Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3899
Peer-review started: March 17, 2023
First decision: April 11, 2023
Revised: April 24, 2023
Accepted: April 27, 2023
Article in press: April 27, 2023
Published online: June 6, 2023
Processing time: 77 Days and 7.6 Hours
Perinatal brain injury may lead to later neurodevelopmental disorders, whose outcomes may vary due to neuroplasticity in young children. Recent neuro
We report the case of an 8-year-old boy who presented with reading difficulty following a perinatal injury in the parieto-temporal-occipital lobes. The patient was born at term and was treated for hypoglycemia and seizures during the neonatal period. Diffusion-weighted brain magnetic resonance imaging on postnatal day 4 revealed cortical and subcortical hyperintensities in the parieto-temporo-occipital lobe. At the age of 8 years, physical examination was unremarkable, aside from mild clumsiness. Despite occipital lobe injury, the patient had adequate visual acuity, normal eye movement, and no visual field defects. Full-scale intelligence quotient and verbal comprehension index on Wechsler Intelligence Scale for Children-Fourth Edition were 75 and 90, respectively. Further assessment revealed adequate recognition of Japanese Hiragana letters. However, he had significantly slower reading speed in the Hiragana reading test than control children. The phonological awareness test revealed significant errors (standard deviation +2.7) in the mora reversal task.
Patients with perinatal brain injuries in the parietotemporal area require attention and may benefit from additional reading instructions.
Core Tip: Limited research on the effect of perinatal cerebral injury on the development of reading ability in childhood is available. Herein, we report the case of an 8-year-old boy presenting with reading difficulty (dyslexia) following perinatal injury in the parieto-temporal-occipital lobes. Despite occipital lobe injury, the patient had adequate visual acuity, normal eye movement, and no visual field defects. His verbal comprehension index on the Wechsler Intelligence Scale for Children-Fourth Edition and ability to adequately recognize Japanese Hiragana letters were adequate. However, he showed remarkably poor reading fluency and phonological awareness. Careful attention should be paid to patients with perinatal brain injury in the parietotemporal region.
- Citation: Kurahashi N, Ogaya S, Maki Y, Nonobe N, Kumai S, Hosokawa Y, Ogawa C, Yamada K, Maruyama K, Miura K, Nakamura M. Reading impairment after neonatal hypoglycemia with parieto-temporo-occipital injury without cortical blindness: A case report. World J Clin Cases 2023; 11(16): 3899-3906
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3899.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3899
Reading is crucial for academic and social success. For reading acquisition, developing phonological awareness and decoding skills in early childhood is vital[1]. Phonological awareness is the ability to recognize, identify, and manipulate syllables and phonemes in language[2]. Decoding is the process of using vowel and consonant combinations to determine word pronunciation[1].
Previous studies using diffusion tensor imaging have demonstrated that microstructural differences in the left parietotemporal region correlate with reading proficiency in the general population[3,4]. In addition, studies using functional magnetic resonance imaging have demonstrated that the left parietotemporal area is essential for decoding each letter to its respective sound, even for Japanese Hiragana letters[5,6]. Currently, literature describing the effect of prenatal injury to the parietotemporal area on reading ability in later life is limited.
An 8-year-old boy with reading difficulties was referred to our hospital. His mother reported that he struggled with chunking letters when reading, while accurately differentiating between similar Hiragana letters.
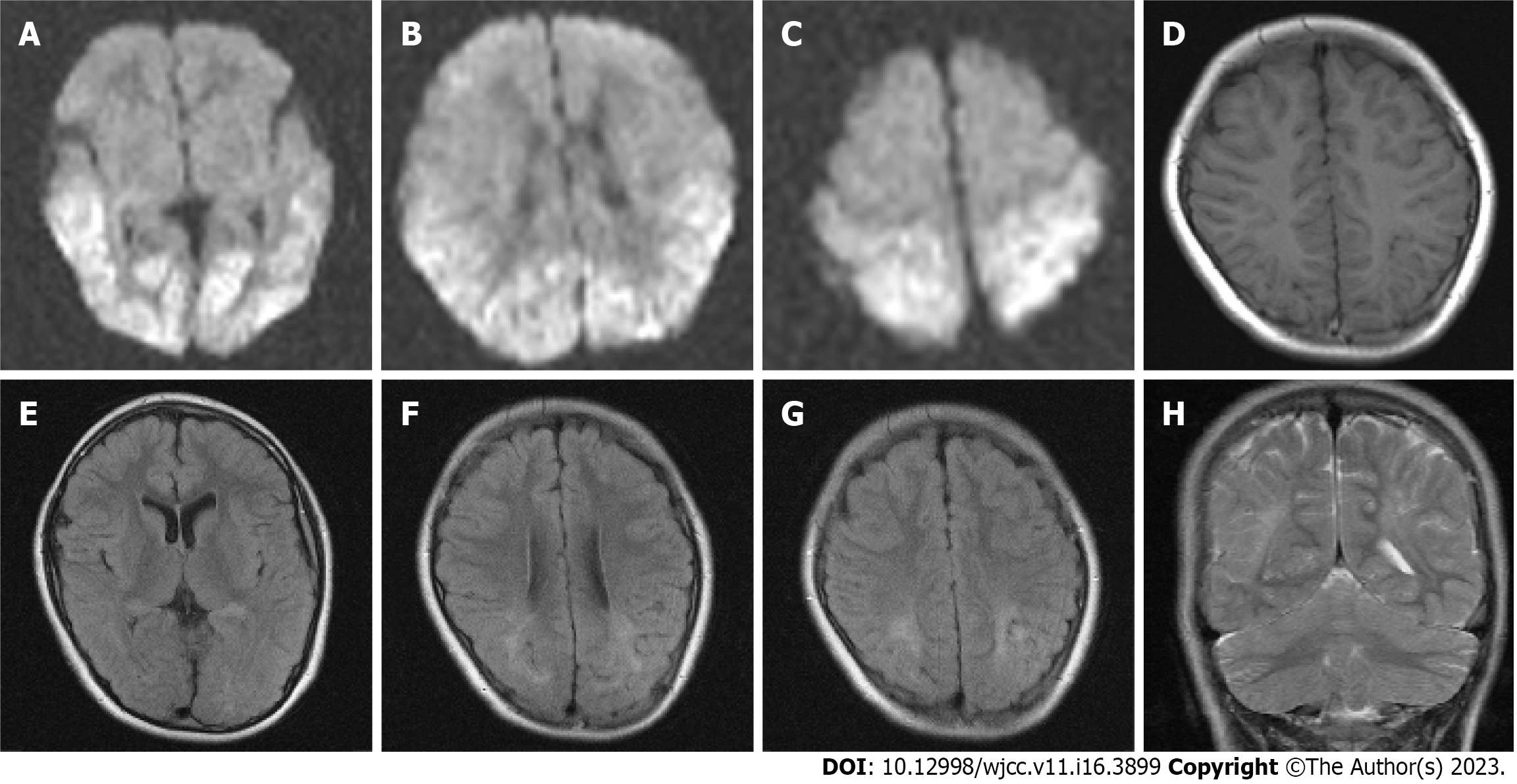
The patient was born at term via emergency cesarean section due to pregnancy-induced hypertension, with a birth weight of 2628 g and Apgar scores of 10 (at 1 min and 5 min). His mother had finished junior college and did not have diabetes mellitus. On day 2, he was admitted to the neonatal intensive care unit for poor feeding, apnea, and hypoglycemia (25 mg/dL), and was treated with oxygen and intravenous glucose. On day 3, he experienced neonatal seizures and was administered midazolam for 1 wk. No signs of infection or inborn metabolic errors were observed. Diffusion-weighted imaging on day 4 revealed cortical and subcortical hyperintensities in the bilateral occipital, parietal, and temporal lobes (Figures 1A-C), which diminished on day 12. The patient was discharged after 2 wk.
His developmental milestones were mildly delayed during infancy; he walked, uttered single words, and said two-word sentences at 18, 16, and 27 mo, respectively. His head circumference during youth was within the normal range and his intelligence quotient on the Tanaka-Binet Intelligence Scale at the age of 4 years was 76. He received temporary educational support for simple calculations and clock reading in the first grade, but required no educational support in the second grade, although he received private tutoring after school. His reading problems emerged in the third grade, where he struggled with longer sentences. He had no difficulties solving simple calculations for his age and could solve mathematical problems when the words were read aloud.
No other relevant history was noted.
No dyslexia or other psychological problems were reported within the family.
Ophthalmological examination showed that his vision was spared (Table 1). Neurological examination showed some soft neurological signs, which were otherwise normal. He had no dysarthria.
| Physical examination parameters | |
| BCVA, LogMAR (OD/OS) | 0.00/0.10 |
| SER(D), (OD/OS) | -2.875/0.375 |
| Eye movement | Normal |
| Strabismus | No |
| Fundus oculi | Normal |
| Confrontational visual field test | Not defected |
| Neurologic examination | Mildly poor performance in diadochokinesis and finger opposition test |
| Psychological test parameter | |
| Wechsler Intelligence Scale for Children-IV | |
| Full scale intelligence quotient | 75 |
| VCI/PRI/WMI/PSI | 90/76/76/73 |
| Subtest digit span, SD | -0.67 |
| Developmental test for visual perception | |
| Perceptional quotient | 64 |
| Subtest I/II/III/IV/V, perceptual age equivalent (year:month) | 5:03/5:00/4:06/5:08/6:06 |
| Kaufman Assessment Battery for Children II | |
| Cognitive ability | 62 |
| Sequential processing/simultaneous processing/planning/learning | 68/60/66/69 |
| Academic achievement | 84 |
| Knowledge/reading/writing/arithmetic | 88/96/66/102 |
| Subtest reading/decoding | 13 |
| Subtest reading and comprehension | 6 |
| Subtest verbal knowledge | 11 |
| Subtest expressive vocabulary | 6 |
| Hiragana reading test | |
| Reading time/error, SD | |
| Single mora task | +5.17/+6.63 |
| Word task (words) | +4.06/+0.86 |
| Word task (non-words) | +2.95/+3.25 |
| Sentence task | +5.52/+2.00 |
| Phonological awareness task (mora reversal task) | |
| Error, SD | |
| Three-mora word | +0.66 |
| Four-mora word | +2.70 |
Laboratory test values were within normal limits, including those for thyroid function.
Magnetic resonance imaging scans obtained at 8 years of age showed mild volume loss in the parieto-temporal region compared with the frontal region, with minimal cortical changes (Figures 1D and F). Fluid-attenuated inversion images obtained at 8 years of age showed a high-intensity area in the white matter of the bilateral parieto-temporo-occipital lobes, which included the periventricular region at the trigone of the lateral ventricles and centrum semiovale (Figures 1E-G). Coronal T2-weighted imaging showed ulegyria in the bilateral parieto-temporal regions (Figure 1H).
To assess his comorbid neurodevelopmental disorders, his mother rated him using the attention-deficit/hyperactivity disorder (ADHD) Rating Scale-IV. His inattention and hyperactivity-impulsivity subscale scores were in the 80th and 50th percentiles, respectively. Additional interviews did not indicate comorbid ADHD or autism spectrum disorder.
The psychological test results are presented in Table 1. His full-scale IQ on the Wechsler Intelligence Scale for Children-Fourth Edition was subnormal, while his verbal comprehension index was normal. The patient showed impaired cerebral visual perception. His ability to recognize Japanese hiragana letters was adequate for his age, as shown in the Reading/Decoding subtest of the Kaufman Assessment Battery for Children-Second Edition (K-ABC II), which analyzes only the accuracy of letter recognition and is unable to detect the disability of the decoding speed (Table 1). We focused on reading speed for the screening of reading disabilities, as Japanese hiragana characters are phonograms whose letter-to-sound correspondence is extremely clear[7]. In such languages, reading speed is more sensitive than reading errors as an indicator of reading ability[8]. Using the hiragana reading test, a time trial test to evaluate both the accuracy and speed of Japanese hiragana, it was revealed that his hiragana reading speed was significantly impaired. Moreover, his phonological weakness was indicated by significant errors in the Mora reversal test[9,10].
The findings of this evaluation resembled those associated with dyslexia, with poor decoding skills and phonological weakness. Dyslexia is characterized by reading disabilities, which are typically caused by impaired decoding skills and phonological weakness[1].
We recommended support using a strategy for children with dyslexia[11]. We insisted to use special textbooks or paper materials with enlarged letters and wider line spacing for dyslexic children. However, the patient refused to do so as he preferred to use the same equipment and materials as other children. Thus, instead, he was given a reduced load of homework and was also assigned an additional support caregiver for his class so that written materials could be read aloud for him.
Before his medical assessment, he was not an active participant in the class, and he had lost his confidence. After his disability was explained to the patient, his parents, and his teachers and classmates, and after his study environment was improved, his willingness to participate in class increased and he showed better adaptation to school activities and works.
A range of long-term developmental deficits may become apparent after perinatal brain injury, depending on the lesions present, and neuroplasticity may modify developmental outcomes[12-14]. Neonatal encephalopathy associated with hypoglycemia commonly affects the occipital lobes and posterior parietotemporal regions. It is reported to cause cortical visual impairment related to occipital lobe injury, intellectual disabilities, cerebral palsy, and intractable epilepsy[15-18]. These individuals are also reported to be at risk of learning and behavioral problems, hyperactivity, attention deficits, and autistic features at school age; however, little has been reported regarding their reading skills[16,18].
In the present case, the patient’s reading disability was evident from his significantly impaired reading speed. Although the development of his phonological awareness was significantly delayed compared to that of control children, as shown by significant errors in the mora reversal task, his phonological working memory was spared, as demonstrated by the digit span subtest score on the Wechsler Intelligence Scale for Children-Fourth Edition[9]. In addition, his reading disability was not based on ophthalmological problems or inadequate letter recognition and was demonstrated by his adequate ability to recognize Japanese Hiragana characters shown in the K-ABC II, a test that is not time-limited. Based on these details, we suspected that his reading impairment was related to decoding skill impairment caused by perinatal brain injury that involved the bilateral parietotemporal area.
Little is known regarding the effects of perinatal brain injury on reading ability in later life. Previous studies have shown that at school age, perinatal brain injuries, such as intraventricular hemorrhage, ventriculomegaly, and periventricular leukomalacia, are significant risk factors for lower academic skills (including reading performance) in extremely low birth weight children, independent of gestational age[19,20]. However, this study did not conduct further analysis of the relationship between the affected brain region and impaired reading ability. Recently, we reported a three-case series of Japanese preterm-born school-aged children with Hiragana-reading deficits. Their reading deficit was strongly suspected to be related to decoding impairment due to perinatal brain injury, specifically periventricular leukomalacia in the parietotemporal region[10]. In contrast, a case report of a girl with periventricular leukomalacia described the possibility of neuroplasticity in young children. The patient’s reading and phonological processing skills were spared, although her arcuate fasciculi were destroyed. Based on findings of multiple analyses of diffusion tensor imaging, it was speculated that her spared skills were related to other intact white matter tracts[13]. Our case findings add that perinatal injury in the parietotemporal area is a risk factor for decoding impairment, even in children born at term, while neuroplasticity may modify their outcomes.
Other factors may have affected the patient’s reading ability. First, his impaired cerebral visual perception might have affected his reading speed. Poor decoding skills and phonological awareness are recognized as typical characteristics of dyslexia, even in Japanese Hiragana readers[9,21]. However, cerebral visual impairment may affect reading ability when a visual attentional disorder or simultanagnosia are present, as well as when vision clarity, the visual field, or the ability to recognize the spelling materials are impaired[22]. We did not investigate the presence of a visual attentional skill disorder or simultanagnosia in our patient. However, it should be noted that our patient had adequate skill to recognize Hiragana letters, while his phonological awareness was poor. Second, we did not test his naming-speed skills, such as rapid automatized naming tasks. Naming-speed skill is an important factor that affects decoding fluency, reduction of which may affect reading ability even in Japanese Hiragana users[23,24]. Third, we did not exclude the possibility of spontaneous learning disorders. Finally, we did not compare his reading speed with that of full scale intelligence quotient-matched children or with those with a similar perceptual reasoning index or processing speed index. His borderline full scale intelligence quotient score may have affected his reading speed or mora reversal test results; however, this effect may be limited because a recent study has shown no difference in Hiragana non-word fluency reading scores among Japanese students with dyslexia with normal or borderline intelligence quotient[25].
School-age survivors of neonatal encephalopathy in the posterior region, involving the parietotemporal area, may be at risk of impaired reading ability even when their ophthalmologic findings are spared. Thorough assessments, including ophthalmological evaluation, psychological tests, and reading tests, are required to assess any educational support needs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nambi G, Saudi Arabia; Surani S, United States S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Shaywitz SE, Morris R, Shaywitz BA. The education of dyslexic children from childhood to young adulthood. Annu Rev Psychol. 2008;59:451-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychol Bull. 1987;101:192-212. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1882] [Cited by in RCA: 1881] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 3. | Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Kim SK. Recent update on reading disability (dyslexia) focused on neurobiology. Clin Exp Pediatr. 2021;64:497-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 560] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 6. | Sakurai Y, Momose T, Iwata M, Sudo Y, Ohtomo K, Kanazawa I. Different cortical activity in reading of Kanji words, Kana words and Kana nonwords. Brain Res Cogn Brain Res. 2000;9:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Wydell TN, Butterworth B. A case study of an English-Japanese bilingual with monolingual dyslexia. Cognition. 1999;70:273-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Paulesu E, Démonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. Dyslexia: cultural diversity and biological unity. Science. 2001;291:2165-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 530] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 9. | Seki A, Kassai K, Uchiyama H, Koeda T. Reading ability and phonological awareness in Japanese children with dyslexia. Brain Dev. 2008;30:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kurahashi N, Futamura Y, Nonobe N, Ogaya S, Maki Y, Yoshimura I, Suzuki T, Hosokawa Y, Yamada K, Aso K, Maruyama K, Nakamura M. Is hiragana decoding impaired in children with periventricular leukomalacia? Brain Dev. 2018;40:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Research Group for Formulation of Diagnostic Criteria and Medical Guideline for Specific Developmental Disorders. Diagnostic Criteria and Medical Guideline for Specific Developmental Disorders (in Japanese). Tokyo: ShindanToChiryosha, 2010: 45-62. |
| 12. | Ballantyne AO, Spilkin AM, Hesselink J, Trauner DA. Plasticity in the developing brain: intellectual, language and academic functions in children with ischaemic perinatal stroke. Brain. 2008;131:2975-2985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Yeatman JD, Feldman HM. Neural plasticity after pre-linguistic injury to the arcuate and superior longitudinal fasciculi. Cortex. 2013;49:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Gaberova K, Pacheva I, Timova E, Petkova A, Velkova K, Ivanov I. An Individualized Approach to Neuroplasticity After Early Unilateral Brain Damage. Front Psychiatry. 2019;10:747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Barkovich AJ, Ali FA, Rowley HA, Bass N. Imaging patterns of neonatal hypoglycemia. AJNR Am J Neuroradiol. 1998;19:523-528. [PubMed] |
| 16. | Yalnizoglu D, Haliloglu G, Turanli G, Cila A, Topcu M. Neurologic outcome in patients with MRI pattern of damage typical for neonatal hypoglycemia. Brain Dev. 2007;29:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Tam EW, Widjaja E, Blaser SI, Macgregor DL, Satodia P, Moore AM. Occipital lobe injury and cortical visual outcomes after neonatal hypoglycemia. Pediatrics. 2008;122:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Karaoğlu P, Polat Aİ, Yiş U, Hız S. Parieto-occipital encephalomalacia in children; clinical and electrophysiological features of twenty-seven cases. J Pediatr Neurosci. 2015;10:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, Ment LR. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111:e340-e346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Downie AL, Frisk V, Jakobson LS. The impact of periventricular brain injury on reading and spelling abilities in the late elementary and adolescent years. Child Neuropsychol. 2005;11:479-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Kita Y, Yamamoto H, Oba K, Terasawa Y, Moriguchi Y, Uchiyama H, Seki A, Koeda T, Inagaki M. Altered brain activity for phonological manipulation in dyslexic Japanese children. Brain. 2013;136:3696-3708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Chokron S, Kovarski K, Dutton GN. Cortical Visual Impairments and Learning Disabilities. Front Hum Neurosci. 2021;15:713316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. J Educ Psychol. 1999;91:415-438. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1062] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 24. | Kobayashi MS, Haynes CW, Macaruso P, Hook PE, Kato J. Effects of mora deletion, nonword repetition, rapid naming, and visual search performance on beginning reading in Japanese. Ann Dyslexia. 2005;55:105-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Yamaguchi D, Hiratani M, Matsuura N, Fujisawa TX, Takiguchi S, Fujioka T, Kono T, Ishizaka I, Tomoda A. The influence of intelligence and cognitive abilities on the reading ability of Japanese students with developmental disorders. Brain Dev. 2022;44:361-371. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |