Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3847
Peer-review started: February 10, 2023
First decision: April 10, 2023
Revised: April 14, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: June 6, 2023
Processing time: 112 Days and 5.4 Hours
Primary adenoid cystic carcinoma in the trachea (TACC) is a rare tumour. Tracheal bronchoscopy is always chosen as a routine approach to obtain a pathological diagnosis, but it can be associated with an increased risk of asphyxia.
We describe a case of TACC in a patient evaluated by chest computed tomography (CT) with three-dimensional reconstruction imaging and diagnosed by transoesophageal endoscopic ultrasonography. The pathological diagnosis confirmed tracheal adenoid cystic carcinoma.
We highlight the importance of CT and provide a successful exploration of transoesophageal biopsy as a safe alternative approach.
Core Tip: For large tracheal masses, tracheal bronchoscopy has certain limitations. This case highlights the importance of chest computed tomography for radiological assessment and provides a successful exploration of transoesophageal endoscopic ultrasound biopsy as a safe alternative biopsy approach.
- Citation: Pu XX, Xu QW, Liu BY. TACC diagnosed by transoesophageal endoscopic ultrasonography: A case report. World J Clin Cases 2023; 11(16): 3847-3851
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3847.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3847
Adenoid cystic carcinoma (ACC) is a rare malignant tumour of the salivary gland, especially in the trachea. In tracheal tumours, the incidence is secondary to squamous cell carcinoma[1], which is mostly located in the distal trachea, while ACC is usually located in the proximal trachea[2]. ACC is slow growing and locally aggressive with a propensity for perineural invasion and haematogenous spread. Surgery is often the first choice unless the difficulty and risk of surgery are assessed to be high.
A 64-year-old male was admitted to our hospital with the chief complaint of paroxysmal cough for more than 10 mo.
The main clinical manifestations of this patient with no smoking history were paroxysmal without sputum production and wheezing for more than 10 mo.
Physical examination showed no abnormalities.
Laboratory examinations showed no abnormalities.
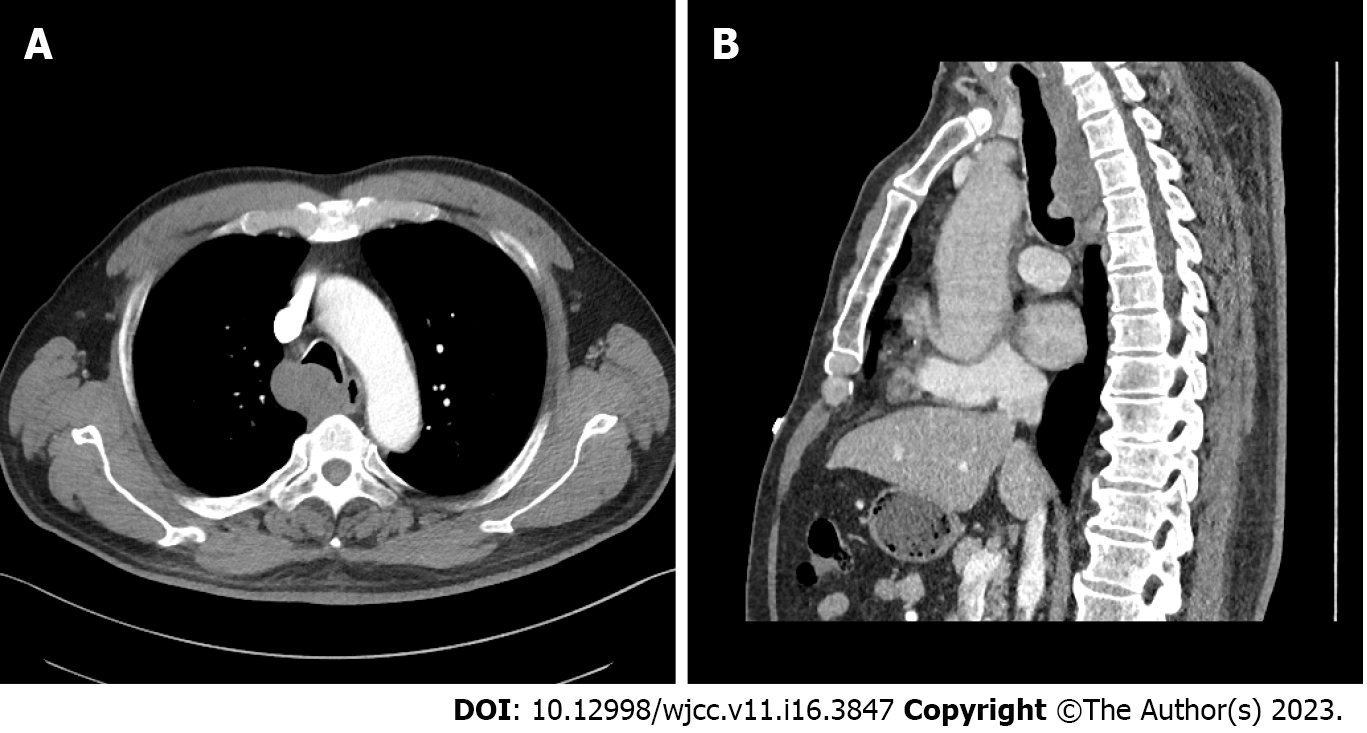
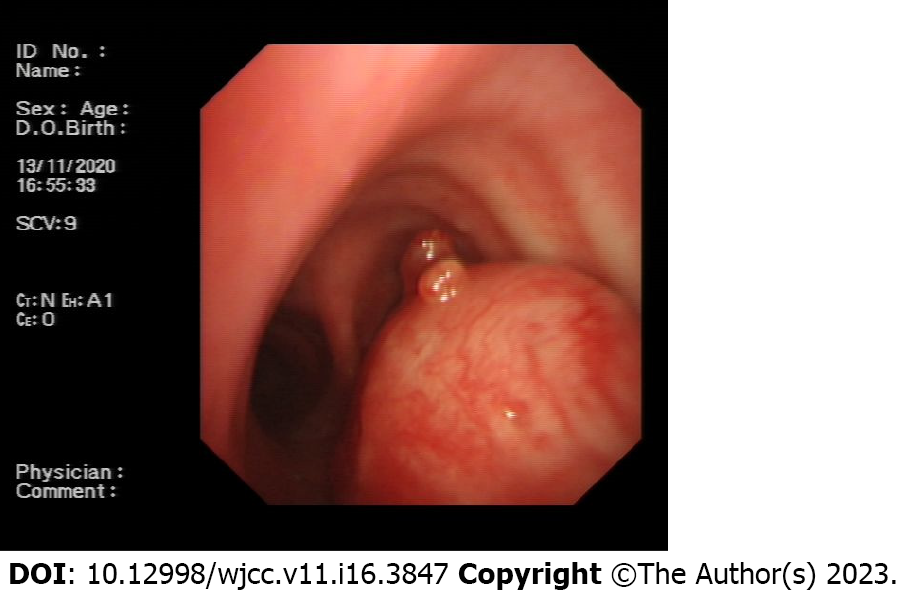
The chest X-ray showed no abnormal signs. Then, we performed a chest computed tomography (CT), which revealed airway occupation (Figure 1A). After a multidisciplinary consultation, we performed CT with three-dimensional (3D) airway reconstruction (Figure 1B) and completed tracheal bronchoscopy. However, bronchoscopy (Figure 2) showed that in the upper part of the trachea, the tumour protruded into the lumen from the membrane and extended to the carina, and the bronchoscope could barely pass through. The left and right main bronchi were unobstructed. As shown by bronchoscopy, the surface of the mass showed abundant vascularity.
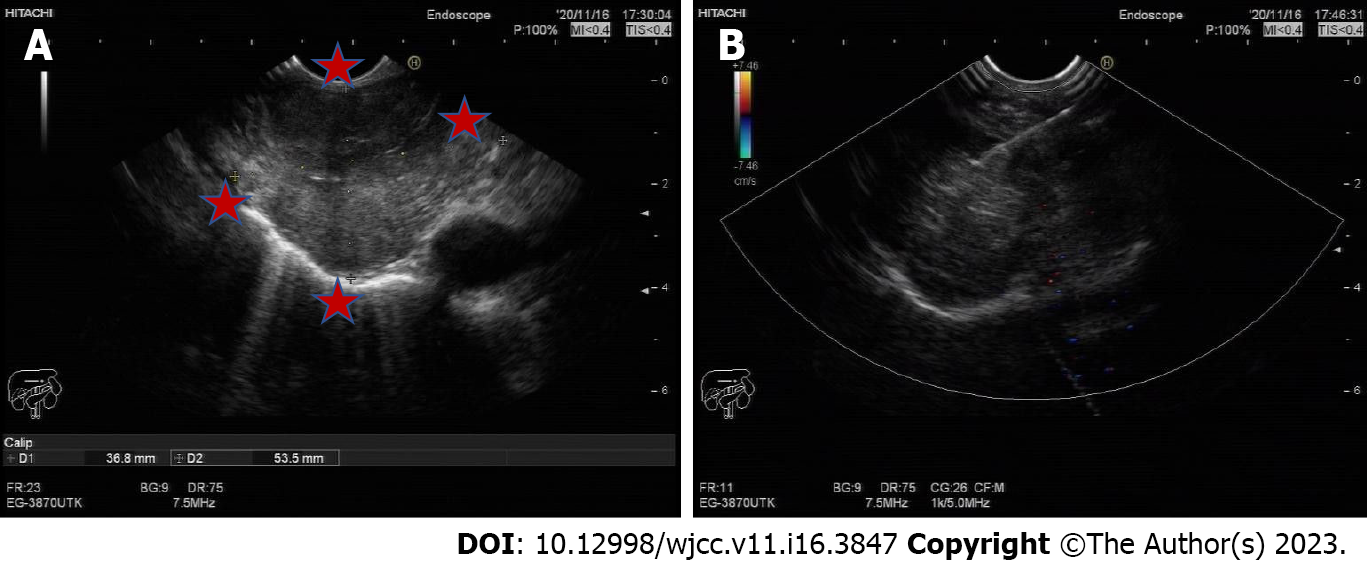
Considering bleeding and the possibility of asphyxia, we decided to perform transoesophageal endoscopic ultrasonography after multidisciplinary treatment. A mucous eminence lesion was found on the right side of the oesophagus 25 cm away from the incisor. An ultrasound scan (Figure 3) revealed an extranuclear medium- and low-echo mass with an unclear boundary and uneven internal echo, with a size of 3.7 cm × 5.4 cm. Colour Doppler flow imaging showed few blood flow signals in this part of the mass, and a 22G puncture needle was used to repeatedly puncture the mass under ultrasound guidance. Finally, the tissue was successfully obtained.
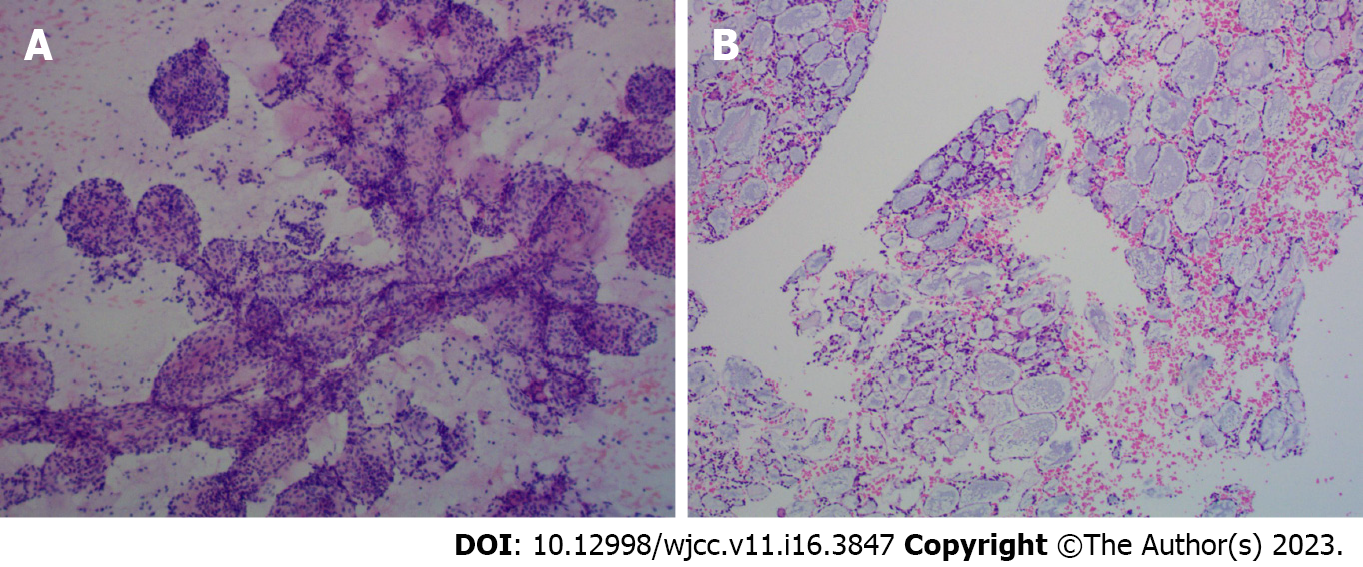
Cytological examination of the tracheal tumours (Figure 4A) showed small lamellar cells arranged in sacs and follicles containing myxoid substances and a few eosinophilic epithelioid cells forming tubules, which were considered salivary gland epithelial-myoepithelial tumours. Histological findings (Figure 4B) of the tracheal tumours revealed that the tumour cells arranged in the puncture tissue were microcystic, contained thin mucus and were surrounded by eosinophilic tubules. Morphological and immunomarker results supported epithelial-myoepithelial-derived tumours, which tend to be adenoid cystic carcinoma. Combined with CT scans, the diagnosis confirmed adenoid cystic carcinoma of the trachea.
Considering that aggressive resection of tracheal lesions may cause fatal complications and that the tumour was slow growing, after consulting other medical institutions, our patient received local radiotherapy in another hospital.
Our patient lost contact one year later.
Tracheal adenoid cystic carcinoma (TACC), originating in the tracheal submucous gland, is relatively rare[3,4]. The low incidence of TACC may be due to the vigorous cough reflex and the cleaning action of airway cilia. TACC is low-grade malignant, grows slowly, and can be polypoid or nodular and protrude into the trachea with a broad base. TACC can also invade the trachea and cause a soft tissue mass inside and outside the lumen, cause stenosis and obstruction of the lumen, and grow along the long axis of the tubular wall.
Microscopically, the tumour tissues show similar sieve and tubular structures, and the tumour cells are small and consistent, with little cytoplasm, an increased ratio of nucleus to cytoplasm, hyperchromatic nuclei, and rare mitosis. Pink-stained basement membrane-like substances can be seen in some lumens, blue-stained mucin-like substances can be seen in some lumens, and both substances can be found in some lumens[5]. Differential diagnoses includes squamous cell carcinoma, carcinoid, mucoepidermoid carcinoma, nonsquamous bronchial carcinoma, lymphoma, etc.[6].
The diagnosis is often delayed and early diagnosis is not obtained because of the absence of specific symptoms until the mass occludes most of the luminal diameter, and the tumour may cause symptoms such as cough, wheezing and exertional dyspnoea. TACC is often misdiagnosed as chronic obstructive pulmonary disease, asthma or airway inflammation. It is often difficult to detect lumps or eminences of the track on chest X-ray. Our case may be a good example. At the beginning, the patient presented with only a mild cough, and our community doctors thought it was bronchitis after completing the chest X-ray. Ten months later, we found the mass via a chest CT scan. Therefore, CT is particularly important[7]. In particular, CT with 3D reconstruction was used to assess airway involvement, and a combined enhancement scan was used to assess infiltration.
To obtain a pathological diagnosis and provide a better follow-up assessment, tissue biopsy was necessary. Tracheal bronchoscopy is always chosen as a routine approach to achieve this aim. The site can be confirmed visually, and enough prior information can be provided, including the appropriate size and position of the endotracheal intubation once asphyxia occurs. Conversely, the risk of asphyxia can be increased due to bleeding and mucosal oedema, especially when the tumour already causes almost 50% central airway obstruction; therefore, the bronchial approach may not be suitable. TEUS may be a safe alternative approach.
Surgical resection is the main treatment method at present, local mass resection is the main principle of radical treatment, and postoperative radiotherapy can reduce the recurrence rate[8]. However, due to the special location of the trachea, it is difficult to perform extended or complete resection, and aggressive resections may cause unpredictable and even fatal consequences, such as tracheoesophageal leakage. Therefore, partial resections with adjuvant therapy may help these patients obtain a promising prognosis. In addition to conventional radiation therapy, other treatment approaches have been attempted, such as radiotherapy with photons and hadron irradiation with carbon ions (C12)[9]. After consulting other medical institutions, the patient chose radiation therapy instead of a palliative operation.
There are several limitations in our report. First, rigid bronchoscopy may have been an alternative treatment option, but our team had limited experience with this approach. Second, our patient may have benefitted from undergoing palliative operation to treat the mass. Furthermore, we only provided short-term follow-up, and more detailed follow-up is needed.
In summary, we report a case of TACC. For tracheal masses, tracheal-bronchial biopsy is routine, but for large tracheal masses, tracheal bronchoscopy has certain limitations. This case highlights the importance of chest CT for radiological assessment and provides a successful exploration of transoesophageal endoscopic ultrasound biopsy as a safe alternative biopsy approach.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gokce E, Turkey; Zharikov YO, Russia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Junker K. Pathology of tracheal tumors. Thorac Surg Clin. 2014;24:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | El Marjany M, Arsalane A, Sifat H, Andaloussi K, Oukabli M, Hadadi K, Kabiri el H, Mansouri H. Primary adenoid cystic carcinoma of the trachea: a report of two cases and literature review. Pan Afr Med J. 2014;19:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Djaković Ž, Janevski Z, Cesarec V, Slobodnjak Z, Stančić-Rokotov D. Adenoid cystic carcinoma of distal trachea: A case report. Acta Clin Croat. 2019;58:777-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Desai HM, Thakare R, Amonkar GP, Karkhanis V, Joshi JM. Adenoid cystic carcinoma of the trachea. Indian J Pathol Microbiol. 2015;58:516-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Gao F, Zang L, He J, Xu W. A Case of Solid Variant of Adenoid Cystic Carcinoma from Trachea: A Case Report and Review of Literature. Onco Targets Ther. 2021;14:1997-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Gaissert HA, Grillo HC, Shadmehr BM, Wright CD, Gokhale M, Wain JC, Mathisen DJ. Laryngotracheoplastic resection for primary tumors of the proximal airway. J Thorac Cardiovasc Surg. 2005;129:1006-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Gupta D, Singh I, Sakthivel P. Adenoid Cystic Carcinoma of Trachea: A Diagnostic and Therapeutic Challenge. Indian J Otolaryngol Head Neck Surg. 2016;68:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Calzada AP, Miller M, Lai CK, Elashoff DA, Abemayor E, St John MA. Adenoid cystic carcinoma of the airway: a 30-year review at one institution. Am J Otolaryngol. 2012;33:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Högerle BA, Lasitschka F, Muley T, Bougatf N, Herfarth K, Adeberg S, Eichhorn M, Debus J, Winter H, Rieken S, Uhl M. Primary adenoid cystic carcinoma of the trachea: clinical outcome of 38 patients after interdisciplinary treatment in a single institution. Radiat Oncol. 2019;14:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |