg/h. The activated clotting time can guide the dosage of heparin, with a target of 180–220 s or maintaining the activated partial thromboplastin time at 1.5–2.5 times the normal value.
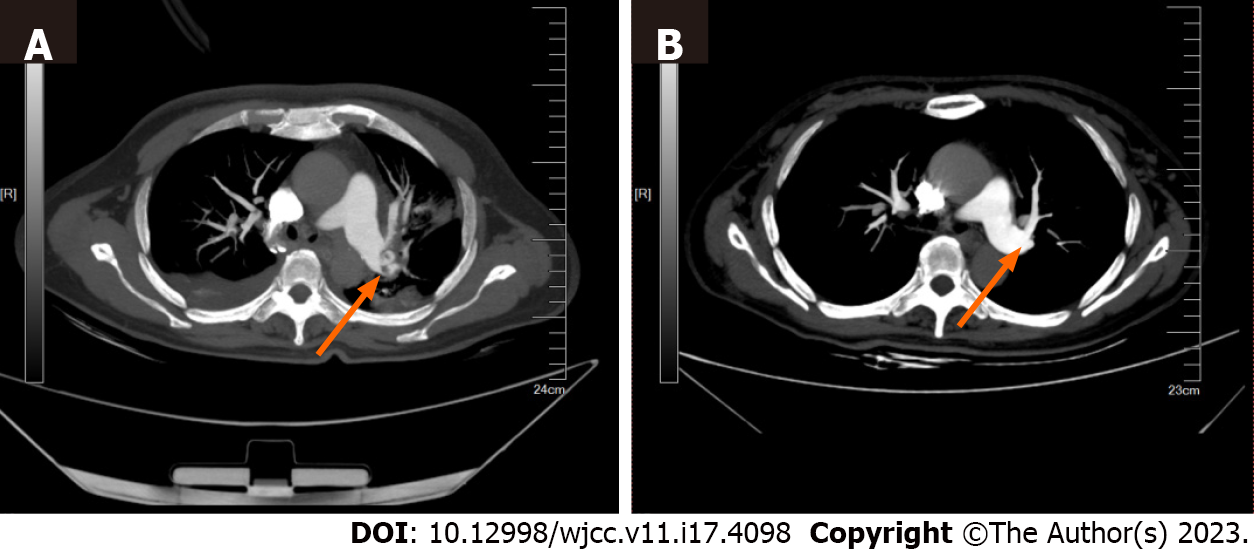
The median low-flow time of the six patients was 41.5 min [interquartile range (IQR): 29.5–52.00 min]. The median duration of ECMO support was 61 h (IQR: 40.00–228.75 h). The median duration of ICU stay was 18.5 d (IQR: 9.75–27.75 d). Five patients (83.33%) were successfully weaned from ECMO. Four patients (66.67%) survived for 30 d after discharge; of these, two patients (33.33%) had good neurological outcomes (Table 3). Three patients (50%) had ECMO complications, including two cases of hemorrhage (33.33%). Emboli in the pulmonary arteries of survivors were decreased after 30 d of therapy (an example is shown in Figure 2).
| Case | Low-flow time (min) | Duration of ECMO support (h) | ECMO complications | Weaned from ECMO | Duration of ICU stay (days) | 30-d CPC score | 30-d survival |
| 1 | 50 | 19 | DIC, intra-abdominal hemorrhage | Y | 17 | 3 | Y |
| 2 | 30 | 218 | None | Y | 42 | 2 | Y |
| 3 | 40 | 54 | DIC, airway hemorrhage | Y | 23 | 3 | Y |
| 4 | 58 | 68 | Ischemia of right first metatarsal | Y | 6 | 5 | N |
| 5 | 28 | 47 | None | Y | 20 | 1 | Y |
| 6 | 43 | 261 | None | N | 11 | 5 | N |
Despite recent advances in techniques such basic and advanced life support, the overall survival rate from CA remains very low[6]. VA-ECMO is an effective means of providing mechanical circulatory assistance. In patients with CA secondary to PE, ECMO not only provides near-full-flow support and improves systemic circulatory perfusion, but also reduces right heart afterload and pulmonary artery pressure by bypassing the native pulmonary circulation and returning blood into the systemic circulation. This typically occurs via the femoral artery, thus assisting right heart function, improving hypoxemia[7], and providing more time for the diagnosis and treatment of PE. However, the use of ECMO for the treatment of patients with CA secondary to massive PE is still in the exploratory phase, and whether systemic thrombolysis, surgical thrombolysis, or percutaneous catheter-directed therapy should be performed remains a matter of debate. According to the 2019 European guidelines for the diagnosis and management of acute PE[8], ECMO combined with surgical thrombolysis or per
Thrombosis or hemorrhage is a common complication in patients with CA undergoing ECPR due to the presence of ischemia-reperfusion injury, exposure of the blood to the surface of a non-endothelialized artificial extracorporeal circuit, a systemic inflammatory response, and activation of the coagulation system, all of which disrupt the balance of the coagulation system in the body[11]. In such patients, anticoagulation is a top priority in clinical management. Heparin can activate the body’s own fibrinolytic mechanism and promote thrombolysis while effectively preventing re-thrombosis. After all patients in the present study were successfully initiated onto ECMO, their coagulation status was evaluated. Cerebral hemorrhage was ruled out by cranial CT examination, and heparin therapy was initially administered by continuous intravenous infusion at 4–10 U/kg/h. Activated clotting time is the primary method for immediate monitoring of heparinization during ECMO support. Repeated monitoring of the activated clotting time can guide the dosage of heparin, with a target of 180–220 s or maintaining the activated partial thromboplastin time at 1.5–2.5 times the normal value, depending on the risk of hemorrhage[12,13]. Heparin therapy was discontinued midstream in cases 1 and 3 due to secondary disseminated intravascular coagulation and active hemorrhage. After the disseminated intravascular coagulation and hemostasis was corrected, anticoagulation therapy was resumed, and the patients survived.
Massive PE can lead to severe respiratory distress and shock rapidly followed by CA. VA-ECMO can provide adequate circulation and gas exchange support. Our findings indicate that the treatment protocol of applying ECPR in conjunction with heparin anticoagulation provides improved outcomes for the resuscitation of patients with CA secondary to PE. Small sample size of this single center case series limits interpretation of the neurologically intact survival, however this preliminary data is encouraging.
All the authors would like to express their gratitude to the patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Richardson ASC, Australia S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Aissaoui N, Konstantinides S, Meyer G. What's new in severe pulmonary embolism? Intensive Care Med. 2019;45:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Assouline B, Assouline-Reinmann M, Giraud R, Levy D, Saura O, Bendjelid K, Combes A, Schmidt M. Management of High-Risk Pulmonary Embolism: What Is the Place of Extracorporeal Membrane Oxygenation? J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Hobohm L, Sagoschen I, Habertheuer A, Barco S, Valerio L, Wild J, Schmidt FP, Gori T, Münzel T, Konstantinides S, Keller K. Clinical use and outcome of extracorporeal membrane oxygenation in patients with pulmonary embolism. Resuscitation. 2022;170:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Mandigers L, Scholten E, Rietdijk WJR, den Uil CA, van Thiel RJ, Rigter S, Heijnen BGADH, Gommers D, Dos Reis Miranda D. Survival and neurological outcome with extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest caused by massive pulmonary embolism: A two center observational study. Resuscitation. 2019;136:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Guglin M, Zucker MJ, Bazan VM, Bozkurt B, El Banayosy A, Estep JD, Gurley J, Nelson K, Malyala R, Panjrath GS, Zwischenberger JB, Pinney SP. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73:698-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 302] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 6. | Gräsner JT, Herlitz J, Tjelmeland IBM, Wnent J, Masterson S, Lilja G, Bein B, Böttiger BW, Rosell-Ortiz F, Nolan JP, Bossaert L, Perkins GD. European Resuscitation Council Guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation. 2021;161:61-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 395] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 7. | Abrams D, Brodie D. Extracorporeal Membrane Oxygenation for Adult Respiratory Failure: 2017 Update. Chest. 2017;152:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Áinle FN, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 816] [Article Influence: 136.0] [Reference Citation Analysis (0)] |
| 9. | Meneveau N, Guillon B, Planquette B, Piton G, Kimmoun A, Gaide-Chevronnay L, Aissaoui N, Neuschwander A, Zogheib E, Dupont H, Pili-Floury S, Ecarnot F, Schiele F, Deye N, de Prost N, Favory R, Girard P, Cristinar M, Ferré A, Meyer G, Capellier G, Sanchez O. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases. Eur Heart J. 2018;39:4196-4204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Loyalka P, Ansari MZ, Cheema FH, Miller CC 3rd, Rajagopal S, Rajagopal K. Surgical pulmonary embolectomy and catheter-based therapies for acute pulmonary embolism: A contemporary systematic review. J Thorac Cardiovasc Surg. 2018;156:2155-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Andrews J, Winkler AM. Challenges with Navigating the Precarious Hemostatic Balance during Extracorporeal Life Support: Implications for Coagulation and Transfusion Management. Transfus Med Rev. 2016;30:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Rajsic S, Breitkopf R, Jadzic D, Popovic Krneta M, Tauber H, Treml B. Anticoagulation Strategies during Extracorporeal Membrane Oxygenation: A Narrative Review. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 13. | Bedeir K, Seethala R, Kelly E. Extracorporeal life support in trauma: Worth the risks? A systematic review of published series. J Trauma Acute Care Surg. 2017;82:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |