Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3736
Peer-review started: March 23, 2023
First decision: April 11, 2023
Revised: April 25, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: June 6, 2023
Processing time: 70 Days and 23.8 Hours
Diabetes mellitus (DM) is one of the chronic metabolic noncommunicable diseases that has attained worldwide epidemics. It threatens healthy life around the globe, with mild-to-severe secondary complications and leads to significant illness including nephropathy, neuropathy, retinopathy, and macrovascular abnor
Core Tip: Most of the review articles and meta–analysis on diabetes and ocular science are focused on diabetic retinopathy and posterior segment only. To the best of our knowledge, this is the first such review article on the effect of diabetes on the anterior segment of the eye. It gives a detailed insight into the intricate pathophysiology of the adverse effect of diabetes on the eye-appendages, cornea, lens and uvea of the eye.
- Citation: Morya AK, Ramesh PV, Kaur K, Gurnani B, Heda A, Bhatia K, Sinha A. Diabetes more than retinopathy, it’s effect on the anterior segment of eye. World J Clin Cases 2023; 11(16): 3736-3749
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3736.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3736
Diabetes mellitus (DM) is characterized by chronic hyperglycemia secondary to lack of or diminished efficacy of endogenous insulin and causes significant morbidity and mortality in multiple systems of the body. It is no longer a disease of affluent or industrialized nations. It has the highest prevalence among the populations of developing countries and in migrant and minority communities in industrialized countries. Diabetic eye disease is becoming a problem in developed and developing countries due to longer life expectancy and a sedentary lifestyle. In the eye, manifestations are found in almost every parts including orbit, lids, anterior and posterior segments. Most previous authors have concentrated on diabetic retinopathy (DR), but this disease can affect virtually every part of the eye and sometimes can significantly affect vision. Primary eye care workers and other professionals can easily examine the anterior segment of the eye for features of DM if trained to do so.
Orbital and lid features include boils, chalazia, xanthelasma, cranial nerve palsies (seventh, sixth, third, and fourth), and cellulitis. Conjunctival features include tortuous and dilated vessels commonly found in the inferior bulbar region, pinguecula, and pterygia. Tortuosity and dilatation of veins are part of the microvascular abnormalities found in diabetes. Recent advances in ocular surface imaging techniques have enabled the study of microstructural effects of DM on the ocular surface, which include tear film, cornea and conjunctiva. Identifying the microstructural abnormalities related to DM with routine slit lamp examination is difficult. In vivo confocal microscopy (IVCM) has recently become the standard cornea assessment tool. It is being used to detect and monitor the progress of DM and its complications. This instrument has been shown to produce precise and accurate results. The spectrum of abnormalities associated with ocular surface complications includes epithelial fragility, punctate keratopathy, persistent epithelial defects and decreased corneal sensitivity. The corneal endothelium is also shown to be affected in long-standing diabetic patients. Increased corneal endothelial pleo
Diabetes also influences lens transparency and pharmacological pupil dilatation. A cataract occurring in diabetic patients can be due to diabetes itself or to accelerated senile cataract, in which case the cataract occurs earlier than normal. As with retinopathy, the duration and control of the diabetes are important factors in cataract development and management. Changes in the eye’s refractive state may indicate the onset of diabetes. These may be myopia or hypermetropia. Myopia may be due to an increase in the thickness and curvature of the crystalline lens. Cranial nerve palsies are common in DM, with the facial nerve (seventh) being the most commonly affected, followed by the abducens, oculomotor, and trochlear. The association between DM and glaucoma in the literature is equivocal. Some researchers have not found an association, whereas others in Australia found the prevalence of open-angle glaucoma greater in people with diabetes than in the normal population. Iris atrophy, ectropion uvea, and rubeosis iridis are also seen in diabetic patients. This manuscript will throw light on understanding the disease process of diabetes affecting the anterior segment of the eye as well as its pathology and treatment options.
We collected highly cited articles in PubMed, Scopus database, Google Scholar, Web of Science, Cochrane Library, and Embase database on Diabetes effect on the anterior segment of the eye published between the year 1984 to 2022. We diligently used Reference Citation Analysis (RCA) for searching the keywords and articles were ranked based on the “Impact Index Per Article”. The latest highlighted articles were selected for review. Only articles published in English were considered and the rest were rejected.
The eyelid is the anterior most part of both eyes, comprising structures like the upper and lower tarsal conjunctiva, meibomian gland, punctum, and lashes. Eyelid’s function involves blinking mechanism, protection of both eyes, maintaining ocular surface health, tear/oxygen supply, etc.
DM is a systemic disease that mainly affects microcirculation and can affect the ocular surface integrity through a different mechanism. It is the most complicated disease to manage systematically and from an ophthalmology point of view, as many pathologies are related to it, including regular eyelid inflammatory abnormalities[1,2].
Regarding meibomian gland function in diabetics, there is a significant rise in lipid layer thickness in people with diabetes compared to non-diabetics, and there is also meibomian gland dysfunction present in diabetics, most severely in an asymptomatic diabetic population who needed dry eye treatment[3]. Xanthelasma is a yellowish plaque, benign lesion present on superonasal parts of the upper eyelid, usually bilateral in occurrence can also be triggered by systemic diseases such as diabetes, thyroid, etc
Acute bacterial infection is the most common complication of DM. Microvascular abnormalities due to long-standing diabetes will predispose the conjunctiva to infections. Conjunctival angiopathy includes increased microvessel dilation, increased tortuosity and leakage of conjunctival capillaries. These changes mimic vessel changes observed in the retina.
Macro vessel dilation associated with diabetes may result in vessel engorgement and straightening, especially among those with longer disease durations. Increased tortuosity associated with diabetes among conjunctival capillaries mirrors established vessel changes observed in the retina. Conjunctival angiopathy associated with diabetes may contribute to susceptibility to anterior eye disease among patients with diabetes.
Patients with long-standing diabetes typically complain of dry eye symptoms such as burning and foreign body sensations. In more severe cases, diabetic neurotrophic keratopathy occurs. The stability, secretion and lipid layer quality of tear film are reduced in diabetes due to decreased trophic effect of trigeminal sensory nerves on the cornea. The tear fluid in diabetic patients contains higher glucose concentrations due to conjunctival angiopathy and vessel leakage. It decreases the corneal epithelium’s wound healing capacity and damages the microvascular supply to lacrimal glands, leading to reduced lacrimation.
Decreased tear film stability is the typical manifestation in people with diabetes. It is found to be due to a reduced number of goblet cell densities, which are the main source of tear film mucins that protect the cornea and maintain a stable preocular tear film. Trigeminal nerve dysfunction also disrupts lacrimal gland function and decreases basal tear production. Schirmer test used to assess the function of the lacrimal gland, shows lower tear production in diabetic patients. The corneal limbus is a narrow band of tissue that encircles the cornea. Under physiological conditions, corneal limbal epithelial stem cells give rise to progeny, differentiating into mature corneal epithelium during their radial migration towards the centre.
The tear film is the primary interface between the ocular surface and the external environment and plays a pivotal role in maintaining the morphological and functional integrity of the cornea. In addition, the lacrimal glands, lacrimal drainage system, and interconnecting innervation work together as the lacrimal function unit (LFU). DM is also associated with film abnormality and LFU insufficiency, which can deteriorate corneal components. Owing to abnormal tear dynamics, diabetic patients are more prone to suffer from dry eye syndrome (DES). DES is ubiquitous in diabetic patients, especially those with Dr. DES is a potential visual impairment syndrome that can lead to superficial punctuate keratopathy, secondary bacterial infection, and even perforation. The decrease in lacrimal gland secretory function is the cardinal problem in DES.
Many mechanisms, notably chronic inflammation and peripheral neuropathy, contribute to the onset and progression of the tear film abnormality in diabetic patients in DES. Chronic hyperglycemia is the primary causative mechanism underlying the pathogenesis of tear film abnormality. In addition, there was a significant elevation of inflammation or pre-inflammation markers in the tears and conjunctiva of diabetic patients, such as interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor-α. As previously stated, matrix metalloproteinases (MMP) are an essential mediator of inflammation in diabetes and contribute to tissue impairment. It was reported that elevated MMP-9 was significantly correlated with ocular surface inflammation. In addition, the level of substance P was considerably lower in the tears of diabetic patients. A recent study showed that increasing metallic elements in patients with DM’s tears might indicate ocular damage. In addition, oxidative stress in the diabetic rat model leads to pathological alteration of the lacrimal gland acinar cells. An experimental study demonstrated that overexpression of SIRT1 in the diabetic dry eye model was evident for the DES oxidative stress mechanism.
Furthermore, chronic hyperglycemia may eventually lead to tearing film hyperosmolarity. Exposure of corneal structures, including the corneal epithelium and corneal limbus, to tear film hyperosmolarity leads to a cascade of inflammatory reactions. Additionally, the elevated volume of the tear film of patients with DM may be attributed to tear film instability and rapid evaporation of the tear, which leads to tear secretion in a reflex action. Usually, the secretion of tears in patients with DM is reduced. Furthermore, tear film instability and hyperosmolarity play significant roles in the vicious cycle of diabetic tear film abnormality.
Lacrimal nerve fibres play a pivotal role in the maintenance of tear production and integrity of the LFU. Diabetic neuropathy may compromise the innervation of the LFU. Moreover, impairment of the LFU sensory nerve may also inhibit tear secretion associated with the reduced corneal sensitivity threshold. Interestingly, using IVCM, the number of corneal sub-basal nerves was significantly correlated with Schirmer test values. Such a phenomenon may indirectly reveal alterations in the corneal innervations in DES patients with diabetes. Furthermore, exposure to high glucose levels is deleterious for human meibomian gland epithelial cells and may help explain the importance of hyperglycemia for LFU in patients with DM.
Uncontrolled diabetes or diabetic alteration can cause clinically significant changes in the cornea[7]. The corneal changes seen are epithelial defect, fragile epithelium, recurrent corneal erosion syndrome, superficial punctate keratitis, increased corneal thickness, corneal infiltrate, oedema, delayed corneal healing, and reduced corneal sensation leading to neuropathy[8]. Another common corneal change seen in people with diabetes is dry eye syndrome. Diabetic corneal neuropathy is characterized by decreased corneal sensitivity, reduced sub-basal nerve fibre and branch density, tortuous and thickened stromal nerves and slow nerve regeneration after any traumatic injury[9].
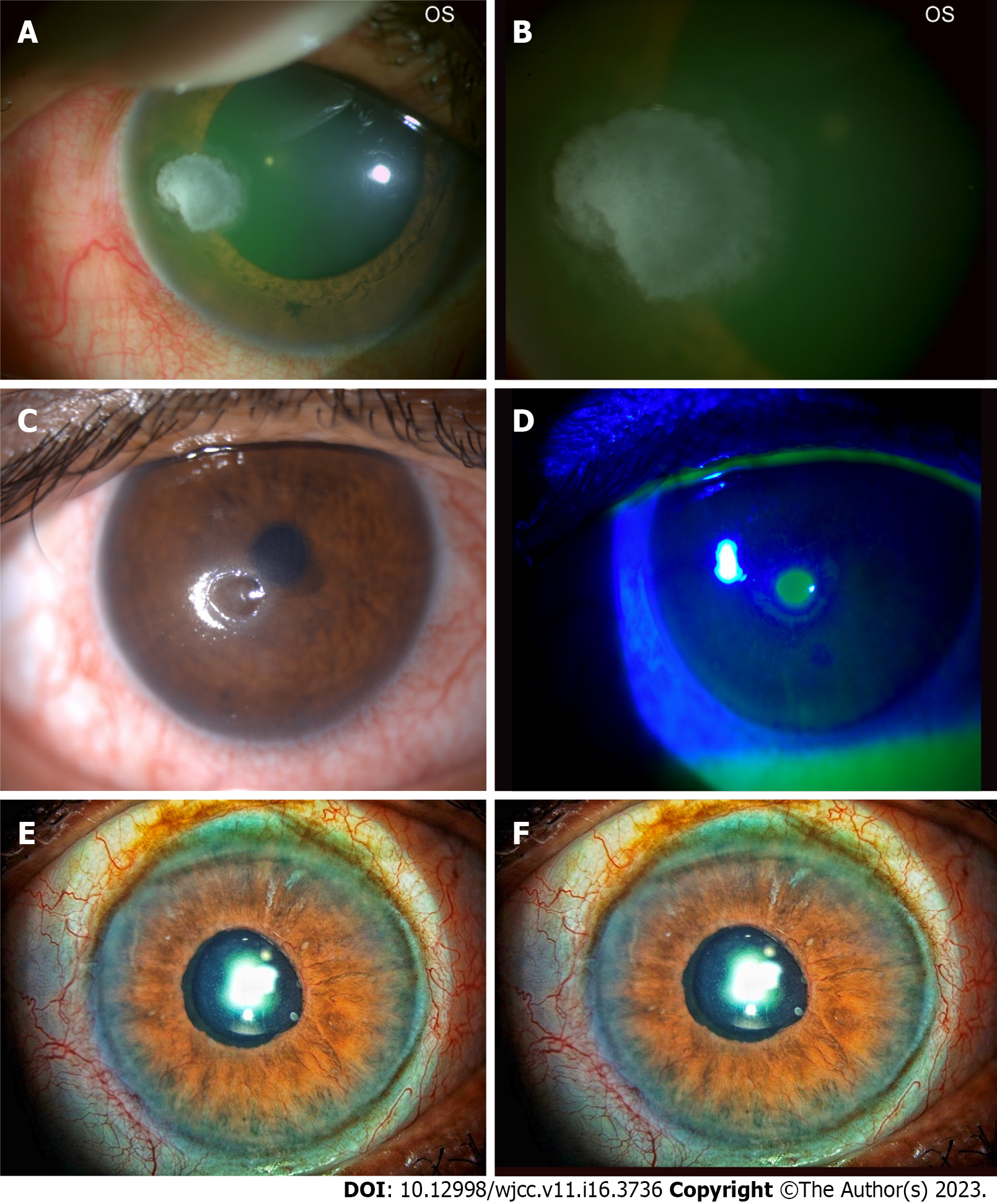
The corneal epithelial changes secondary to diabetes are often labelled as diabetic keratopathy. Common epithelium changes are epithelial defect, fragility, recurrent corneal erosions, superficial punctate keratitis, delayed wound healing, and corneal ulceration[8] (Figure 1A and B).
Uncontrolled diabetes (especially type 2 DM) has been linked to diabetic keratopathy, and the changes are seen more often in females. The presence of diabetic keratopathy is an alarm sign for occult peripheral neuropathy and should alert the physician. Neurotrophic keratopathy has also been reported but is comparatively a rare complication (Figure 1C and D).
Uncontrolled diabetes also causes structural and functional alternation in the epithelium, which may not be directly linked to neuropathy. As per previous studies, hyperglycemia has a direct impact on corneal epithelial cells and also on cultured corneal epithelial cells reducing cell adhesions and slowing down the healing process[10].
The first findings on decreased corneal sensitivity in diabetics were reported in 1970. Since then, with continued understanding and growing research, it is now well documented that corneal sensations are reduced in diabetic patients, and the severity may increase with age. Reduction in sensations is related to abnormalities of corneal nerve structure and function in diabetes[11]. In vivo IVCM performed in diabetic cornea reveals structural alterations in nerve fibre density, branch density, length of fibres, tortuosity nerves, and thickened nerves. It has been noted that these changes deteriorate after pan retinal photocoagulation (PRP) in proliferative DR (PDR). In most diabetics, the maximum reduction in nerve fibre density is seen in the sub-basal nerve plexus adjacent to the corneal epithelium indicating a close correlation between diabetic keratopathy and corneal neuropathy. Once the sub-basal nerve plexus is damaged due to injury, regeneration occurs slower in the diabetic cornea than non-diabetic one[12]. In mice models, sub-basal nerve alterations are associated with alteration in dendritic cells that play a crucial role in neurotrophic functions. As per the literature review, corneal neuropathy can manifest early in diabetes, even before the onset of DR. In some rat models, the corneal nerve damage was noted in obese and non-diabetics, indicating the onset of hyperglycemia[13].
There is only limited literature available on corneal stromal changes in diabetics. In patients with non-insulin-dependent DM, the corneal stroma reveals abnormal bundles of collagen fibrils of varying thickness[14]. It has also been noticed that in diabetic corneas, there is an accumulation of advanced glycation end products (AGES) which may lead to collagen cross-linking and increase central corneal thickness. In mice and rat models, AGES accumulation changes type 4 collagen expression, altered cell adhesion, and increased keratocyte apoptosis. Stromal oedema has also been reported in diabetic corneas. The stromal nerves also appear thickened and tortuous. The levels of MMPs, MMP-3 and MMP-10, were also found to be elevated in corneal stroma of diabetics but not in keratoconus patients[15].
Previous studies have intricately studied the endothelial cell count, function, and morphology in diabetic patients. It has been noted that endothelial cell show increased pleomorphism and polymegathism in people with diabetes. Few studies have indicated normal endothelial cell morphology, whereas other recent analyses have quoted reduced endothelial cell count in the cornea of patients with IDDM and NIDDM[16]. Studies on endothelial function also found that oedema was reduced compared to non-diabetic corneas. Compared to other complications of diabetes, corneal endothelial diabetics are a relatively small number. Moreover, there is a high risk of endothelial compromise following corneal surgery[17].
Biomechanical alterations in the cornea result in increased central thickness, altered basement membrane composition, structural changes in the stromal collagen, and accumulation of age-related glycation products that may result in corneal collagen crosslinking[18]. Biomechanical changes have been studied with the help of an ocular response analyzer by measuring corneal resistance factor, which is a measure of corneal elasticity and resistance offered by the cornea (CRF), and corneal hysteresis (CH), which is a measure of viscoelastic property and strength of the cornea. The studies have also reported an increase in corneal thickness with diabetes. The CH and CRF have been reported variably by the studies[19].
Structural and functional alteration in diabetic cornea predisposes the cornea to an increased risk of surgical complications; even with the best surgical techniques available, the major keratorefractive complications, non-resolving vitreous haemorrhage, and after PRP occur in diabetics. In a few previous reports, diabetic keratopathy has been reported after ocular surgery[20]. In some cases which require epithelial scraping for better intraoperative visualization, in diabetics, the healing is delayed. The sub-basal nerve plexus is damaged and hence grows slower than usual. The damage to epithelial cells and sub-basal nerve plexus cause slow wound healing and reduction in corneal sensations postoperatively. The autologous serum has been tried in diabetic corneas to accelerate wound healing. Cataract surgery causes increased corneal thickness and more loss of endothelial cells in people with diabetes than non-diabetics. It has been seen that the incidence of corneal oedema is higher after phacoemulsification in people with diabetes than non-diabetics, and these patients are also at higher risk for Descemet’s membrane keratoplasty[21].
Patients with DR and neuropathy usually remain symptomatic. A large number of drugs have been tried, but they are still in the experimental stage. Some of the experimental drugs in the diabetic cornea are summarized below (Table 1).
| No. | Drug | Indication |
| 1 | Topical thymosin β4 | Non-healing epithelial defect |
| 2 | Topical autologous serum | Promote corneal wound healing |
| 3 | Topical CT-112 | Reduction in corneal barrier effect |
| 4 | Topical insulin | Quicker reepithelization after epithelial scraping for vitreoretinal surgeries |
| 5 | Topical insulin | Prevent sub-basal nerve plexus loss |
| 6 | Topical insulin | Promote wound healing |
| 7 | Topical ranirestat | Promote wound healing, control the expression of MMP-10 and integrin α3 expression |
| 8 | Injection IGF-1 | Prevention of stem cell loss and improve sub-basal nerve plexus density |
| 9 | Topical naltrexone | Normalize corneal epithelial wound healing, tear film, and corneal sensations |
| 10 | Nateglinide and glibenclamide | Inhibit descemet membrane changes |
| 11 | Ciliary neurotrophic factor | Improve epithelial stem cells, increase nerve density and promote epithelial healing |
| 12 | Topical nerve growth factor | Reduced apoptosis and inflammation |
| 13 | IL-1 antagonists | Reduced apoptosis, faster wound healing, sensory stimulation, improved Akt signaling |
| 14 | Substance P | Improved wound healing, reinnervation, and reactivation of EGFR/Akt signaling |
Uncontrolled diabetes is a contraindication for refractive surgery. Phototherapeutic keratectomy, Laser in situ keratomileusis (LASIK), and small incision lenticule extraction are riskier and have unfavourable outcomes in diabetics because they can result in recurrent corneal erosions, non-healing epithelial defect, corneal ulcer, epithelial down growth, poor wound healing and increased risk of infections. A previous report quoted an increased risk of DR in LASIK. Hence, it is better to attempt LASIK in non-diabetics having no DR. Few Ophthalmologists prefer non-touching diabetics patients for refractive surgery at all[22].
Diabetic patients using contact lenses are at risk for dry eyes, corneal erosion, neovascularization, and microbial keratitis. Hb1Ac levels are a good indicator of precise diabetic control, and most ophthalmologists take a cut off of 7 for prescribing contact lenses. Daily disposable contact lenses are the best ones for diabetic corneas. Vision can also fluctuate due to changing refractive errors in people with diabetes. Hence, contact lenses should be prescribed only when fasting and postprandial levels are under control[23].
Chronic hyperglycemia has been linked with myopic refraction, but as sugar levels are under control, the refraction becomes less myopic or more hyperopic. Some researchers have suggested that acute changes in plasma glucose for a month or two will result in hyperopia. In contrast, some have mentioned myopia or hyperopia will occur when plasma glucose increases or decreases. Hence, the exact refractive changes in a diabetic eye have not yet been established, and the mechanism remains to be elucidated[24].
Three different mechanisms have been implicated in the pathogenesis of cataracts in diabetics. These include the polyol pathway, osmotic and oxidative stress, and autoimmunity. In the polyol pathway, the enzyme aldol reductase catalyzes the reduction of glucose into sorbitol which is the primary mechanism in cataract development[25]. The extra sorbitol accumulation causes the hyperosmotic effect, resulting in the degeneration of hydropic lens fibres and cataract formation. Osmotic stress causes rapid swelling of the cortical lens fibres, which is a significant factor in the rapid development of cataracts, especially in type 1 DM patients. Osmotic stress accumulates sorbitol in the endoplasmic reticulum (ER), the leading site of protein synthesis and forms free radicals. Fluctuating glucose levels also initiate ER stress, producing reactive oxygen species and causing oxidative damage to lens fibres. Elevated glucose in aqueous, AGEs, elevated hydrogen peroxide, free radicals from nitrogen peroxide, and superoxide radicals are other mechanisms that cause damage to the lens fibres and lead to cataract formation. Another mechanism linked with bilateral type 1 cataract development is the autoimmune mechanism[26]. Autoantibodies are present in the blood within three months of treatment, which correlates with cataract development. Senile and snowflake cataracts are common forms linked with DM, especially type 1 cataracts. Posterior Subcapsular Cortical Cataract has also been linked to diabetes. Elevated Hb1Ac has been linked with cortical and nuclear cataracts. As per previous studies, the duration of diabetes has been associated with cortical cataracts[27].
It has been well proven that cataract incidence is higher and occurs at an earlier age in diabetics compared to non-diabetics. The reported incidence is four times higher in diabetics before age 65. In patients above 65, the incidence is twice that of non-diabetics. The major risk factors for cataract development are more prolonged duration of diabetes and poor metabolic control. Strict metabolic control can reverse snowflake cataracts in young diabetics. The Beaver Dam Study showed the increased incidence and progression of cortical and posterior subcapsular cataracts in DM patients. Increased nuclear and cortical cataract incidence was reported with elevated glycated haemoglobin levels[28]. The incidence of cataract surgery is also higher in diabetics. The Wisconsin Epidemiologic Study of DR reported that 8.3% of type 1 diabetics and 24.9% of type 2 diabetics had an incidence of diabetes. The other risk factors related to diabetes are age, DR severity, proteinuria, type 2 DM, and the use of insulin. The Blue Mountain Study assessed the relationship between nuclear, cortical, and posterior subcapsular cataracts and DM. The Barbados Eye Study evaluated the association between DM and lens changes and found that a history of DM was related to all the changes, especially at a young age[29].
Counselling is crucial in cataract surgery in diabetic patients. Excellent glycaemic control is mandatory, and no evidence of any infection. If the refractive error is changing, it should be documented. Changes in topography during uncontrolled DM can lead to errors in intraocular lens (IOL) power calculation. Complete anterior and posterior segment examination, best corrected visual acuity, relative afferent pupillary defect, neovascularization of iris (NVI), tonometry, dilated fundus, and gonioscopy is mandatory. In selected cases, fundus fluorescein angiography, ocular coherence tomography, and a B scan will be needed. An experienced surgeon should attempt surgery. A thorough evaluation by a retina surgeon is a must. PRP[30] is required in patients with PDR because of rapid progression after cataract surgery, and in cases with dense cataracts can be performed after cataract surgery. Some cases require combined surgery in the form of cataract, vitrectomy, and endolaser for tractional retinal detachment.
Moreover, maculopathy should be controlled pre-operatively to prevent deterioration of macular oedema (ME). The cataract surgery approach in diabetic patients is changing worldwide. The majority of surgeons now prefer early surgery in diabetic patients. As per previous studies, ME is the leading cause of poor visual outcomes in these patients. Hence, cataract surgery was preferred at a visual acuity of 6/30 to 6/36. The cataract patient may wish to postpone surgery if there is severe DR or PDR. Early surgery offers a good chance for PRP and treatment for Diabetic ME[31].
Phacoemulsification has good outcomes in diabetic patients as it yields better results compared to manual small incision cataract surgery and extracapsular cataract extraction (ECCE). A common sequela in diabetic patients is anterior capsular phimosis. The capsulorhexis size is should be larger but smaller than the optic to prevent anterior capsular phimosis, posterior capsular opacification, and movement of the lens in the sulcus. A larger diameter IOL is also important as it helps diagnose and treat peripheral retinal pathologies. Retinopathy can progress in patients with DR. The duration of diabetes and cataract complexity are primary reasons for retinopathy progression. Pupillary dilatation will be poor due to reduced parasympathetic supply and elevated prostaglandin levels. Pupil dilatation is poor. Hence, pupillary expansion devices, such as iris hooks, B-HEX pupil dilators, etc., are required. Amsler’s sign with bleeding in the anterior chamber can be seen during the surgery in patients having NVI. DM causes changes in corneal stem cells, epithelial cells, and endothelial cells. This results in epithelial defects post-surgery, which heal slowly. Endothelial cell loss is higher in diabetic corneas as compared to non-diabetics; hence routine specular microscopy is recommended[31].
IOL implantation is imperative in patients with DR as it helps visualise and treat patients with Non-PDR and PDR. Posterior capsular opacification (PCO) is another challenge after cataract extraction. The onset and severity of PCO are accelerated in DM patients compared to non-diabetics. Square edge IOL inhibits lens epithelial cell proliferation and therefore prevents PCO formation. The biocompatibility of three different types of IOL has been studied to assess the rate of PCO formation. Hydrophilic IOL has good capsular biocompatibility but causes more anterior chamber flare. They have a low tendency for silicon oil adhesion, meaning they are the IOL of choice for diabetic patients. Silicon IOL is contraindicated in patients who have undergone vitreoretinal surgery[32]. Hydrophilic IOLs can experience opacification in patients with PDR, as elevated serum phosphorus levels combined with aqueous humor of diabetics result in opacification. Progressive IOL calcification has been reported for hydrophilic IOLs in diabetic patients. Multifocal and accommodative IOLs should be avoided in people with diabetes as they pose difficulty because of the optics of these lenses. Moreover, ME may cause visual dissatisfaction for these patients with pre-existing maculopathy. The IOL should be implanted in the capsular bag as sulcus fixated, iris claw and angle fixated ones cause iritis, NVI, and increased risk of Cystoid ME.
Diabetics are at increased risk for PCO, diabetic ME or cystoid ME, and progression of retinopathy. Few of the previous studies have quoted a high incidence of PCO in diabetics; others have shown fewer cases of PCO in diabetics, irrespective of retinopathy, over two years[33]. Hayashi et al[34] reported that PCO rates were more in diabetics after 18 mo post cataract surgery, although the rates were comparable after the first 12 mo of the surgery. The severity of retinopathy didn’t reveal any impact on the development of PCO. Diabetic ME, cystoid ME or Irvin Gas syndrome, and pseudophakic ME cause reduced vision in diabetics. Various angiogenic factors have been implicated, which aggravate maculopathy. Increased macular thickness has been documented on OCT in eyes without retinopathy as compared to non-diabetics. The risk factor for DR progression is male sex, disease duration, and poor DM control. Progression of retinopathy is more with ECCE and intra capsular cataract extraction as compared to phacoemulsification.
Endophthalmitis is a grave complication post cataract surgery in diabetics. It progresses faster in diabetics, and diabetics are more prone to irreversible visual sequelae. This has been linked to changes in immune and inflammatory factors that intervene with wound healing and local adnexal ocular bacterial flora[35].
Glaucoma is the leading cause of worldwide irreversible blindness, as defined by best-corrected central visual acuity of less than 3/60 or a visual field of less than 10° in the better-seeing eye, characterized by pathognomonic optic nerve changes which result in progressive visual field loss over the period of time[36]. Association between diabetes and glaucoma has always been in debate, and there is an increase in the evidence to suggest that diabetic patients have a greater risk for glaucoma as well.
A meta-analysis by Zhao et al[37] which included 47 studies, reported a pooled relative risk of glaucoma of 1.48 in patients with diabetes compared to those without diabetes. Duration of diabetes has a direct impact on the risk for glaucoma. Diabetic patients had a pooled average increase in intra ocular pressure (IOP) of 0.09 mmHg for every 10 mg/dL increase in fasting glucose. Goldacre and colleagues found that the rate ratio for glaucoma among patients admitted for diabetes was substantially increased at 2.47 compared to the reference cohort[38].
Though the pathophysiology of glaucoma is not entirely understood, diabetes and glaucoma appear to share some common risk factors. The pathophysiologic similarities with studies also report that diabetes and elevated fasting glucose levels are associated with elevated IOP. Diabetes and hyperglycaemia are related to the glycation of lipids and abnormalities in lipid metabolism. This, in turn, increases oxidative stress and promotes cellular apoptosisthe same mechanism by which retinal ganglion cell loss occurs in glaucoma. Vascular dysregulation has been described in both diabetic eye disease and glaucoma. Upregulation of nitric oxide, a potent vasodilator, has been reported in both conditions[39-42]. Protein kinase-C (PKC) plays a role in the pathophysiology of DR. At the same time, there is evidence to suggest that elevated PKC may be associated with abnormalities of matrix metalloprotease in the trabecular meshwork that causes impaired aqueous outflow and ultimately elevated IOP[43,44]. Dysfunction of the glial cells is also evidenced to contribute to neuroinflammatory pathways of apoptosis in both diabetes and glaucoma. It has also been proven that alterations in connective tissue remodeling due to diabetes may affect both the lamina cribrosa and the trabecular meshwork, thereby potentially increasing susceptibility to glaucoma through biomechanical changes at the optic nerve and impairment of aqueous humour outflow affecting IOP homeostasis[45].
Alterations in neurotrophic factor expression, such as insulin-like growth factor and neurotrophin-3, are also seen in the presence of elevated IOP, the primary risk factor for glaucomatous optic neuropathy[46]. Retrospective cohort of diabetic patients with open-angle glaucoma reported that metformin, a first-line agent used to treat insulin resistance in type 2 diabetes, is associated with a decreased risk of developing open-angle glaucoma even after accounting for variations in glycaemic control[47].
Risk factors common for diabetes and glaucoma can be listed as follows: Dyslipidaemia; hypertension; vascular dysregulation; and hypoxia. Other risk factors for glaucoma: Age over 40; family history of glaucoma; race-African, hispanic, or Asian heritage; high IOP; and thin cornea.
Glaucoma is called the silent killer of the eye as the affected individuals are not symptomatic, especially in the early stage of the disease. So, an opportunistic, case-finding approach to glaucoma screening may be of value in a high-risk population. This highlights the implications of the purported significant glaucoma risk associated with diabetes. Glaucoma that can be diagnosed commonly can be primary or secondary. Primary glaucoma can be primary open-angle or closed-angle glaucoma. Secondary glaucoma, especially neovascular glaucoma (NVG), is common in advanced diabetic eye disease (Figure 1E and F).
Ischaemia leads to neovascularization of the iris and neovascularization of the angle or both, ultimately leading to a rise in IOP due to various mechanisms leading to optic nerve damage. The formation of a fibrovascular membrane can be seen histologically in these eyes. This membrane initially obstructs the aqueous outflow through the trabecular meshwork, resulting in open-angle glaucoma. At this stage, pharmacological management of the elevated IOP is possible. PRP of the underlying DR is essential. As the disease progresses, the proliferating myofibroblasts of the fibrovascular membrane contract, leading to ectropion uveae, peripheral anterior synechiae and, ultimately, total synechial angle-closure. The resultant secondary glaucoma is often refractory to pharmacological management and requires surgical intervention.
Aqueous suppressants can be of great help in reducing raised IOP. Anticholinergics should be avoided as they can increase inflammation and worsen synechial closure. Prostaglandins are relatively contraindicated in these eyes since they can increase inflammation, and the presence of synechiae limits the flow of aqueous via the uveoscleral pathway. Topical corticosteroids can be used for inflammation in a controlled manner. Cycloplegic agents can be used to relieve the ciliary spasm and to control the pain. Topical glycerine may help to clear corneal edema, facilitating accurate diagnosis and delivery of PRP when required. Osmotic agents may provide acute but transient lowering of IOP by reducing vitreous volume. Anti-vascular endothelial growth factor (VEGF) agents can be used as an additional measure.
Trabeculectomy has limited success in the setting of NVG. It is usually complicated by intraoperative bleeding and the progression of the fibrovascular membrane postoperatively. By reducing active NVI and neovascularization of the angle, PRP treatment may decrease intra- and post-operative complications. The use of antimetabolites may improve the outcome. Tsai and colleagues reported a success rate of approximately 30 percent at five years with the help of 5-fluorouracil during trabeculectomy in cases with NVG[48].
Aqueous drainage implants have been experimented with in NVG since last many decades with variable success. Success rate with glaucoma drainage device (GDD) also decreases, as with glaucoma filtration surgery, over time. No significant differences have been noted among the various types of implants. Mermoud and co-workers used Molteno implants in 60 eyes with NVG. They reported a success rate of 62 percent at one year but only 10 percent at five years[49]. Sidoti and colleagues reported a success rate of nearly 80 percent at one year but only 56 percent at 18 mo using Baerveldt implants in 36 eyes[50].
Cyclodestructive procedures using either Nd: YAG or diode lasers have been used to destroy the ciliary body to reduce aqueous humor production. This method can reduce IOP with fewer complications, but the long-term success rate and treatment protocol are not well established.
These treatment options are reserved for the eyes that are not responding to the conventional treatment options. Despite exhaustive measures to lower IOP, most eyes have a poor visual outcome.
Mucormycosis, previously known as Zygomycosis, is a severe but rare fungal pathology caused by a group of moulds known as Mucormycetes. Commonly found in our environment, the common route of infection is inhalation, ingestion and traumatic inoculation. The disease is rare among the healthy population. Predisposing factors are immunocompromised conditions such as HIV/AIDS patients, organ transplant patients, cancer patients, stem cell transplant patients, also uncontrolled diabetes.
DM creates an ideal environment for Mucorales as they often exhibit impaired innate and adaptive immunity increasing the susceptibility to any infection, particularly mucormycosis. In a study by Corzo-León et al[51], which was a retrospective study corresponding to the clinical presentation of 181 patients of diabetes with mucormycosis, 159 (88%) cases had a sinus infection, 5% had a skin infection, 4% with pulmonary presentation and 2% had disseminated with the intra-abdominal presentation[51]. In a study by Bhansali et al[52], which was a retrospective, non-comparative, interventional analysis of a cohort of 23 men and 12 with a mean (SD) age of 47.3 years were studied, five patients had type 1 diabetes, and 29 had type 2 diabetes. Nine patients had Rhino-orbital-cerebral mucormycosis as the first clinical manifestation of diabetes. This study reported the ophthalmic symptoms and signs as follows: External ophthalmoplegia (89%), proptosis (83%), visual loss (80%), chemosis (74%), and eyelid gangrene (14%). The study concluded that in patients with diabetes and rhino-orbito-cerebral mucormycosis was the presenting feature in one-fourth of patients[52].
A case reported by Bavikar et al[53], a 38-year-old female, presented with complaints of headache, fever, inability to open right eyelid and seizures. The microbiologist confirmed the growth of the micromycetes from the nasal swabs[53]. In the case of a patient diagnosed with diabetes and contracting mucormycosis, a rapidly corrected underlying metabolic derangement is the most important criterion for considering the conservation of the orbit, even in the presence of total ophthalmoplegia and central retinal artery obstruction.
Snaith et al[54] documented the first hematogenous cerebral spread in rhino-orbital-mucormycosis in a patient presenting with diabetic ketoacidosis (DKA) and sinusitis. The patient had a history of recurrent DKA, Foot ulcers with osteomyelitis, retinopathy and albuminuria. A right orbital exen
Despite the recommendation for an annual eye exam in DM patients as an innovative, inexpensive way to avoid blindness, screening is ineffectively carried out and unable to meet the rising demand from the expanding population of newly diagnosed DM patients[55]. The introduction of advanced digital technologies, including artificial intelligence (AI), and telehealth technology has created new opportunities in screening, diagnosis, and management of DM-related ocular complications. Novel hybrid telemedicine systems have been well introduced to allow a wider range of DM-related eye screening. It carries diagnostic sets including a combination of mobile ultra-field mounted cameras on vehicles in vans[56-58]. Recently, two technologies for community-based teleophthalmology DR screening were successfully deployed in India: The MII RetCam and the Remidio Fundus on Phone[59,60].
The use of deep learning techniques in AI, in particular, has increased in large data management and automated image-recognition tasks, which is beneficial for the early diagnosis of DR and other ocular problems associated with DM[61-63].
The use of remote monitoring technology has increased throughout the COVID-19 pandemic and has been recognized as an efficient way to provide timely pathology identification and appropriate management for patients with DM-related ocular disorders outside of hospitals and clinics[64,65].
Recently, a brand-new anti-VEGF therapy was introduced to treat diabetic ME. Conbercept (Chengdu Kanghong Biotech, Sichuan Province, China) is a recombinant human VEGF receptor-Fc fusion protein that efficiently treats ME. It has a considerably stronger affinity to VEGF than bevacizumab and ranibizumab and inhibits VEGF-A/B/C isoforms and PGF[66].
Though there is more controversy in treating DM-related ocular complications effectively, new technologies and treatment strategies have been evolving to enhance the treatment protocol and screening concept.
DM not only led to major posterior segment abnormalities but also lead to various anterior segment abnormalities involving conjunctiva, cornea, lens and iris. Anterior segment abnormalities associated with systemic uncontrolled DM abnormalities like reduced tear secretion and unstable tear film, decreased sub-basal nerve plexus density and corneal sensitivity, lens abnormalities, and other problems can occur before the clinical evidence of any major ocular diseases such as DR, NVG, etc., with DM. To predict DM problems earlier, these characteristics have the potential to be employed as non-invasive biomarkers for starting the treatment of DM[67].
Although many treatment modalities for treating and preventing anterior segment disorders linked to DM have been developed, more research is still required to create more effective treatment plans. A proper guideline for screening ocular surface pathologies resulting from uncontrolled DM should also be developed and established. For the best management of DM, it is crucial that patients and healthcare professionals, particularly diabetologists, ophthalmologists, and paramedical personnel, have a better awareness of the effects of DM on the anterior portion of the eye.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; He Z, China S-Editor: Chen YL L-Editor: A P-Editor: Zhao S
| 1. | Adeoti C, Isawumi M, Ashaye A, Olomola B. The anterior segment of the eye in diabetes. Clin Ophthalmol. 2012;6:667-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Ozdemir M, Buyukbese MA, Cetinkaya A, Ozdemir G. Risk factors for ocular surface disorders in patients with diabetes mellitus. Diabetes Res Clin Pract. 2003;59:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Lin X, Xu B, Zheng Y, Coursey TG, Zhao Y, Li J, Fu Y, Chen X, Zhao YE. Meibomian Gland Dysfunction in Type 2 Diabetic Patients. J Ophthalmol. 2017;2017:3047867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Nair PA, Singhal R. Xanthelasma palpebrarum - a brief review. Clin Cosmet Investig Dermatol. 2018;11:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Eissa IM, Khalil NM, El-Gendy HA. A Controlled Study on the Correlation between Tear Film Volume and Tear Film Stability in Diabetic Patients. J Ophthalmol. 2016;2016:5465272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Inoue K, Okugawa K, Amano S, Oshika T, Takamura E, Egami F, Umizu G, Aikawa K, Kato S. Blinking and superficial punctate keratopathy in patients with diabetes mellitus. Eye (Lond). 2005;19:418-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Shih KC, Lam KS, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes. 2017;7:e251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular Complications of Diabetes and Therapeutic Approaches. Biomed Res Int. 2016;2016:3801570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Beckman KA. Characterization of dry eye disease in diabetic patients versus nondiabetic patients. Cornea. 2014;33:851-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Tomomatsu T, Takamura Y, Kubo E, Akagi Y. Aldose reductase inhibitor counteracts the attenuated adhesion of human corneal epithelial cells induced by high glucose through modulation of MMP-10 expression. Diabetes Res Clin Pract. 2009;86:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 2007;30:1895-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Gao N, Yan C, Lee P, Sun H, Yu FS. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J Clin Invest. 2016;126:1998-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. 2017;15:15-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 14. | Zou C, Wang S, Huang F, Zhang YA. Advanced glycation end products and ultrastructural changes in corneas of long-term streptozotocin-induced diabetic monkeys. Cornea. 2012;31:1455-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Saghizadeh M, Brown DJ, Castellon R, Chwa M, Huang GH, Ljubimova JY, Rosenberg S, Spirin KS, Stolitenko RB, Adachi W, Kinoshita S, Murphy G, Windsor LJ, Kenney MC, Ljubimov AV. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: a possible mechanism of basement membrane and integrin alterations. Am J Pathol. 2001;158:723-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Matsuda M, Ohguro N, Ishimoto I, Fukuda M. Relationship of corneal endothelial morphology to diabetic retinopathy, duration of diabetes and glycemic control. Jpn J Ophthalmol. 1990;34:53-56. [PubMed] |
| 17. | Larsson LI, Bourne WM, Pach JM, Brubaker RF. Structure and function of the corneal endothelium in diabetes mellitus type I and type II. Arch Ophthalmol. 1996;114:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Rehany U, Ishii Y, Lahav M, Rumelt S. Ultrastructural changes in corneas of diabetic patients: an electron-microscopy study. Cornea. 2000;19:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Scheler A, Spoerl E, Boehm AG. Effect of diabetes mellitus on corneal biomechanics and measurement of intraocular pressure. Acta Ophthalmol. 2012;90:e447-e451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Foulks GN, Thoft RA, Perry HD, Tolentino FI. Factors related to corneal epithelial complications after closed vitrectomy in diabetics. Arch Ophthalmol. 1979;97:1076-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Greiner MA, Rixen JJ, Wagoner MD, Schmidt GA, Stoeger CG, Straiko MD, Zimmerman MB, Kitzmann AS, Goins KM. Diabetes mellitus increases risk of unsuccessful graft preparation in Descemet membrane endothelial keratoplasty: a multicenter study. Cornea. 2014;33:1129-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Spadea L, Paroli MP. Laser refractive surgery in diabetic patients: a review of the literature. Clin Ophthalmol. 2012;6:1775-1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Bussan KA, Robertson DM. Contact lens wear and the diabetic corneal epithelium: A happy or disastrous marriage? J Diabetes Complications. 2019;33:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Li HY, Luo GC, Guo J, Liang Z. Effects of glycemic control on refraction in diabetic patients. Int J Ophthalmol. 2010;3:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Kador PF, Wyman M, Oates PJ. Aldose reductase, ocular diabetic complications and the development of topical Kinostat(®). Prog Retin Eye Res. 2016;54:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 26. | Klein BE, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1995;2:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Klein BE, Klein R, Moss SE. Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Am J Ophthalmol. 1995;119:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Nielsen NV, Vinding T. The prevalence of cataract in insulin-dependent and non-insulin-dependent-diabetes mellitus. Acta Ophthalmol (Copenh). 1984;62:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Javadi MA, Zarei-Ghanavati S. Cataracts in diabetic patients: a review article. J Ophthalmic Vis Res. 2008;3:52-65. [PubMed] |
| 30. | Sonmez B, Bozkurt B, Atmaca A, Irkec M, Orhan M, Aslan U. Effect of glycemic control on refractive changes in diabetic patients with hyperglycemia. Cornea. 2005;24:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol. 2004;18:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Kim CJ, Choi SK. Analysis of aqueous humor calcium and phosphate from cataract eyes with and without diabetes mellitus. Korean J Ophthalmol. 2007;21:90-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Nishi O, Nishi K, Osakabe Y. Effect of intraocular lenses on preventing posterior capsule opacification: design versus material. J Cataract Refract Surg. 2004;30:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Hayashi Y, Kato S, Fukushima H, Numaga J, Kaiya T, Tamaki Y, Oshika T. Relationship between anterior capsule contraction and posterior capsule opacification after cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2004;30:1517-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | El-Mollayess GM, Saadeh JS, Salti HI. Exogenous endophthalmitis in diabetic patients: a systemic review. ISRN Ophthalmol. 2012;2012:456209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5390] [Cited by in RCA: 4435] [Article Influence: 403.2] [Reference Citation Analysis (4)] |
| 37. | Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015;122:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 38. | Goldacre MJ, Wotton CJ, Keenan TD. Risk of selected eye diseases in people admitted to hospital for hypertension or diabetes mellitus: record linkage studies. Br J Ophthalmol. 2012;96:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26:205-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 40. | Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front Biosci (Landmark Ed). 2009;14:3974-3987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Cavet ME, Vittitow JL, Impagnatiello F, Ongini E, Bastia E. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest Ophthalmol Vis Sci. 2014;55:5005-5015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 42. | Husain S, Abdul Y, Singh S, Ahmad A, Husain M. Regulation of nitric oxide production by δ-opioid receptors during glaucomatous injury. PLoS One. 2014;9:e110397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4983] [Cited by in RCA: 4446] [Article Influence: 247.0] [Reference Citation Analysis (0)] |
| 44. | Alexander JP, Acott TS. Involvement of protein kinase C in TNFalpha regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2001;42:2831-2838. [PubMed] |
| 45. | Wong VH, Bui BV, Vingrys AJ. Clinical and experimental links between diabetes and glaucoma. Clin Exp Optom. 2011;94:4-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Rudzinski M, Wong TP, Saragovi HU. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J Neurobiol. 2004;58:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Lin HC, Stein JD, Nan B, Childers D, Newman-Casey PA, Thompson DA, Richards JE. Association of Geroprotective Effects of Metformin and Risk of Open-Angle Glaucoma in Persons With Diabetes Mellitus. JAMA Ophthalmol. 2015;133:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | Tsai JC, Feuer WJ, Parrish RK 2nd, Grajewski AL. 5-Fluorouracil filtering surgery and neovascular glaucoma. Long-term follow-up of the original pilot study. Ophthalmology. 1995;102:887-92; discussion 892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Mermoud A, Salmon JF, Alexander P, Straker C, Murray AD. Molteno tube implantation for neovascular glaucoma. Long-term results and factors influencing the outcome. Ophthalmology. 1993;100:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Sidoti PA, Dunphy TR, Baerveldt G, LaBree L, Minckler DS, Lee PP, Heuer DK. Experience with the Baerveldt glaucoma implant in treating neovascular glaucoma. Ophthalmology. 1995;102:1107-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Corzo-León DE, Chora-Hernández LD, Rodríguez-Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56:29-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 52. | Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, Chakarbarti A, Dash RJ. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Bavikar P, Mehta V. Rhino-Orbital-Cerebral Mucormycosis: A Fatal Complication of Uncontrolled Diabetes Mellitus. Cureus. 2017;9:e1841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Snaith J, Burns K, Kok J, Chen S, Cheung NW. A case of rhino-orbital mucormycosis in diabetes with haematogenous cerebral spread. Med Mycol Case Rep. 2016;13:22-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Li JO, Liu H, Ting DSJ, Jeon S, Chan RVP, Kim JE, Sim DA, Thomas PBM, Lin H, Chen Y, Sakomoto T, Loewenstein A, Lam DSC, Pasquale LR, Wong TY, Lam LA, Ting DSW. Digital technology, tele-medicine and artificial intelligence in ophthalmology: A global perspective. Prog Retin Eye Res. 2021;82:100900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 280] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 56. | Afshar AR, Oldenburg CE, Stewart JM. A Novel Hybrid Fixed and Mobile Ultra-Widefield Imaging Program for Diabetic Teleretinopathy Screening. Ophthalmol Retina. 2019;3:576-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Rajalakshmi R, Prathiba V, Arulmalar S, Usha M. Review of retinal cameras for global coverage of diabetic retinopathy screening. Eye (Lond). 2021;35:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | Karakaya M, Hacisoftaoglu RE. Comparison of smartphone-based retinal imaging systems for diabetic retinopathy detection using deep learning. BMC Bioinformatics. 2020;21:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Kaur R, Singh H, Samria S, Kumar N, Parachuri N, Sharma R, Bandello F, Loewenstein A, Bilong Y, Hafeez Faridi M, Sharma A. MII RetCam assisted smartphone-based fundus imaging (MSFI)-A boon for paediatric retinal imaging. Eye (Lond). 2020;34:1307-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Natarajan S, Jain A, Krishnan R, Rogye A, Sivaprasad S. Diagnostic Accuracy of Community-Based Diabetic Retinopathy Screening With an Offline Artificial Intelligence System on a Smartphone. JAMA Ophthalmol. 2019;137:1182-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 61. | Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 62. | Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 739] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 63. | Ramesh PV, Ramesh SV, Subramanian T, Ray P, Devadas AK, Ansar SM. Customised artificial intelligence toolbox for detecting diabetic retinopathy with confocal truecolor fundus images using object detection methods. TNOA J Ophthalmic Sci Res. 2023;61:57-66. [DOI] [Full Text] |
| 64. | Keenan TDL, Goldstein M, Goldenberg D, Zur D, Shulman S, Loewenstein A. Prospective, Longitudinal Pilot Study: Daily Self-Imaging with Patient-Operated Home OCT in Neovascular Age-Related Macular Degeneration. Ophthalmol Sci. 2021;1:100034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 65. | Maloca P, Hasler PW, Barthelmes D, Arnold P, Matthias M, Scholl HPN, Gerding H, Garweg J, Heeren T, Balaskas K, de Carvalho JER, Egan C, Tufail A, Zweifel SA. Safety and Feasibility of a Novel Sparse Optical Coherence Tomography Device for Patient-Delivered Retina Home Monitoring. Transl Vis Sci Technol. 2018;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | White NH, Sun W, Cleary PA, Tamborlane WV, Danis RP, Hainsworth DP, Davis MD; DCCT-EDIC Research Group. Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes. 2010;59:1244-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 67. | Ford JA, Lois N, Royle P, Clar C, Shyangdan D, Waugh N. Current treatments in diabetic macular oedema: systematic review and meta-analysis. BMJ Open. 2013;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |