Published online Jun 16, 2023. doi: 10.12998/wjcc.v11.i17.4072
Peer-review started: February 6, 2023
First decision: April 26, 2023
Revised: May 9, 2023
Accepted: May 16, 2023
Article in press: May 16, 2023
Published online: June 16, 2023
Processing time: 126 Days and 1.7 Hours
Angioimmunoblastic T-cell lymphoma (AITL), a unique subtype of peripheral T-cell lymphoma, has relatively poor outcomes. High-dose chemotherapy with autologous stem cell transplantation (ASCT) can achieve complete remission and improve outcomes. Unfortunately, subsequent T-cell lymphoma-triggered hemophagocytic lymphohistiocytosis (HLH) has a worse prognosis than B-cell lymphoma-triggered HLH.
We here report a 50-year-old woman with AITL who achieved a favorable outcome after developing HLH 2 mo after receiving high-dose chemotherapy/ ASCT. The patient was initially admitted to our hospital because of multiple enlarged lymph nodes. The final pathologic diagnosis, made on biopsy of a left axillary lymph node was AITL (Stage IV, Group A). Four cycles of the following chemotherapy regimen were administered: Cyclophosphamide 1.3 g, doxorubicin 86 mg, and vincristine 2 mg on day 1; prednisone 100 mg on days 1-5; and lenalidomide 25 mg on days 1-14. The interval between each cycle was 21 d. The patient received a conditioning regimen (busulfan, cyclophosphamide, and etoposide) followed by peripheral blood stem cell infusion. Unfortunately, she developed sustained fever and a low platelet count 17 d after ACST, leading to a diagnosis of HLH after ASCT. During treatment, she experienced thrombocytopenia and Pneumocystis carinii pneumonia. The patient was successfully treated with etoposide and glucocorticoids.
It is possible that development of HLH is related to immune reconstitution after ASCT.
Core Tip: Angioimmunoblastic T cell lymphoma (AITL), a subtype of mature peripheral T cell lymphoma, is characterized by intense inflammatory and immune reactions. Although high-dose chemotherapy with autologous stem cell transplantation (ASCT) is currently considered the optimal treatment option for AITL, subsequent development of hemophagocytic lymphohistiocytosis (HLH), a rare, life-threatening immunological syndrome, may still result in poor outcomes. In the present case, we speculate that HLH was likely attributable to immune reconstitution after ASCT. Successful immune reconstitution after ASCT is essential to improving overall survival and preventing opportunistic infections in patients with AITL.
- Citation: Zhang ZR, Dou AX, Liu Y, Zhu HB, Jia HP, Kong QH, Sun LK, Qin AQ. Hemophagocytic lymphohistiocytosis after autologous stem cell transplantation in angioimmunoblastic T-cell lymphoma: A case report. World J Clin Cases 2023; 11(17): 4072-4078
- URL: https://www.wjgnet.com/2307-8960/full/v11/i17/4072.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i17.4072
Angioimmunoblastic T-cell lymphoma (AITL), a unique subtype of peripheral T-cell lymphoma, is the most frequent subtype of mature T-cell and natural killer (NK) cell neoplasm listed in the World Health Organization classification of lymphoid neoplasms[1]. The distinct clinicopathological features of AITL and treatment purely with conventional cyclophosphamide, hydroxyl daunorubicin, vincristine, and prednisone (CHOP-R) regimens always result in poor outcomes.
High-dose chemotherapy with autologous stem cell transplantation (HDC/ASCT) is a more effective treatment option for AITL. Advani et al[2] performed a subset analysis of 282 patients with AITL and recorded improved outcomes in patients who achieved complete remission (CR) after ASCT. Upfront ASCT was linked to better outcomes in patients with AITL and CR than in those with partial responses. Specifically, patients with CR exhibited better 5-year overall survival (OS) and progression-free survival rates of 44% and 32%, respectively[2,3]. However, successful treatment of T/NK cell lymphoma is frequently followed by development of hemophagocytic lymphohistiocytosis (HLH), the incidence being 35.2% in patients with T-cell or NK cell lymphoma or leukemia.
HLH, a potentially life-threatening hyperinflammatory state, is characterized by sustained fever, cytopenia, hypertriglyceridemia, hyperferritinemia, and hemophagocytosis in the bone marrow, liver, spleen, or lymph nodes. The co-trigger of Epstein-Barr virus infection is present in approximately 33% of patients with peripheral T-cell lymphomas, including AITL, and can result in high rates of misdiagnosis and mortality. In this context, T-cell lymphoma-triggered HLH usually has a worse prognosis than B-cell lymphoma-triggered HLH[2]. We here describe a patient with AITL who developed HLH 2 mo after undergoing HDCT/ASCT and achieved a favorable outcome when treated with etoposide (VP16) and glucocorticoids (GCs).
A 50-year-old woman was admitted to our hospital because she had had multiple enlarged lymph nodes for 4 mo.
The patient had found a small, soybean-sized lump in the right supraclavicular region 4 mo previously. The lump had gradually enlarged, accompanied by bilateral enlargement of lymph nodes in the axillary and inguinal areas.
The patient had been diagnosed with high blood pressure 4 years prior to this presentation.
The patient had been diagnosed with high blood pressure 4 years prior to this presentation.
Examination revealed bilateral enlarged lymph nodes in the supraclavicular, axillary, and inguinal areas.
Bone marrow biopsy revealed lymphomatous infiltration shown by immunophenotyping to denote T-cell lymphoma. Pathological examination by Beijing Cancer Hospital of a biopsy of a left axillary lymph node indicated non-Hodgkin lymphoma and vascular immunoblastic T-cell lymphoma.
Ultrasound and enhanced computed tomography (CT) detected bilateral enlarged lymph nodes in the supraclavicular, subclavian, axillary, and inguinal areas and in the mediastinum and retroperitoneal area.
The final pathologic diagnosis was AITL (Stage IV, Group A).
Four cycles of CHOP-R chemotherapy were administered. The regimen consisted of cyclophosphamide 1.3 g, doxorubicin 86 mg, and vincristine 2 mg on day 1; prednisone 100 mg on days 1-5; and lenalidomide 25 mg on days 1-14. The interval between each cycle was 21 d. Thereafter, we performed enhanced CT and bone marrow biopsy to evaluate the therapeutic effect. All enlarged lymph nodes had reduced in size by more than 90% compared with baseline sizes and no lymphomatous infiltration was found on bone marrow biopsy with immunohistochemistry. Thus, the patient was considered to have achieved a CR.
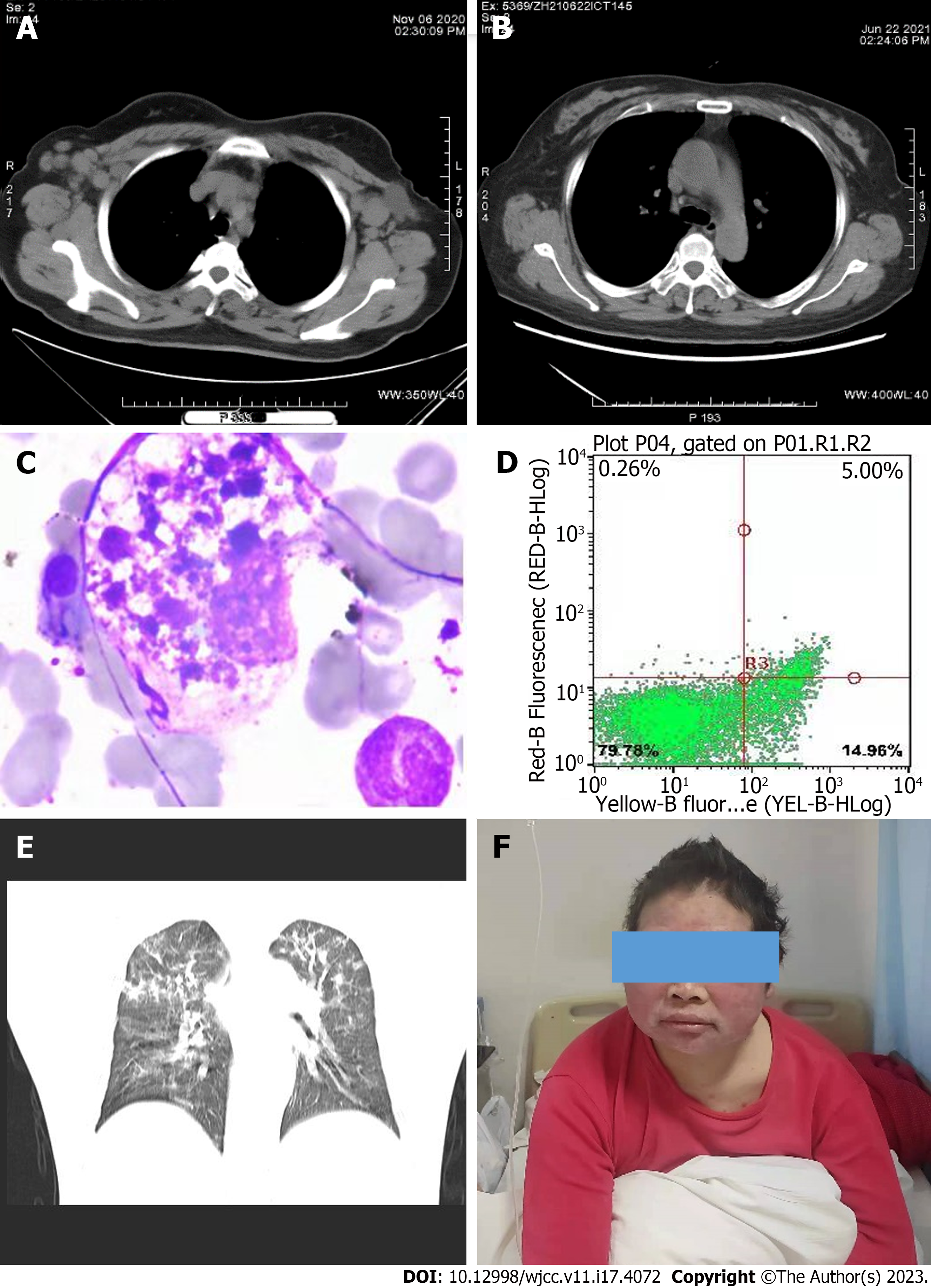
The treatment team reached a consensus that ASCT would likely be effective and safe as a follow-up to HDC in this patient[4]. Hematopoietic stem cell mobilization was performed with high dose-cyclophosphamide plus plerixafor, as previously reported[5]. The counts of CD34+ and TNC cells were 1.5 × 106/kg and 5.0 × 108/kg, respectively. A conditioning regimen (busulfan, cyclophosphamide, and etoposide) was administered, followed by peripheral blood stem cell infusion. The patient recovered well and was discharged 11 d after ASCT. Enhanced CT scans showed that the enlarged lymph nodes that had been present at baseline (Figure 1A) had disappeared after HDC/ASCT (Figure 1B).
Unfortunately, the patient developed sustained fever and a low platelet count 17 d after ACST. Initially, we used eltrombopag to stimulate the dysfunctional megakaryocytes and increase the platelet count. A bone marrow aspiration smear showed hemophagocytosis and phagocytic platelets (Figure 1C) with high concentrations of serum ferritin (FERR) and soluble CD25. Specifically, the soluble CD25 concentration was 11094 pg/mL (twice the upper limit of normal). The FERR concentration of 16500 ng/mL also exceeded the upper limit of normal. The results of an NK cell activity test were also abnormal (Figure 1D). Thus, HLH after ASCT was diagnosed and VP16 with GCs administered[6].
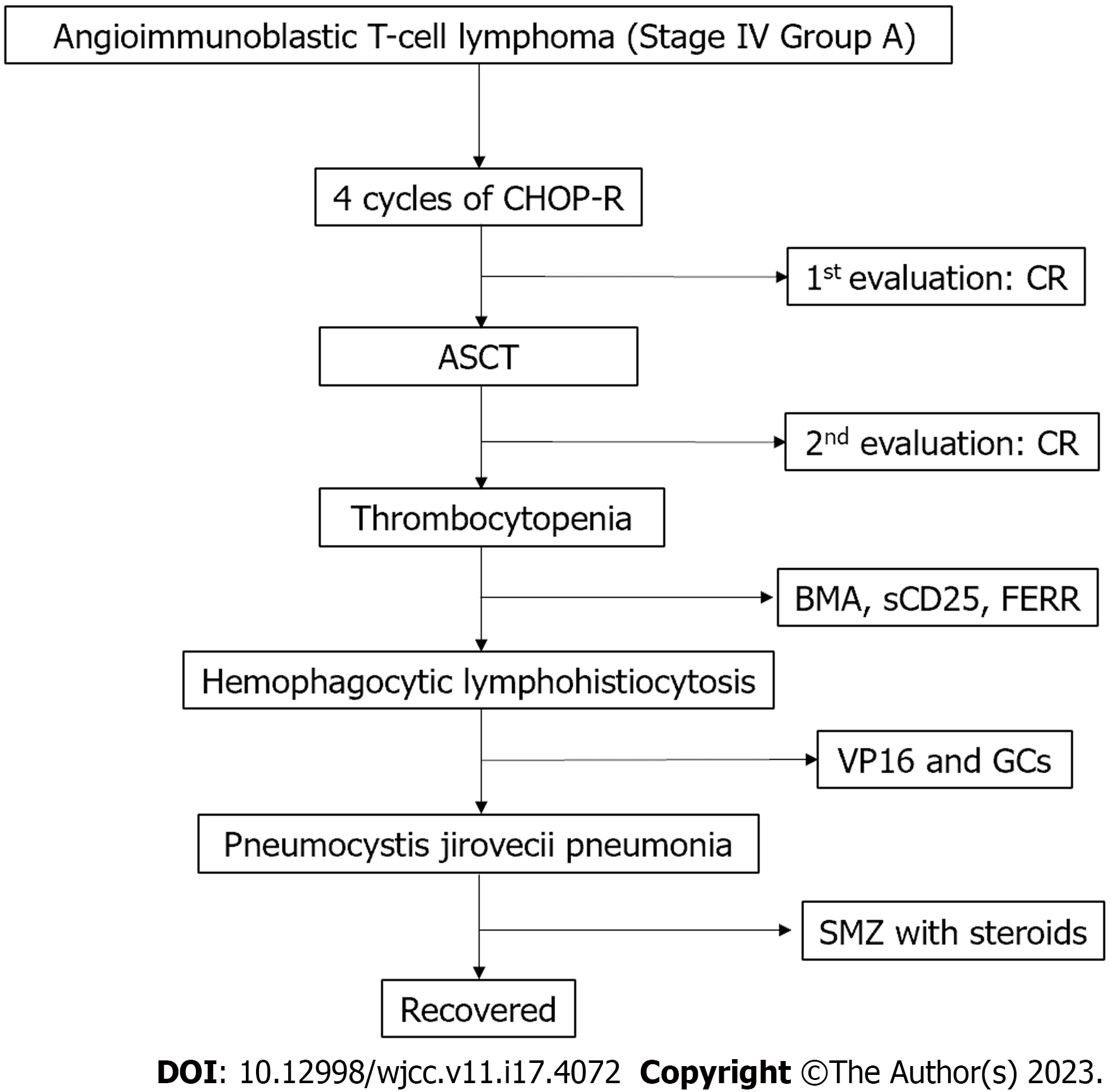
Although the platelet count was extremely low at 7 × 109/mL, no hemorrhagic events occurred. Treatment with GCs, VP16, and supportive care gradually normalized our patient’s body temperature and platelet count. Unfortunately, she manifested dyspnea and hypoxemia on day 30 after commencing standard treatment. Thus, she was urgently transferred to the intensive care unit, where she underwent intubation and mechanical ventilation. Pneumocystis jirovecii (P. jirovecii) pneumonia was diagnosed by microscopic examination of the patient’s bronchoalveolar lavage fluid. The chest CT findings are presented in Figure 1E. The patient’s serum beta-d-glucan concentration exceeded 300 pg/mL and positive reactivities were observed for P. jirovecii. Trimethoprim-sulfamethoxazole 9 g plus steroids achieved rapid improvement in symptoms and laboratory findings; however, the high dose of trimethoprim-sulfamethoxazole caused a transient rash (Figure 1F). The flowchart of treatment process of this case was demonstrated in Figure 2.
Thrombocytopenia is the first adverse event to manifest after ASCT. Huang et al have reported that dysfunctional megakaryopoiesis hinder platelet production. Macrophages are crucial components of the bone marrow microenvironment, M1 and M2 macrophages having opposite effects on dysfunctional megakaryopoiesis through the PI3K-AKT pathway[7]. Promotion of megakaryopoiesis in patients with poor megakaryocyte engraftment after ASCT requires an effective strategy.
Our patient exhibited sustained fever and AITL-associated HLH was diagnosed in a timely manner on the basis of high FERR concentrations and positive findings on bone marrow aspiration. VP16 with GCs achieved rapid improvement in her symptoms and laboratory findings. Considering the lack of evidence of relapse of AITL or of severe infection in this case and the fact that HLH is usually caused by the uncontrolled proliferation and activation of lymphocytes and macrophages, we speculated that her HLH was likely attributable to the immune reconstitution that occurs after ASCT[8].
GCs caused various adverse effects, including moon face, buffalo back, and weight gain, in our case. One month after commencing standard GC treatment, severe dyspnea and hypoxemia caused by P. jirovecii pneumonia were detected in this patient. In this context, we believe that erythema multiforme and eczema can be signs of immune rebuilding. Of note, intravenous calcium channel blockers have been recommended for treatment of AITL-associated HLH in other cases[9].
Successful immune reconstitution after ASCT is critical for promoting OS and preventing opportunistic infections in patients with AITL. Such reconstitution involves complex biological processes with continuous interplay between multiple immune cell populations[10]. T-cell reconstitution after ASCT is mediated by the homeostatic proliferation and generation of newborn T-cells through thymopoiesis. Restoration in the early post-transplant period is predominantly mediated by the homeostatic proliferation of CD8+ memory T-cells[11,12].
HLH is likely under-recognized and mortality remains high, especially in adults. Thus, prompt diagnosis and treatment are essential. Given that sustained, aberrant activation of cytotoxic CD8+ T cells and resultant inflammatory cytokine release are core pathogenic mechanisms of HLH, immune reconstitution after ASCT may be the main mechanism underlying development of HLH. We should therefore pay more attention to immune reconstitution after ASCT. This is very important for the prognosis of patients who have undergone ASCT.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Nwabo Kamdje AH, Cameroon S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Chiba S, Sakata-Yanagimoto M. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia. 2020;34:2592-2606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Advani RH, Skrypets T, Civallero M, Spinner MA, Manni M, Kim WS, Shustov AR, Horwitz SM, Hitz F, Cabrera ME, Dlouhy I, Vassallo J, Pileri SA, Inghirami G, Montoto S, Vitolo U, Radford J, Vose JM, Federico M. Outcomes and prognostic factors in angioimmunoblastic T-cell lymphoma: final report from the international T-cell Project. Blood. 2021;138:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 3. | Yamasaki S, Chihara D, Kim SW, Kawata T, Mizuta S, Ago H, Chou T, Yamane T, Uchiyama H, Oyake T, Miura K, Saito B, Taji H, Nakamae H, Miyamoto T, Fukuda T, Kanda J, Atsuta Y, Suzuki R. Risk factors and timing of autologous stem cell transplantation for patients with peripheral T-cell lymphoma. Int J Hematol. 2019;109:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Kameda K, Kako S, Kim SW, Usui Y, Kato K, Fukuda T, Uchida N, Kobayashi H, Wakayama T, Sakaida E, Yano S, Imada K, Nara M, Ikeda T, Fuchida SI, Ishikawa J, Sugahara H, Kanda J, Kimura T, Ichinohe T, Atsuta Y, Kondo E. Autologous or allogeneic hematopoietic cell transplantation for relapsed or refractory PTCL-NOS or AITL. Leukemia. 2022;36:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Karres D, Ali S, van Hennik PB, Straus S, Josephson F, Thole G, Glerum PJ, Herberts C, Babae N, Herold R, Papadouli I, Pignatti F. EMA Recommendation for the Pediatric Indications of Plerixafor (Mozobil) to Enhance Mobilization of Hematopoietic Stem Cells for Collection and Subsequent Autologous Transplantation in Children with Lymphoma or Malignant Solid Tumors. Oncologist. 2020;25:e976-e981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Gómez CH, Vargas-Hernández DA, Largo J, Hernández S, Faccini-Martínez ÁA. Hemophagocytic lymphohistiocytosis and acute Chagas disease, Colombia. Travel Med Infect Dis. 2021;44:102213. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Zhao HY, Zhang YY, Xing T, Tang SQ, Wen Q, Lyu ZS, Lv M, Wang Y, Xu LP, Zhang XH, Kong Y, Huang XJ. M2 macrophages, but not M1 macrophages, support megakaryopoiesis by upregulating PI3K-AKT pathway activity. Signal Transduct Target Ther. 2021;6:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 8. | Jiang M, Wan JH, Tu Y, Shen Y, Kong FC, Zhang ZL. Angioimmunoblastic T-cell lymphoma induced hemophagocytic lymphohistiocytosis and disseminated intravascular coagulopathy: A case report. World J Clin Cases. 2023;11:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Yamauchi N, Hongo T, Kawakami M, Inoguchi K, Oguni S, Momoki N, Ueno A, Ikeda F, Fujioka S, Yamamoto K. Successful Recovery from Severe Fever with Thrombocytopenia Syndrome and Hemophagocytic Lymphohistiocytosis with Standard Treatment and a Calcium Channel Blocker of Nicardipine Hydrochloride. Intern Med. 2023;62:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Parmar H, Gertz M, Anderson EI, Kumar S, Kourelis TV. Microenvironment immune reconstitution patterns correlate with outcomes after autologous transplant in multiple myeloma. Blood Adv. 2021;5:1797-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Williams KM, Chakrabarty JH. Imaging haemopoietic stem cells and microenvironment dynamics through transplantation. Lancet Haematol. 2020;7:e259-e269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2021;21:277-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |