Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3907
Peer-review started: March 16, 2023
First decision: April 11, 2023
Revised: April 18, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: June 6, 2023
Processing time: 78 Days and 5.5 Hours
Pyogenic granuloma (PG) is a localized, reddish and vascularized hyperplastic lesion of the connective tissue which occurs in the oral cavity. In most cases, the presence of this lesion does not show alveolar bone resorption. The pathology is diagnosed clinically with some caution. However, the diagnosis and treatment are usually corroborated with histopathological evidence.
Three clinical cases of PG associated with bone loss were described in this study. The three patients presented tumor-like growth which bled on touch, and were associated with local irritant factors. Radiographs showed bone loss. All cases were treated with conservative surgical excision. The scarring was satisfactory, and there was no case of recurrence. The diagnoses were based on clinical findings, and were confirmed histopathologically.
The occurrence of oral PG with bone loss is unusual. Therefore, clinical and radiographic evaluations are important for the diagnosis.
Core Tip: Pyogenic granuloma is a soft tissue tumor of the oral cavity which frequently does not present alveolar bone resorption. However, these three clinical cases of pyogenic granuloma were associated with bone loss, an unusual feature of this pathology. The patients were treated with conservative surgical excision. The diagnoses were based on the clinical findings which were confirmed with histopathology. These cases underline the importance of clinical and radiographic evaluation as guides for accurate diagnosis so as to enhance the development of an appropriate treatment plan in unusual cases.
- Citation: Lomelí Martínez SM, Bocanegra Morando D, Mercado González AE, Gómez Sandoval JR. Unusual clinical presentation of oral pyogenic granuloma with severe alveolar bone loss: A case report and review of literature. World J Clin Cases 2023; 11(16): 3907-3914
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3907.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3907
Pyogenic granuloma (PG) is a soft tissue tumor of the oral cavity[1-3]. The etiology of the tumor is unknown, although it is thought that it probably originates from exacerbated response of the connective tissue to trauma, local irritation or hormonal imbalances[1,4]. Pyogenic granuloma has also been considered an "infectious" entity, due to the presence of botryomycosis[1].
PG presents clinically as a smooth mass with a lobular architecture which is usually pedunculated, although some lesions are sessile[5,6]. Radiographic evaluation of this mass has not been considered as a diagnostic strategy. However, the characteristic bone loss associated with PG has been reported only in few clinical cases in India[7-9]. In this study, we report three cases of female patients who presented PG associated with bone loss.
Case 1: A 32-year-old woman presented with a lump on the palate that bled frequently and interfered with chewing.
Case 2: A 42-year-old woman presented with a bulge near the upper lip. The patient complained of pain when chewing and mobility of the central incisor.
Case 3: A 38-year-old woman had two enlargements which bled frequently and interfered with chewing.
Case 1: The patient stated that the lesion appeared approximately 3 mo prior to the clinical diagnosis.
Case 2: In the course of anamnesis, it was revealed that the lesion had 5 mo of evolution. It bled on touch, and it had a firmer consistency than before.
Case 3: The patient said that the lesion had 6 mo evolution period, during which it grew until chewing became an uncomfortable exercise.
Case 1: The patient did not have any history of systemic disease. Thus, the medical record was not relevant.
Case 2: The medical record was not relevant, since the patient denied any pathology or systemic disease.
Case 3: The patient did not mention presence of any systemic disease, and she was not undergoing any medical treatment.
Case 1: None.
Case 2: Father was diagnosed with Type 2 diabetes mellitus 5 years earlier.
Case 3: Mother was diagnosed with high bold pressure in 2017.
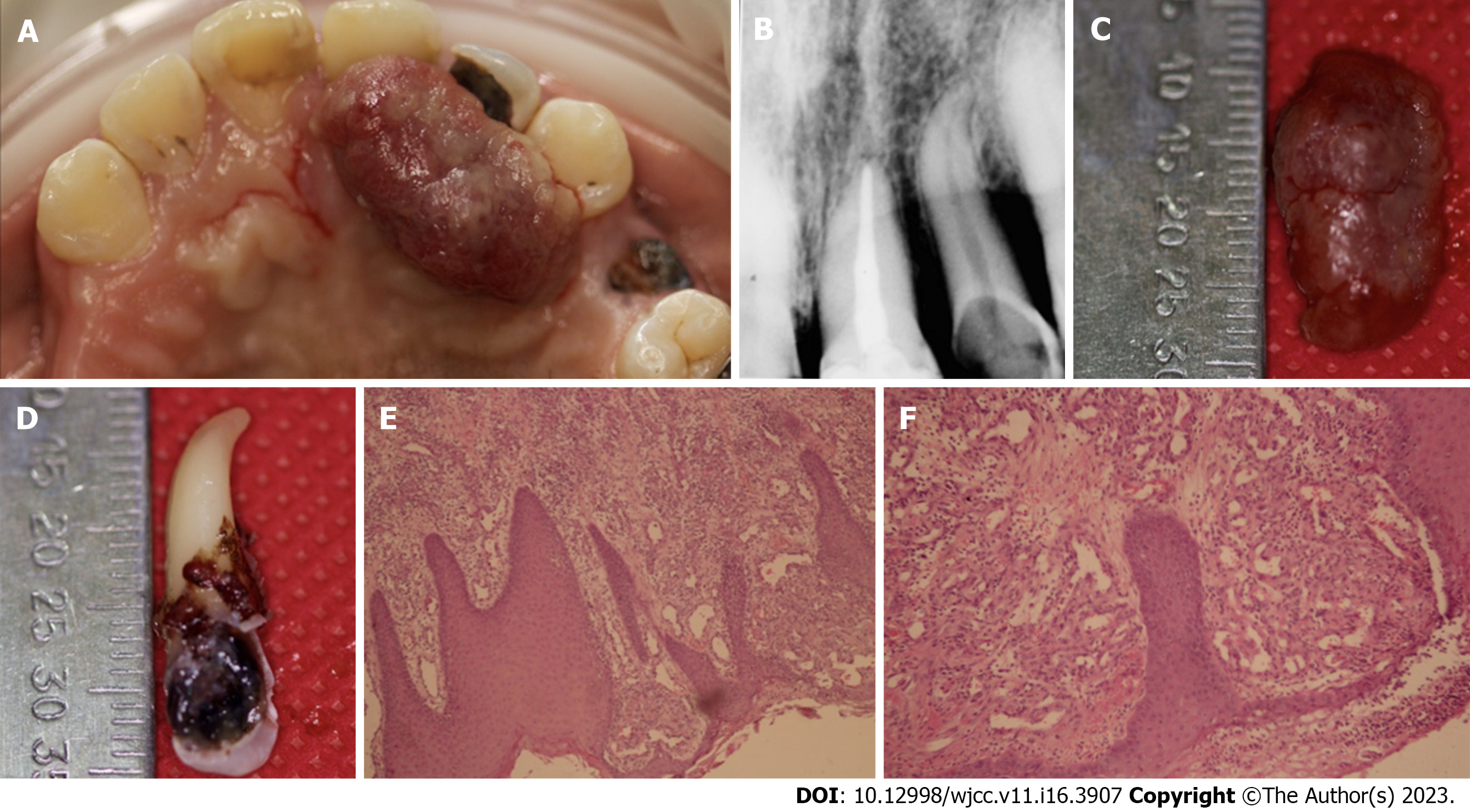
Case 1: Oral examination revealed a localized exophytic lesion manifested as a 25 mm × 12 mm erythematous mass with smooth consistency which bled on provocation. The lesion was pedicled and attached to the marginal gingiva of dental organ 22, and it extended to the middle third of palatal surface of dental organs 21 and 23 (Figure 1A). In addition, dental organ 22 presented grade-three mobility and extensive caries along the palatine surface.
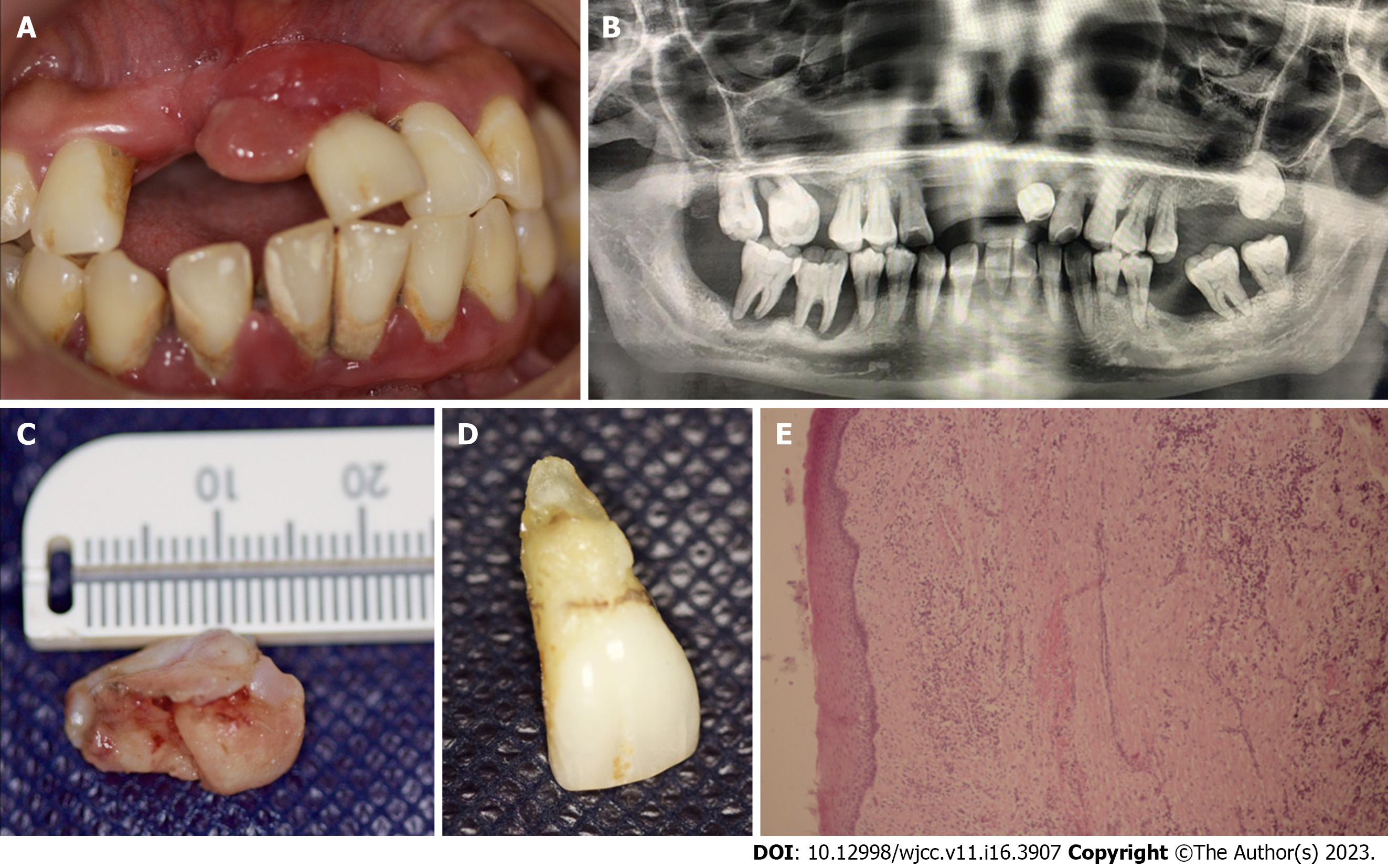
Case 2: During the oral examination, a semi-ovoid 16 mm × 10 mm formation of gingival mucosa was found. The surface was smooth and reddish in color. The growth was pedicled and attached to the marginal gingiva of dental organ 21 (Figure 2A). This dental organ presented grade-three mobility, supra and sub-gingival calculus, and probing depth of 8 mm.
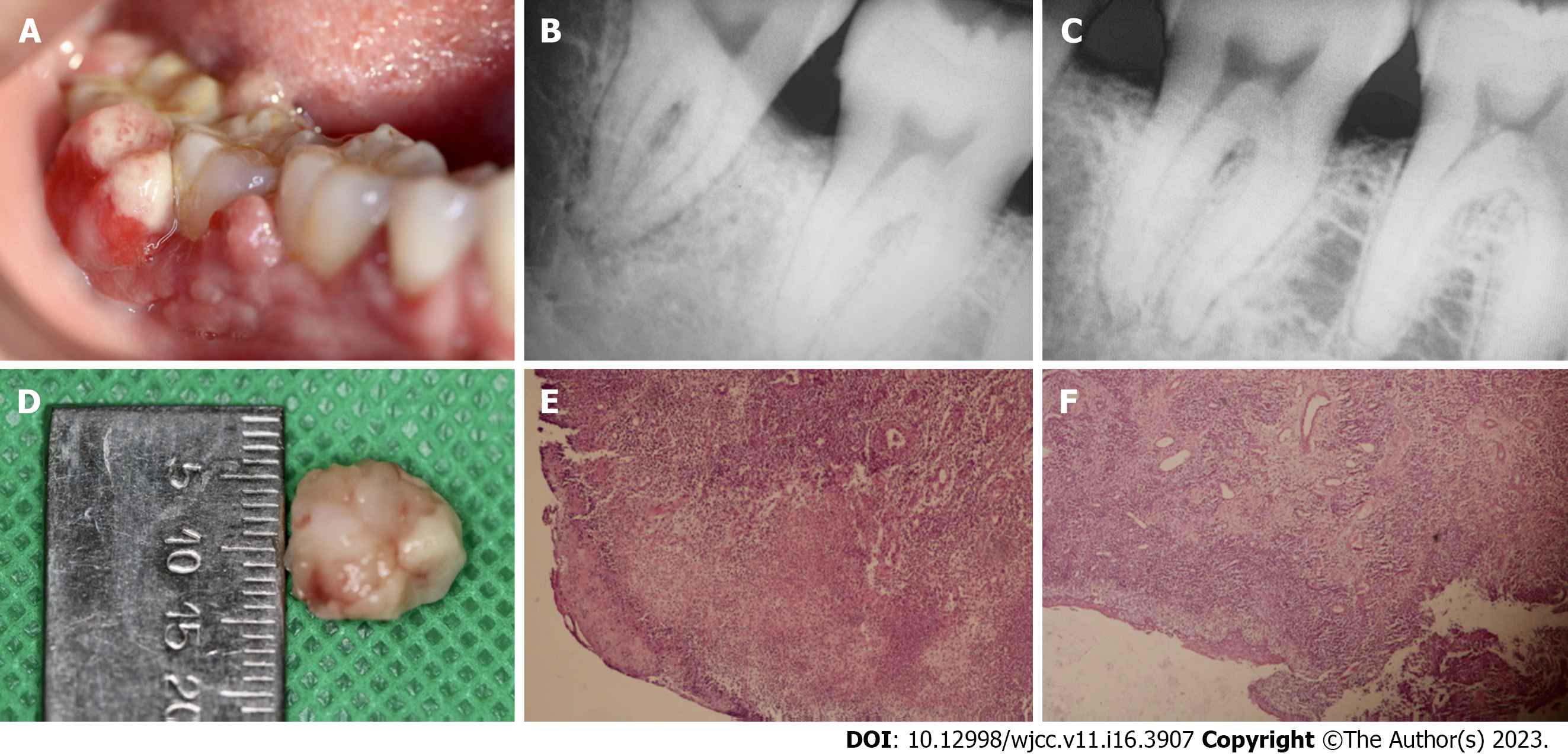
Case 3: During the clinical examination, two reddish exophytic lesions with smooth surfaces were identified. The lesions were about 20 mm × 15 mm and 10 mm × 12 mm in size. They were pedicled and attached to the interproximal zone of dental organs 46 and 47 (Figure 3A). Supra- and sub-gingival calculus were identified in the two dental organs.
Case 1: Hemoglobin level was slightly low (11.8 g/dL). The other results were within normal limits.
Case 2: Neutrophil count was slightly high (72%). However, the levels of the other white cells were within normal limits [platelet level (309 × 103/µL) was normal, and hemoglobin level (14.5 g/dL) was normal].
Case 3: Hemoglobin, neutrophil and platelet levels were within normal limits.
Case 1: Periapical radiography showed horizontal bone loss up to the middle third region of dental organ 22 (Figure 1B).
Case 2: Panoramic radiography showed generalized alveolar bone loss in both arches (Figure 2B).
Case 3: Periapical radiography showed interproximal bone loss between dental organs 47 and 48 (Figure 3B); and between dental organs 46 and 47 (Figure 3C).
Based on all the information obtained from anamnesis, and from radiographic imaging, clinical features of lesion such as ulcerated surface, bleeding on provocation, as well as the adjacent local irritants, a presumptive diagnosis of pyogenic granuloma was made.
The information obtained from the clinical, radiographic examination, and the history of evolution of the lesion led to a presumptive diagnosis of pyogenic granuloma.
Based on all the information obtained during the clinical and radiographic examinations, as well as the clinical features of the lesion, and the adjacent local irritants, the presumptive diagnosis was pyogenic granuloma.
Microscopic examination revealed a segment of buccal mucosa with fibrous stroma and a diffuse lymphoplasmacytic-type inflammatory infiltrate. There was evidence of old and recent stromal hemorrhages around newly-formed, congested blood vessels having irregular contours and varied diameters, with hyperplastic endothelial cells covered by epithelium with extensive areas of erosion. This histological examination confirmed the diagnosis of PG (Figures 1E and F).
Histological analysis revealed a segment of mucosal stratified squamous epithelium with underlying fibrovascular stroma and a dense infiltrate of chronic inflammatory cells, stromal hemorrhage, large number of budding capillaries, fibroblasts, and areas of extravasated blood. Therefore, the diagnosis of PG with gingival hyperplasia was confirmed (Figure 2E). Along with this final diagnosis, and based on the results of intraoral clinical examination, periodontal chart, and X-ray, a stage III grade generalized periodontitis was also diagnosed.
On microscopic examination, sections showed stratified squamous epithelium with non-neoplastic endothelial cell proliferation, formation of new blood cells, as well as acute and chronic inflammatory cell infiltration in a collagenous matrix around the newly formed, congested blood vessels with irregular contours and varied diameters. The blood vessels were covered by epithelium with extensive areas of erosion. These features corroborated the presumptive diagnosis of PG (Figures 3E and F).
The exophytic lesion was removed with excisional biopsy (Figure 1C). The procedure was performed under local infiltrated anesthesia [2% mepivacaine with epinephrine (10 µg/mL)]. The incision was made with a 15C scalpel blade on the base of the pediculated lesion. The gingival tissues were remodeled with LaGrange scissors. Due to the extent of bone loss, degree of mobility, and the extensive caries in dental organ 22, a decision was made to remove it (Figure 1D). Oral prophylaxis was performed with an ultrasonic device and Gracey 1-2 curettes in order to remove the local irritants on the adjacent teeth. After the procedures, 500-mg amoxicillin tablets were prescribed for the patient, to be taken 3 times daily for 5 d, and post-surgery indications were given.
The exophitic mass was removed through an excisional biopsy (Figure 2C), along with the extraction of dental organ 21. The extraction was performed without complications (Figure 2D). The procedure was performed under local infiltrated anesthesia [2% mepivacaine with epinephrine (10 µg/mL)]. The incision was made with a 15C scalpel blade on the base of the pediculated lesion. Oral prophylaxis was also performed with ultrasonic device and McCall 13-14 and 17-18 curettes. After the procedures, amoxicillin tablets (500 mg) were administered to the patient 3 times daily for 5 d, and post-surgery indications were given.
An excisional biopsy was performed under local infiltrated anesthesia [2% mepivacaine with epinephrine (10 µg/mL)]. The incision was made with a 15C scalpel blade on the based of the lesion. The incision was extended to the periosteum, and a 2-mm margin was included from the adjacent soft tissues (Figure 3D). Oral prophylaxis was performed with an ultrasonic device and McCall 17-18 curettes in order to remove the calculus on dental organs 46, 47 and 48. Post-surgery indications were given, and the patient was placed on 500 mg tablets of amoxicillin 3 times daily for 5 d.
After three months of follow-up, the scarring was satisfactory, and there was no recurrence.
The scarring was satisfactory after three months of follow up, and there was no recurrence. Additional therapy was started for the periodontitis.
After three months of follow-up, the scarring was satisfactory, and there was no recurrence
PG is a localized, reddish and vascularized gingival hyperplastic lesion[10-12]. It has been reported that oral PG accounts for an incidence of 52.71% among all non-neoplastic lesions[13]. It occurs more frequently in women than in men, and the major site of predilection is the gingiva[14]. Other lesion sites are the buccal mucosa, upper lip, lower lip, tongue, paladar and labial mucosa[9-12].
PG is considered a reactive self-limited pathology that presents as an exuberant proliferation of connective tissue in response to stimulation by some local irritant factor. Tripathi et al[8] and Verma et al[9] concluded, in their clinical case reports, that poor oral hygiene leading to abundant biofilm and dental calculus accumulation causes chronic irritation and contributes to the development of oral PG, similar to the cases presented. In the third case the presence of supra and sub gingival calculus was identified in the dental organs, which could be associated with the appearance of PG, and the periapical radiograph showed interproximal bone loss. However, in the first clinical case, we reported the presence and extent of dental caries in dental organ 12. Dental caries is caused by etiologic agents, such as Staphylococci and Streptococcus which produce colonies with fungal characteristics[15]. Chronic trauma provides a pathway for invasion of these microorganisms that induce proliferation of vascular connective tissue. Thus, it can be inferred that these pathogens also act as stimuli that favour the formation and growth of pyogenic granuloma[1,16].
In the oral cavity, PG appears a smooth or lobulated exophytic and red erythematous papule on a pedunculated or sessile base, and it is usually hemorrhagic and compressible[1,2,16]. The surface of PG is characteristically ulcerated[17], and its color varies from red, reddish purple to pink, depending on the degree of vascularity and the age of the lesion[16,17]. Newly -formed lesions are highly vascular, and appear more reddish in color, relative to old lesions. In contrast, mature lesions are pink in color and firm in consistency due to presence of high level of collagen fibers[3,17].
Most researchers have reported that radiographic evaluation of pyogenic granuloma does not show relevant features because the lesion arises from soft tissues[1,18-20]. However, pyogenic granuloma is a benign inflammatory lesion that expresses significantly more vascular endothelial growth factors and basic fibroblast growth factors than healthy gingiva and periodontitis[21]. In all three clinical cases described, bone tissue loss is observed and, although infrequent, PG can cause significant bone loss on rare occasions, as reported by Mastammanavar et al[7], Tripathi et al[8], and Verma et al[9]. Growth factors such as fibroblast growth factor-2, growth arrest-specific gene 6, and tumor necrosis factor-α stimulate mature osteoclast function and survival through extracellular signal-regulated kinase (ERK) activation, resulting in degradation or resorption of organic and inorganic bone components[22]. We may reasonably hypothesize that the severe alveolar bone loss in the three clinical cases presented was due to the activation of ERK signal pathway. Therefore, we suggest that bone loss should be considered as a possible diagnostic feature of pyogenic granuloma, notwithstanding the fact that the lesion arises from soft tissues.
In all clinical cases evaluated, the recommended management of this pathology involves surgical excision. However, different treatment modalities have been applied. These comprise Nd: YAG laser[23], CO2 laser[24], flashlamp pulsed dye lasers[25,26], cryotherapy[27,28] and intra-lesional steroids[29]. The rate of recurrence of PG is 16%. This is due to incomplete excision of lesion and failure to remove local etiologic factors such as plaque, calculus and source of trauma[30,31]. For the latter, we recommend oral prophylaxis and elimination of local irritant factors, in addition to simultaneous resection of the lesion. It is essential to continuously implement complete dental evaluation involving preventive measures consisting of elimination of local irritant factors (dental biofilm, dental calculus, over contoured restorations, etc.) and meticulous oral hygiene care [brushing after eating (at least twice a day), using toothpaste on a soft-bristled toothbrush, and daily flossing]. These strategies decrease the possibility of appearance and development of pyogenic granuloma.
We presented three cases of oral PG with an unusual feature i.e., alveolar bone loss. The clinical cases showed various etiological factors such calculus and periodontitis, which generate or contribute to the formation of PG. This hyperplastic lesion may occur as a single entity, or it may be associated with a non-neoplastic reactive proliferative process such as gingival hyperplasia. Surgical excision, oral prophylaxis and the elimination of local irritant factors are procedures that may be effectively implemented to minimize the recurrence of this lesion. Likewise, diagnosis, treatment plan and continuous dental evaluation are very essential. These case reports indicate that the clinical diagnosis of PG is a complex process. Therefore, it is important to know the clinical characteristics of the lesion at various locations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gica N, Romania; Kukiattrakoon B, Thailand S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Kamal R, Dahiya P, Puri A. Oral pyogenic granuloma: Various concepts of etiopathogenesis. J Oral Maxillofac Pathol. 2012;16:79-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Gowda D, Owens C. Pyogenic Granuloma in an Unusual Site. Glob Pediatr Health. 2017;4:2333794X17704607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Nejad ES, BigomTaheri J, Azimi S. Frequency of gingival pregnancy tumor in iran (confirmed by biopsy). J Int Oral Health. 2014;6:72-76. [PubMed] |
| 4. | Mussalli NG, Hopps RM, Johnson NW. Oral pyogenic granuloma as a complication of pregnancy and the use of hormonal contraceptives. Int J Gynaecol Obstet. 1976;14:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Brunet-LLobet L, Miranda-Rius J, Lahor-Soler E, Mrina O, Nadal A. A Gray-purple Mass on the Floor of the Mouth: Gigantic Mucogingival Pyogenic Granuloma in a Teenage Patient. Open Dent J. 2014;8:125-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Bhaskar SN, Jacoway JR. Pyogenic granuloma--clinical features, incidence, histology, and result of treatment: report of 242 cases. J Oral Surg. 1966;24:391-398. [PubMed] |
| 7. | Mastammanavar D, Hunasgi S, Koneru A, Vanishree M, Surekha R, Vardendra M. Aggressive Pyogenic Granuloma: A Case Report. IJOMP. 2014;5. |
| 8. | Tripathi AK, Upadhaya V, Kumar V, Saimbi CS. Hyperactive lesions of gingiva associated with severe alveolar bone loss: A rare finding. Contemp Clin Dent. 2015;6:223-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Verma PK, Srivastava R, Baranwal HC, Chaturvedi TP, Gautam A, Singh A. "Pyogenic granuloma - Hyperplastic lesion of the gingiva: case reports". Open Dent J. 2012;6:153-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Sachdeva SK. Extragingival Pyogenic Granuloma: an Unusual Clinical Presentation. J Dent (Shiraz). 2015;16:282-285. [PubMed] |
| 11. | Patil K, Mahima VG, Lahari K. Extragingival pyogenic granuloma. Indian J Dent Res. 2006;17:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Nagarajappa A, Chandrashekar K, Jain N, Pandya D, Kaushal T. Extragingival pyogenic granuloma: report of an unusual case with review of literature. Int J Med Appl Sci. 2016;5:46e52. |
| 13. | Shamim T, Varghese VI, Shameena PM, Sudha S. A retrospective analysis of gingival biopsied lesions in South Indian population: 2001-2006. Med Oral Patol Oral Cir Bucal. 2008;13:E414-E418. [PubMed] |
| 14. | Akyol MU, Yalçiner EG, Doğan AI. Pyogenic granuloma (lobular capillary hemangioma) of the tongue. Int J Pediatr Otorhinolaryngol. 2001;58:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Shafer WG, Hine MK, Levy BM. Shafer’s textbook of oral pathology. 5th ed. Amsterdam: Elsevier Health Sciences 2006. |
| 16. | Gomes SR, Shakir QJ, Thaker PV, Tavadia JK. Pyogenic granuloma of the gingiva: A misnomer? - A case report and review of literature. J Indian Soc Periodontol. 2013;17:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Regezi JA, Sciubba JJ, Jordan RC. Oral Pathology and Clinical Pathological Considerations. 4th ed. Philadelphia, PA: W B Saunders; 2003: 115–116. |
| 18. | Aragaki T, Tomomatsu N, Michi Y, Hosaka H, Fukai Y, Iijima M, Yoda T. Ramucirumab-related Oral Pyogenic Granuloma: A Report of Two Cases. Intern Med. 2021;60:2601-2605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Banjar A, Abdrabuh A, Al-Habshi M, Parambil M, Bastos P, Abed H. Labial pyogenic granuloma related to trauma: A case report and mini-review. Dent Traumatol. 2020;36:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Asnaashari M, Mehdipour M, MoradiAbbasabadi F, Azari-Marhabi S. Expedited removal of pyogenic granuloma by diode laser in a pediatric patient. J Lasers Med Sci. 2015;6:40-44. [PubMed] |
| 21. | Yuan K, Jin YT, Lin MT. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by immunohistochemistry. J Periodontol. 2000;71:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Lee K, Seo I, Choi MH, Jeong D. Roles of Mitogen-Activated Protein Kinases in Osteoclast Biology. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 23. | Powell JL, Bailey CL, Coopland AT, Otis CN, Frank JL, Meyer I. Nd:YAG laser excision of a giant gingival pyogenic granuloma of pregnancy. Lasers Surg Med. 1994;14:178-183. [PubMed] [DOI] [Full Text] |
| 24. | Pagliai KA, Cohen BA. Pyogenic granuloma in children. Pediatr Dermatol. 2004;21:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Meffert JJ, Cagna DR, Meffert RM. Treatment of oral granulation tissue with the flashlamp pulsed dye laser. Dermatol Surg. 1998;24:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Al-Mohaya MA, Al-Malik AM. Excision of oral pyogenic granuloma in a diabetic patient with 940nm diode laser. Saudi Med J. 2016;37:1395-1400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Ishida CE, Ramos-e-Silva M. Cryosurgery in oral lesions. Int J Dermatol. 1998;37:283-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Mirshams M, Daneshpazhooh M, Mirshekari A, Taheri A, Mansoori P, Hekmat S. Cryotherapy in the treatment of pyogenic granuloma. J Eur Acad Dermatol Venereol. 2006;20: 788-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Parisi E, Glick PH, Glick M. Recurrent intraoral pyogenic granuloma with satellitosis treated with corticosteroids. Oral Dis. 2006;12:70-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Taira JW, Hill TL, Everett MA. Lobular capillary hemangioma (pyogenic granuloma) with satellitosis. J Am Acad Dermatol. 1992;27:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Esmeili T, Lozada-Nur F, Epstein J. Common benign oral soft tissue masses. Dent Clin North Am. 2005;49:223-240, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |