Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3791
Peer-review started: February 8, 2023
First decision: March 14, 2023
Revised: March 28, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: June 6, 2023
Processing time: 113 Days and 12.8 Hours
Patients admitted to intensive care unit (ICU) after cardiac surgery develop acute kidney injury (AKI) immediately post-operation. We hypothesized that AKI occurs mainly due to perioperative risk factors and may affect outcome.
To assess peri-operative risk factors for AKI post cardiac surgery and its relationship with clinical outcome.
This was an observational single center, tertiary care setting study, which enrolled 206 consecutive patients, admitted to ICU after cardiac surgery. Patients were followed-up until ICU discharge or death, in order to determine the incidence of AKI, perioperative risk factors for AKI and its association with outcome. Univariate and multivariate logistic regression analysis was performed to assess predictor variables for AKI development.
After ICU admission, 55 patients (26.7%) developed AKI within 48 h. From the logistic regression analysis performed, high EuroScore II (OR: 1.18; 95%CI: 1.06-1.31, P = 0.003), white blood cells (WBC) pre-operatively (OR: 1.0; 95%CI: 1.0-1.0, P = 0.002) and history of chronic kidney disease (OR: 2.82; 95%CI: 1.195-6.65, P = 0.018) emerged as independent predictors of AKI among univariate predictors. AKI that developed AKI had longer duration of mechanical ventilation [1113 (777–2195) vs 714 (511–1020) min, P = 0.0001] and ICU length of stay [70 (28–129) vs 26 (21–51) h, P = 0.0001], higher rate of ICU-acquired weakness (16.4% vs 5.3%, P = 0.015), reintubation (10.9% vs 1.3%, P = 0.005), dialysis (7% vs 0%, P = 0.005), delirium (36.4% vs 23.8%, P = 0.001) and mortality (3.6% vs 0.7%, P = 0.046).
Patients present frequently with AKI after cardiac surgery. EuroScore II, WBC count and chronic kidney disease are independent predictors of AKI development. The occurrence of AKI is associated with poor outcome.
Core Tip: Acute kidney injury (AKI) may develop in patients after cardiac surgery. In this observational study we assessed the incidence, the peri-operative risk factors for AKI occurrence and its association with outcome in patients after cardiac surgery post- intensive care unit (ICU) admission. The results of the study have shown that AKI occurs frequently after cardiac surgery. EuroScore II, history of chronic kidney disease and white blood cell count are independent predictors of AKI development. The presence of AKI was associated with poor outcome in terms of mechanical ventilation duration, ICU length of stay, rate of dialysis, reintubation, ICU-acquired weakness, delirium and mortality.
- Citation: Dimopoulos S, Zagkotsis G, Kinti C, Rouvali N, Georgopoulou M, Mavraki M, Tasouli A, Lyberopoulou E, Roussakis A, Vasileiadis I, Nanas S, Karabinis A. Incidence and peri-operative risk factors for development of acute kidney injury in patients after cardiac surgery: A prospective observational study. World J Clin Cases 2023; 11(16): 3791-3801
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3791.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3791
Each year, more than 2 million cardiac surgeries are performed worldwide. Acute kidney injury (AKI) consists of a frequent and serious complication postoperatively. Cardiac surgery-associated acute kidney injury (CSA-AKI) has an incidence rate that varies from 5% to 42%. Apart from sepsis, it is the most common cause of AKI in the intensive care unit (ICU) setting. CSA-AKI is a major risk factor of death after cardiac surgery resulting to 3-8-fold increase in perioperative mortality, while it is associated with prolonged stay in the ICU and hospital and increased cost of care[1]. 10 years after cardiac surgery, long term mortality remains high, even for patients with complete recovery of renal function and regardless of other risk factors[2]. Many of the predisposing risk factors for AKI, such as advanced age, hypertension, smoking and chronic kidney disease, are non-modifiable[3]. However, the patho
This study aimed to investigate the prevalence of AKI as well as to assess peri-operative risk factors for AKI post cardiac surgery and its relationship with clinical outcome.
We hypothesized that there is a high prevalence of AKI post cardiac surgery and perioperative risk factors play a major role for developing AKI in these patients.
This prospective observational study was conducted at the Cardiac Surgery ICU of Onassis Cardiac Surgery Center during a 3-mo period. The research was approved by Ethics Committee of the Onassis Cardiac Surgery Center (Number Id: 663/12.12.19) with obtained patient`s informed consent and carried out in accordance with the ethical standards set by the Declaration of Helsinki.
Inclusion criteria were consecutive adult (> 20 years old) patients following their admission in the Cardiac Surgery ICU within 24 h of cardiac surgery.
Participants with chronic renal failure requiring dialysis prior to cardiac surgery operation were excluded from the study. Moreover patients who were re-admitted to the ICU were not included twice in the study.
This was a prospective observational study conducted in a single center Cardiac Surgery ICU. All patients were enrolled consecutively to the study post cardiac surgery and they were followed-up until ICU discharge.
Demographic data and baseline clinical perioperative characteristics of all participants and according to AKI development within the first 48 h after cardiac surgery and post ICU admission were prospectively collected and are presented in Table 1. Chronic kidney disease (CKD) was defined by the presence of an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2, that was calculated preoperatively using the CKD-EPI equation[5].
| Total population (%) | N = 206 | AKI | No AKI | P value |
| 206 (100) | 55 (26.7) | 151 (73.3) | ||
| Baseline pre-operative characteristics | ||||
| Gender, female | 62 (30.1) | 13 (23.6) | 49 (32.5) | 0.236 |
| Age (yr) | 70 (59-76) | 74 (59–79) | 68.5 (59–74) | 0.039 |
| BMI (kg/m2) | 27.8 (25.2-31.3) | 27.0 (24.8–30.0) | 28.1 (25.4–31.5) | 0.308 |
| EuroScore II (%) | 1.7 (1.09-2.9) | 2.54 (1.52–4.4) | 1.54 (0.94–2.4) | 0.0001 |
| Hb (gr/dL) | 10.3 (9.6-11) | 10.3 (9.8–10.9) | 10.2 (9.5–11.0) | 0.603 |
| WBC (×103/μL) | 10.3 (7.9-13.1) | 11.4 (8.9–13.7) | 9.5 (7.5–12.8) | 0.015 |
| Creatinine (mg/dL) | 0.9 (0.8-1.1) | 1.0 (0.9–1.3) | 0.9 (0.8–1.1) | 0.009 |
| CKD | 50 (24.3) | 22 (40) | 28 (18.5) | 0.003 |
| eGFR (mL/min/1.73 m2) | 79.1 (60.2-89.9) | 69.1 (52.3-84.2) | 81.3 (63.1-90.8) | 0.007 |
| PPC | 5 (2.4) | 1 (1.8) | 4 (2.6) | 1.0 |
| ICD | 5 (2.4) | 4 (7.3) | 1 (0.7) | 0.019 |
| PAD | 10 (4.9) | 4 (7.3) | 6 (4) | 0.462 |
| Thyroid disease | 36 (17.5) | 7 (12.7) | 29 (19.2) | 0.309 |
| Stroke | 3 (1.5) | 1 (1.8) | 2 (1.3) | 1.0 |
| Intraoperative characteristics | ||||
| CPB (Extracorporeal circulation) | 198 (96.1) | 51 (92.7) | 147 (97.4) | 0.214 |
| Duration of CPB (min), Ν = 198 | 112 (84-145) | 121 (104–171) | 106 (80–141) | 0.009 |
| Aortic cross-clamp time (min), Ν = 193 | 89 (59-108) | 87 (67–111) | 77 (56–107) | 0.067 |
| Duration of general anesthesia in surgery (min) | 240 (190-300) | 273 (213–350) | 229 (183–300) | 0.016 |
| CABG | 116 (56.3) | 29 (52.7) | 87 (57.6) | 0.634 |
| Heart valve repair | 7 (3.4) | 1 (1.8) | 6 (4) | 0.678 |
| Aortic valve replacement | 71 (34.5) | 20 (36.4) | 51 (33.8) | 0.743 |
| Mitral valve replacement | 15 (7.3) | 4 (7.3) | 11 (7.3) | 1.0 |
| Tricuspid valve replacement | 7 (3.4) | 2 (3.6) | 5 (3.3) | 1.0 |
| Mixed valve replacement | 10 (4.9) | 2 (3.6) | 8 (5.3) | 1.0 |
| Aneurysm repair | 23 (11.2) | 5 (9.1) | 18 (11.9) | 0.803 |
| Acute aortic dissection | 4 (1.9) | 3 (5.5) | 1 (0.7) | 0.059 |
| Heart transplant | 4 (1.9) | 3 (5.5) | 1 (0.7) | 0.059 |
| Post-operative characteristics | ||||
| Duration of mechanical ventilation (min) | 780 (591-1198) | 1113 (777–2195) | 714 (511–1020) | 0.0001 |
| Duration of sedation in ICU (min) | 360 (250-600) | 540 (300–810) | 300 (240–480) | 0.0001 |
| Duration of sedation during and post surgery (min) | 593 (480-842) | 754 (540–1245) | 570 (476–740) | 0.0001 |
| Fluid balance 24 h post surgery (mL) | 722 (70-1418) | 1090 (330–1862) | 501 (11–1150) | 0.001 |
| Fluid balance 48 h post surgery (mL), (N = 146) | N (%); 146 (70.9) | 46 (31.5) | 100 (68.5) | 0.526 |
| -202 [(-1146) - 377] | -163 [(-1186) – 531] | -248 [(-1099) – 296] | ||
| Systolic blood pressure (mmHg) | 119 (105-135) | 116 (103 – 134) | 120 (108–135) | 0.162 |
| Diastolic blood pressure (mmHg) | 61 (55-70) | 58 (54–65) | 63 (55–70) | 0.015 |
| Mean arterial blood pressure (mmHg) | 80 (71-88) | 75 (68–84) | 80 (74–89) | 0.005 |
| Vasopressors | 65 (31.6) | 27 (49.1) | 38 (25.2) | 0.002 |
| Inotropes | 130 (63.1) | 41 (74.5) | 89 (58.9) | 0.05 |
The duration of mechanical ventilation, ICU length of stay, the incidence rate of ICU acquired weakness, delirium, reintubation and dialysis required as well as the ICU mortality outcome of enrolled patients were also recorded. (1) The primary endpoint of the present study was the incidence of AKI after cardiac surgery and the assessment of the perioperative risk factors for AKI development; and (2) The secondary endpoint was the association of AKI with clinical outcome.
The medical research council (MRC) scale was used to evaluate muscle strength. Patients proceeded to assessment as soon as were awake and cooperative. Evaluation included the measurement of six muscle groups bilaterally: shoulder abductors, elbow flexors and wrist dorsiflexors for the upper limbs as well as hip flexors, knee extensors and ankle dorsiflexors for the lower limbs. Test was performed in the same order each time. Each muscle group scored from 0, indicating no contraction, to 5, indicating normal power. Total maximum score was 60, whilst MRC score ≤ 48 was defined as ICU-acquired weakness.
The confusion assessment method for the intensive care unit (CAM-ICU) was used to assess delirium among patients during ICU stay.
AKI was defined according to the kidney disease: Improving global outcome (KDIGO) guidelines. For each patient, serum creatinine (sCr) levels were measured within 3 d prior to surgery (as baseline levels) and were monitored during the first 48 h postoperatively. AKI was defined as an absolute increase in sCr of 0.3 mg/dL within 48 h or a 1.5-fold increase from baseline[6].
Descriptive statistics analysis was performed to describe the baseline data. Distribution‘s normality was checked with Kolmogorov-Smirnov test. Normally distributed continuous variables were expressed as mean ± SD and non-normally distributed variables as median with interquartile range, and categorical variables as proportions with percentages and absolute numbers. To analyze continuous variables between patient groups, Mann Whitney test was used for those with non-normal distribution, t test for those with normal distribution and χ2 test for categorical variables. Univariate logistic regression analysis was performed for all variables to assess risk factors for AKI development. A multivariate logistic regression analysis model (enter method) was then applied to detect independent predictors of AKI for those variables with statistical significance in the univariate analysis. A receiver operating characteristics (ROC) analysis was also performed to test predictor variables for AKI development. Level of significance was set at P < 0.05. All statistical analyses were performed with SPSS v.25 software.
The present study enrolled 206 patients during a 3 mo period with a predominance of male gender (69.9%). The incidence of postoperative AKI, as defined by KDIGO guidelines, was 26.7%. The baseline demographic and clinical perioperative characteristics in the entire cohort and according to AKI status are presented in Table 1. We had all types of cardiac surgery including heart transplantation; however we did not have any case of beating heart surgery.
Patients that developed AKI were significantly older, had a higher rate of chronic kidney disease, greater EuroScore II and white blood cells, lower diastolic and mean blood pressure with higher rate of vasopressor and inotrope requirement at ICU admission than those patients without AKI. They had also greater extracorporeal circulation time and a trend to a greater cross-clamp time intra-operatively with longer duration of general anesthesia and ICU sedation than non-AKI patients.
The univariate logistic regression analysis is presented in Table 2. From the multivariate logistic regression analysis performed, high EuroScore II (OR: 1.18; 95%CI: 1.06-1.31, P = 0.003), white blood cells pre-operatively (OR: 1.0; 95%CI: 1.0-1.0, P = 0.002) and history of chronic kidney disease (OR: 2.82; 95%CI: 1.195-6.65, P = 0.018) were independent predictors of AKI (the multivariate analysis model included also univariate predictors such as mean arterial blood pressure, duration of cardiopulmonary bypass, ICU sedation time, duration of general anesthesia, use of vasopressors and inotropes and fluid balance at the 1st ICU day).
| Variable | Exp (B) | 95%CI | P value |
| Preoperative characteristics | |||
| Female sex | 0.64 | 0.32–1.31 | 0.224 |
| Age | 1.02 | 0.99–1.05 | 0.125 |
| BMI | 0.98 | 0.92–1.05 | 0.601 |
| EuroScore II | 1.21 | 1.08–1.34 | 0.001 |
| Hb | 1.01 | 0.76–1.34 | 0.948 |
| WBC | 1.0 | 1.0–1.0 | 0.014 |
| Creatinine | 1.5 | 0.76–2.96 | 0.246 |
| HTN | 0.86 | 0.4–1.85 | 0.699 |
| Diabetes | 0.91 | 0.47–1.75 | 0.777 |
| CKD | 2.93 | 1.49–5.77 | 0.002 |
| CAD | 1.02 | 0.53–1.96 | 0.959 |
| CHF | 0.12 | 0.01–1.14 | 0.64 |
| PCP | 1.47 | 0.16–13.44 | 0.733 |
| PAD | 0.53 | 0.14–1.95 | 0.337 |
| Thyroid disease | 1.63 | 0.67–3.97 | 0.282 |
| Stroke | 0.73 | 0.06–8.16 | 0.794 |
| Intraoperative characteristics | |||
| Duration of CPB | 1.01 | 1.0–1.01 | 0.044 |
| Aortic cross-clamp time | 1.01 | 1.0–1.01 | 0.222 |
| Duration of general anesthesia in surgery | 1.0 | 1.0–1.01 | 0.031 |
| Postoperative characteristics | |||
| Systolic blood pressure | 0.99 | 0.97–1.01 | 0.181 |
| Diastolic blood pressure | 0.97 | 0.94–1.0 | 0.026 |
| Mean arterial blood pressure | 0.97 | 0.94–0.99 | 0.009 |
| Duration of mechanical ventilation | 1.043 | 1.02-1.066 | < 0.001 |
| Duration of sedation in ICU | 1.0 | 1.0–1.0 | 0.004 |
| Duration of sedation during and post surgery | 1.0 | 1.0–1.0 | 0.003 |
| Fluid balance 24 h post surgery | 1.0 | 1.0–1.0 | 0.007 |
| WBC | 1.11 | 1.02–1.21 | 0.014 |
| Vasopressors | 0.35 | 0.18–0.67 | 0.001 |
| Inotropes | 0.49 | 0.25–0.98 | 0.042 |
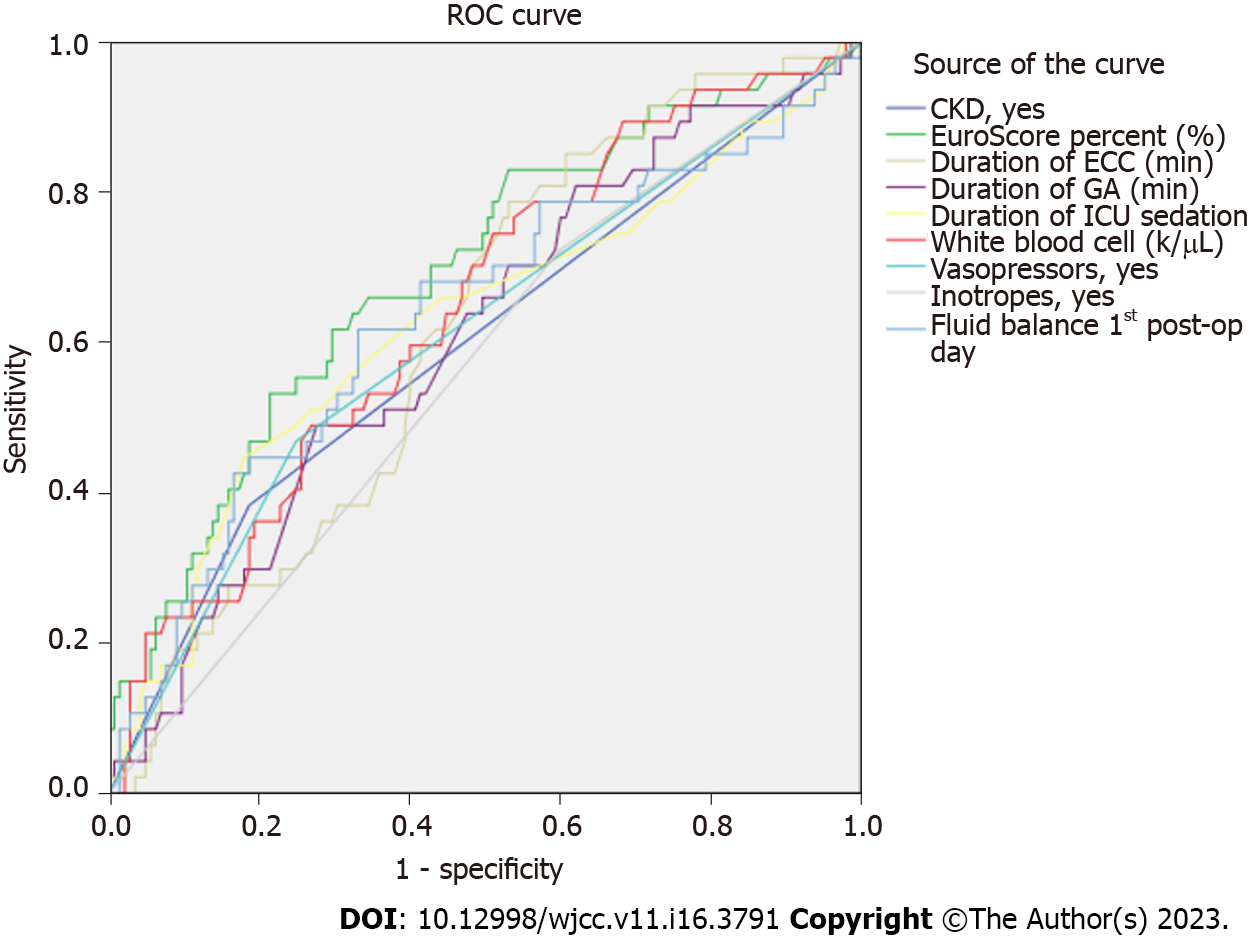
Graphic results from ROC analysis are illustrated in Figure 1 and detailed data are reported in Table 3.
| Test result variable(s) | Area under curve | SE | P value | 95%CI | |
| Lower bound | Upper bound | ||||
| CKD | 0.602 | 0.050 | 0.036 | 0.504 | 0.699 |
| EuroScore II percentage (%) | 0.692 | 0.045 | 0.000 | 0.604 | 0.781 |
| Duration of ECC (min) | 0.618 | 0.044 | 0.015 | 0.532 | 0.704 |
| Duration of GA (min) | 0.607 | 0.047 | 0.028 | 0.515 | 0.699 |
| Duration of ICU sedation (min) | 0.625 | 0.051 | 0.010 | 0.526 | 0.724 |
| White blood cell (k/uL) | 0.643 | 0.046 | 0.003 | 0.554 | 0.733 |
| Vasopressors | 0.610 | 0.049 | 0.024 | 0.514 | 0.706 |
| Inotropes | 0.562 | 0.047 | 0.204 | 0.469 | 0.654 |
| Fluid balance 1st day after cardiac surgery (mL) | 0.634 | 0.050 | 0.006 | 0.537 | 0.731 |
There was a significant association of AKI development and clinical outcome. Patients with AKI had prolonged duration of mechanical ventilation and stay in ICU, higher rate of ICU-acquired weakness, reintubation, delirium, dialysis and mortality (Table 4).
| Outcome | AKI | No AKI | P value |
| Reintubation (n) | 6 (10.9) | 2 (1.3) | 0.005 |
| Dialysis (n) | 4 (7) | 0 | 0.005 |
| Duration of mechanical ventilation (min) | 1113 (777–2195) | 714 (511–1020) | 0.0001 |
| Delirium (n) | 20 (36.4) | 36 (23.8) | 0.001 |
| ICU acquired weakness (n) | 9 (16.4) | 8 (5.3) | 0.015 |
| ICU length of stay (hours) | 70 (28–129) | 26 (21–51) | 0.0001 |
| Mortality (n) | 2 (3.6) | 1 (0.7) | 0.046 |
By this study we have shown that there is a high incidence of AKI development in patients undergoing cardiac surgery. The main results of our study demonstrated also that the pre-operative assessment severity score EuroScore II, white blood cell count and chronic kidney disease were independent predictors of AKI development. Moreover, the occurrence of CSA-AKI was significantly associated with poor outcome. More specifically, patients that developed AKI after cardiac surgery had prolonged duration of mechanical ventilation and ICU stay, higher rate of re-intubation, dialysis and mortality, while they suffered more ICU-acquired weakness and delirium.
Our study results are in line with previous reports. A moderate to high incidence of AKI has been reported previously from other researchers with, however, a wide range of results. This wide range of incidence is partly explained by the differences in study populations and the non-uniform definition of AKI reported in the current literature[4]. Notably, over thirty different arbitrary definitions have been used over time for the diagnosis and staging of AKI. The Risk, Injury, Failure, Loss, and End stage kidney disease (RIFLE) classification criteria were introduced in 2004, defining AKI as an increase in sCr by 1.5 times compared to baseline (or 25% reduction in GFR) and urine output less than 0.5 mL/kg/h for at least 6 h, over a period of 7 d. The Acute Kidney Injury Network (AKIN) group suggested a revision of these criteria in 2007, where GFR was omitted, the period for the change in sCr was reduced to 48 h, while a small increase of 0.3 mg/dL in sCr was used as a cutoff to define AKI[7]. The KDIGO workshop proposed a synthesis of the RIFLE and AKIN criteria in 2012, defining AKI as an 50% increase in SCr in 7 d, increase in SCr over 0.3 mg/dL in 48 h, or oliguria (< 0.5 mL/kg/h for 6-12 h)[6]. The pooled incidence rate of AKI after cardiac surgery was reported to be 22.3% (95%CI: 19.8 to 25.1) by Hu et al[8] in a systematic review and meta-analysis of 91 observational studies with 320068 patients that defined AKI using RIFLE, AKIN or KDIGO criteria, an incidence rate similar to the one we report. Notably, the polled incidence rate in studies using KDIGO criteria was 24.2 % (95%CI: 17.5-32.5), while greater difference was reported in studies using RIFLE [18.9%, (95%CI: 15.7-22.5)] and AKIN criteria [28.0%, (95%CI: 23.6-32.8)]. We have used the KDIGO criteria that has become the new consensus for the definition of AKI as they demonstrate greater sensitivity to detect AKI and predict in-hospital mortality in critically ill patients[9].
Unfortunately, the implementation of the above criteria in patients who have undergone cardiac surgery remains problematic as they have important limitations. Fluid resuscitation and fluid loading from the pump during CPB is very common, resulting in sCr changes by haemodilution[1,2,4]. Taking into account that sCr concentrations may take up to 24-36 h to rise after the initial renal insult, this may lead to AKI being underdiagnosed[10]. Oliguria consist another pitfall in the assessment of AKI, as it typically occurs prior to sCr increase, but often is an appropriate response to intravascular hypovolemia[3]. Significant differences in the incidence of AKI have been reported in cardiac surgery patients by Lagny et al[11], who compared AKI diagnosed by oliguria criteria vs AKI diagnosed by sCr criteria (40.2% vs 9.7%). Given the fact that urine output documentation is frequently poor, clinicians often use sCr measurements alone to diagnose AKI[3]. The use of novel urinary biomarkers, such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin and interleukin 18 (IL-18), has been proposed for the early detection of AKI, prior to the increase of sCr levels. However, they require further validation before being routinely applied in the heterogeneous population of patients who have undergone cardiac surgery[1,10].
In our study, CKD, preoperative elevated WBC count and EuroScore II were found as independent risk factors for the development of CSA-AKI. Chen et al[12], have reported the association between increased sCr and postoperative AKI in a prospective cohort study of 353 patients who received isolated CABG. Moreover, the presence of CKD has been also associated with the severity of AKI. In a retrospective observational study of 156 CKD patients who received valve surgery with CPB, performed by Fu et al[13], every +1 mg/dL increase in baseline sCr was found to result in 111% increase in the incidence of AKI stage 3. Apart from impaired intrarenal hemodynamics and accelerated atherosclerosis, CKD is characterized by a chronic inflammatory state due to proinflammatory cytokines, oxidative stress and uremia that predisposes to the development of AKI[14]. Preoperative WBC count may as well indicate subclinical inflammatory response or multi-organ dysfunction, predisposing to CSA-AKI or other postoperative complications. In a retrospective cohort study of 10979 cardiac surgery patients, excluding individuals with inflammatory syndromes like CKD or cancer, Mahmood et al[15], reported that leucocytosis (WBC > 11000/μL) was a significant predictor of medical complications, including AKI, although it was not associated with 30-day mortality. EuroScore II has been updated in 2011 and has been widely implemented to assess the mortality risk in patients undergoing cardiac surgery[16]. Since then, several studies has reported its association with the development of AKI and it has been included in preoperative scoring systems in order to predict major postoperative complications such as CSA-AKI[12,17].
The prolonged duration of CPB as well as the longer duration of sedation and mechanical ventilation support appeared to be major intra-operative risk factors for the development of CSA-AKI. These associations were confirmed in our study in univariate, but not in multivariate analysis. CPB induces AKI with complex, not completely understood mechanisms such as: (1) Low-flow, low-pressure non-pulsatile perfusion that leads to renal ischemia; (2) inflammation caused by the CPB pump and circuit resulting in formation of free radicals, complement activation and increase of proinflammatory cytokines; (3) intravascular hemolysis and free hemoglobin release causing renal tubular damage; (4) platelet activation resulting in renal ischemia from microemboli; and (5) reperfusion injury after CPB that exacerbates oxido-inflammatory stress[1,3,4,7,18]. The renoprotective effects of off-pump coronary artery bypass grafting (CABG) were evaluated as a secondary outcome in the CORONARY randomized trial, which included 4752 patients that were randomized to undergo CABG, either on-pump or off-pump. The use of off-pump CABG resulted in reduced incidence of AKI in the first 30 d (RR: 0.87, 95%CI: 0.80-0.96), but did not affect the incidence of AKI requiring dialysis[19]. In our study, there was a trend in the association between CSA-AKI and the duration of CPB, as it has been reported in previous studies[11,18]. A meta-analysis of 14 case-control studies that included 2157 patients undergoing cardiac surgery, reported that longer CPB time is associated with increased risk for CSA-AKI (OR: 33.78, 95%CI: 23.15–44.41)[18].
In our study a higher positive fluid balance (FB) 24 h post cardiac surgery was associated with increased incidence of CSA-AKI, but the association did not reach statistical significance. This correlation has been reported previously in several studies. A retrospective observational study performed by Chen et al[20], suggested that a positive FB over 5% was an independent predictor of AKI occurrence (OR: 3.976, P < 0.001). Fluid resuscitation is performed during and after cardiac surgery, in order to avoid volume depletion and hypoperfusion, especially in the case of low cardiac output syndrome. However, fluid overload leads to venous congestion that plays a major role in the development of congestive cardiorenal syndrome and AKI. Yang et al[21], showed that increased central venous pressure (CVP) after CPB operation is related with increased incidence of AKI, especially when CVP exceeds 10 cmH2O, while it is also an independent factor for mortality. On the other hand, the benefit of negative FB is questionable, as its association with decreased incidence of AKI has been reported, in the previously mentioned study by Chen et al[20], as non-significant. Moreover, a “U”-shaped correlation was found between 48h accumulative FB and AKI progression, suggesting that an accumulative FB between -5% and 3% might be a possible safe target window 48 h post cardiac surgery. Fluid management remains challenging, “cut-off” values are difficult to be determined and clinical judgment plays an important role. Efforts for the implementation of “goal-directed therapies” have been made in order to guide fluid and vasoactive drug administration by achieving preset hemodynamic or perfusion goals. Some studies showed reduction in the incidence of CSA-AKI. However, the ad
The development of AKI after cardiac surgery has been associated in several studies with worse outcomes, regarding mortality and morbidity. These associations have been confirmed in our study. In a meta-analysis performed by Hu et al[8], that included 57 observational studies, the presence of CSA-AKI resulted in increased short term mortality (unadjusted OR: 0.144, 95%CI: 0.108-0.192, P < 0.001), and in increased long term mortality (unadjusted OR: 0.342, 95%CI: 0.287-0.407 P < 0.001). Hobson et al[2] reported in a retrospective study that the impact of AKI on reduced short and long term survival was propoptional to its severity. AKI, as well as its severity, were also significantly associated with prolonged stay in ICU and in hospital. The association of CSA-AKI with other postoperative complications is a common finding in several studies. Patients with AKI is more likely to suffer prolonged mechanical ventilation support, compared with patients that did not develop AKI[23]. Moreover, CSA-AKI has been reported to result in increased risk for reintubation in patients undergoing coronary artery bypass grafting[24] as well as for the development of neurological complications like delirium[25].
This observational study, performed in “Onassis” Cardiac Surgery ICU, represents one of the first prospective studies conducted in Greece that investigate the incidence of AKI development and the peri-operative risk factors. The main limitation of this study is that, as an explorative study, its sample size was estimated based on feasibility for a predefined certain period. Hence, it might have been underpowered to demonstrate the association of AKI development during ICU and important perioperative risk factors such as CPB and cross-clamp duration. Despite the relative low sample size, we did find an important association of AKI development and clinical outcome in all outcome parameters assessed in the present study. Another limitation of the study was the cardiac surgery population included with different types of cardiac surgery which might have also underpowered the sample size.
In conclusion, patients undergoing cardiac surgery present frequently with AKI postoperatively. EuroScore II, white blood cell count and history of chronic kidney disease were independent predictors of AKI development. Importantly, AKI occurrence post cardiac surgery was found to be associated with poor outcome in terms of prolonged duration of mechanical ventilation and ICU stay, more ICU-acquired weakness and delirium and higher re-intubation, dialysis and mortality rate.
Acute kidney injury (AKI) is a complication for patients undergoing cardiac surgery that might be associated with adverse outcome.
Perioperative targeted monitoring for possible AKI risk factors remains suboptimal and identification of patients at greater risk requires further investigation.
The study aimed to assess AKI presentation after cardiac surgery, to investigate prognostic factors for its development and its association with clinical outcome.
This is a prospective observational single-center study that included 206 patients admitted in ICU post cardiac surgery followed-up until ICU discharge. Patients were divided in two groups, the AKI group that developed AKI within 48 h and the non-AKI group. Preoperative clinical characteristics, intra-operative factors and outcome were compared between two groups.
Patients presented frequently with AKI post cardiac surgery. High EuroScore II (P = 0.003), white blood cells (WBC) pre-operatively (P = 0.002) and history of kidney disease (P = 0.018) were independent predictors of AKI. AKI is associated with prolonged intensive care unit (ICU) stay, greater duration of mechanical ventilation and higher rate of dialysis, reintubation, ICU-acquired weakness, delirium and mortality.
AKI is a frequent complication post cardiac surgery associated with poor outcome. Preoperative clinical characteristics, such as EuroScore II, preoperative WBC or presence of chronic kidney disease may help in early identification and appropriate management of patients in risk for AKI.
Further investigation is necessary to assess preventive and optimal treatment strategy protocols for AKI presentation.
We would like to thank Aggeliki Dorkofiti, Professional English Translator and Editor for editing our manuscript. We would also like to thank all staff of Cardiac Surgery ICU, Onassis Cardiac Surgery Center for their continuous support throughout the study period.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eccher A, Italy; Glumac S, Croatia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 485] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 2. | Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 738] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 3. | O'Neal JB, Shaw AD, Billings FT 4th. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 4. | Fuhrman DY, Kellum JA. Epidemiology and pathophysiology of cardiac surgery-associated acute kidney injury. Curr Opin Anaesthesiol. 2017;30:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20173] [Article Influence: 1260.8] [Reference Citation Analysis (0)] |
| 6. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3348] [Article Influence: 257.5] [Reference Citation Analysis (0)] |
| 7. | Ortega-Loubon C, Fernández-Molina M, Carrascal-Hinojal Y, Fulquet-Carreras E. Cardiac surgery-associated acute kidney injury. Ann Card Anaesth. 2016;19:687-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Hu J, Chen R, Liu S, Yu X, Zou J, Ding X. Global Incidence and Outcomes of Adult Patients With Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth. 2016;30:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X; Beijing Acute Kidney Injury Trial (BAKIT) workgroup. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014;18:R144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 10. | Wu B, Chen J, Yang Y. Biomarkers of Acute Kidney Injury after Cardiac Surgery: A Narrative Review. Biomed Res Int. 2019;2019:7298635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Lagny MG, Jouret F, Koch JN, Blaffart F, Donneau AF, Albert A, Roediger L, Krzesinski JM, Defraigne JO. Incidence and outcomes of acute kidney injury after cardiac surgery using either criteria of the RIFLE classification. BMC Nephrol. 2015;16:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Chen SW, Chang CH, Fan PC, Chen YC, Chu PH, Chen TH, Wu VC, Chang SW, Lin PJ, Tsai FC. Comparison of contemporary preoperative risk models at predicting acute kidney injury after isolated coronary artery bypass grafting: a retrospective cohort study. BMJ Open. 2016;6:e010176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Fu HY, Chou NK, Chen YS, Yu HY. Risk factor for acute kidney injury in patients with chronic kidney disease receiving valve surgery with cardiopulmonary bypass. Asian J Surg. 2021;44:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Casas A, Mallén A, Blasco-Lucas A, Sbraga F, Guiteras J, Bolaños N, Castaño E, Torras J, Cruzado JM, Navarro E, Hueso M. Chronic Kidney Disease-Associated Inflammation Increases the Risks of Acute Kidney Injury and Mortality after Cardiac Surgery. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Mahmood E, Knio ZO, Mahmood F, Amir R, Shahul S, Mahmood B, Baribeau Y, Mueller A, Matyal R. Preoperative asymptomatic leukocytosis and postoperative outcome in cardiac surgery patients. PLoS One. 2017;12:e0182118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734-44; discussion 744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 2125] [Article Influence: 163.5] [Reference Citation Analysis (0)] |
| 17. | Ortega-Loubon C, Fernández-Molina M, Pañeda-Delgado L, Jorge-Monjas P, Carrascal Y. Predictors of Postoperative Acute Kidney Injury after Coronary Artery Bypass Graft Surgery. Braz J Cardiovasc Surg. 2018;33:323-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Yi Q, Li K, Jian Z, Xiao YB, Chen L, Zhang Y, Ma RY. Risk Factors for Acute Kidney Injury after Cardiovascular Surgery: Evidence from 2,157 Cases and 49,777 Controls - A Meta-Analysis. Cardiorenal Med. 2016;6:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes JC, Novick RJ, Vaijyanath P, Reddy S, Tao L, Olavegogeascoechea PA, Airan B, Sulling TA, Whitlock RP, Ou Y, Ng J, Chrolavicius S, Yusuf S; CORONARY Investigators. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 517] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 20. | Chen X, Xu J, Li Y, Shen B, Jiang W, Luo Z, Wang C, Teng J, Ding X, Lv W. The Effect of Postoperative Fluid Balance on the Occurrence and Progression of Acute Kidney Injury After Cardiac Surgery. J Cardiothorac Vasc Anesth. 2021;35:2700-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Yang Y, Ma J, Zhao L. High central venous pressure is associated with acute kidney injury and mortality in patients underwent cardiopulmonary bypass surgery. J Crit Care. 2018;48:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Johnston LE, Thiele RH, Hawkins RB, Downs EA, Jaeger JM, Brooks C, Ghanta RK, Ailawadi G, Kron IL, Isbell JM; Virginia Interdisciplinary Cardiothoracic Outcomes Research Center. Goal-directed resuscitation following cardiac surgery reduces acute kidney injury: A quality initiative pre-post analysis. J Thorac Cardiovasc Surg. 2020;159:1868-1877.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA, Moldawer LL, Segal MS, Bihorac A. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 295] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 24. | Jian L, Sheng S, Min Y, Zhongxiang Y. Risk factors for endotracheal re-intubation following coronary artery bypass grafting. J Cardiothorac Surg. 2013;8:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Kotfis K, Szylińska A, Listewnik M, Strzelbicka M, Brykczyński M, Rotter I, Żukowski M. Early delirium after cardiac surgery: an analysis of incidence and risk factors in elderly (≥65 years) and very elderly (≥80 years) patients. Clin Interv Aging. 2018;13:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |