Published online Jun 6, 2023. doi: 10.12998/wjcc.v11.i16.3680
Peer-review started: January 31, 2023
First decision: March 14, 2023
Revised: March 31, 2023
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: June 6, 2023
Processing time: 122 Days and 3.4 Hours
Rectal prolapse is a circumferential, full-thickness protrusion of the rectum through the anus. It is a rare condition, and only affects 0.5% of the general population. Multiple treatment modalities have been described, which have changed significantly over time. Particularly in the last decade, laparoscopic and robotic surgical approaches with different mobilization techniques, combined with medical therapies, have been widely implemented. Because patients have presented with a wide range of complaints (ranging from abdominal discomfort to incomplete bowel evacuation, mucus discharge, constipation, diarrhea, and fecal incontinence), understanding the extent of complaints and ruling out differential diagnoses are essential for choosing a tailored surgical procedure. It is crucial to assess these additional symptoms and their severities using preo
Core Tip: Patients with rectal prolapse should be subjected to detailed history taking, thorough physical examinations, and assessments with appropriate scoring systems before deciding to proceed with surgical intervention. The aim of surgery is an anatomical correction to obtain optimal functional outcomes. Magnetic resonance defecography is beneficial for understanding both functional and anatomical pathologies. To date, robotic and laparoscopic ventral mesh rectopexies are the most commonly performed surgeries and achieve better functional and anatomical outcomes than other surgical alternatives.
- Citation: Oruc M, Erol T. Current diagnostic tools and treatment modalities for rectal prolapse. World J Clin Cases 2023; 11(16): 3680-3693
- URL: https://www.wjgnet.com/2307-8960/full/v11/i16/3680.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i16.3680
Rectal prolapse and rectal intussusception are pelvic floor dysfunction-associated anatomical disorders that are characterized by a complete or partial descent of the rectum. External rectal prolapse is defined as a full-thickness protrusion of the rectum through the anal canal. However, in case of intussusception, the protrusion is limited and does not extend through the anal canal. Most patients with rectal prolapse present with obvious manifestations and can be diagnosed based on a physical examination. Conversely, for a small proportion of patients with intussusception, diagnosis can be challenging (even after making them squat or sit).
Patients also present with additional functional disorders that are accompanied by anatomical abnormalities. It is crucial to assess these additional symptoms and their severity using preoperative scoring systems[1]. Radiological and physiological evaluations may explain some unclear symptoms and also reveal concomitant pelvic disorders[2].
Optimal treatment options for rectal prolapse remain controversial; even recent publications and systematic reviews have not recommended the most appropriate treatment option[3]. According to the practice guidelines proposed by the American Society of Colorectal Surgeons, the goal of a rectal prolapse surgery is to correct the prolapse without causing bowel dysfunction and improve the associated functional abnormalities[4].
This review focuses on the current diagnostic methods, additional treatment modalities, and controversial issues regarding surgical techniques for rectal prolapse.
Rectal prolapse has an annual incidence of 2.5% (per 100000 people); their incidence increases after the fifth decade of life[5]. The condition is more common among women, inmates, and patients with mental disorders[6-8]. The most common symptoms are constipation, incontinence, incomplete evacuation, rectal bleeding, pain, and tenesmus[9]. Although the spectrum of symptoms varies with the type of rectal prolapse, 50%-75% and 25%-50% of the patients complain of fecal incontinence and constipation, respectively[10,11].
Although the disease progression is not understood clearly, chronic straining and constipation are the main predisposing factors. The presence of a deep Douglas pouch, redundant sigmoid colon, insufficient rectosacral fixation, and pelvic floor weakness may also contribute to disease progression[12]. Sustained recto-anal inhibition and the dilatator effect of the prolapsed segment may explain the low resting anal pressure and incontinence seen in most patients[13]. Furthermore, the initial increase in external sphincter tonus may be the first factor that initiates outlet obstruction, constipation, and a straining chain.
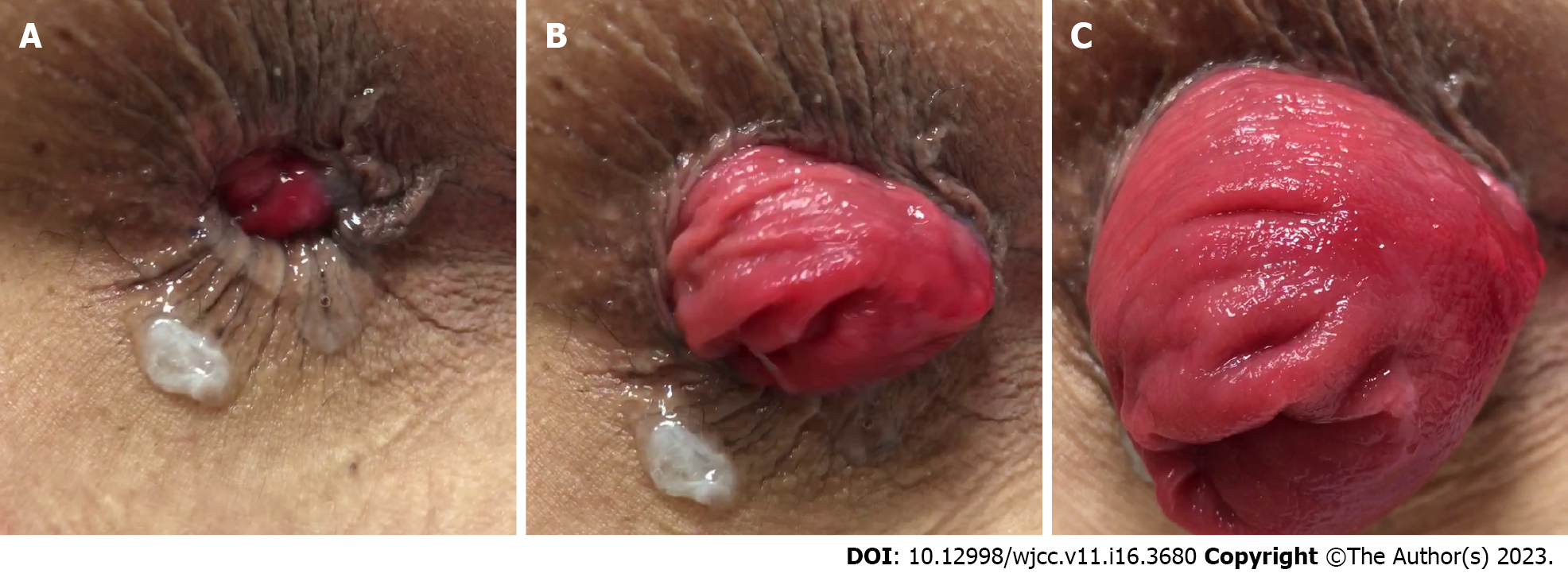
External rectal prolapse is defined as a full-thickness protrusion of the rectum through the anal canal (Figure 1). A full-thickness rectal prolapse has concentric folds of prolapsed tissue, whereas prolapsed hemorrhoids and rectal mucosa have radial invaginations. Diagnoses can be made merely on the basis of history and physical examination findings. No specific test is necessary for diagnosis, except in patients with fecal incontinence. Patients can generally describe the extent of tissue prolapse and whether it reduces spontaneously or requires manual reduction. Most patients report rectal bleeding and pain that can be attributed to solitary rectal ulcers or irritation of the rectal mucosa[14]. Internal rectal prolapse (IRP) is not a precursor of and rarely progresses to external rectal prolapse[15,16].
IRP is characterized by the circular in-folding of the rectal wall into the lumen during straining. Typically appearing 6-8 cm above the anal canal, it has widely varying manifestations. For instance, it can be minimal (such as a 3 mm folding of the wall) or can comprise a circular invagination of all three layers of the rectal wall. In severe cases, IRP may fill the rectal ampulla, which can then obstruct the lumen and hinder stool passage[17]. It is difficult to visualize during a physical examination, and thus, difficult to diagnose[18].
The most common symptoms of IRP are constipation and obstructive defecation (85%), followed by fecal incontinence (56%)[19]. Fecal incontinence is more severe in patients with higher-grade IRP[13].
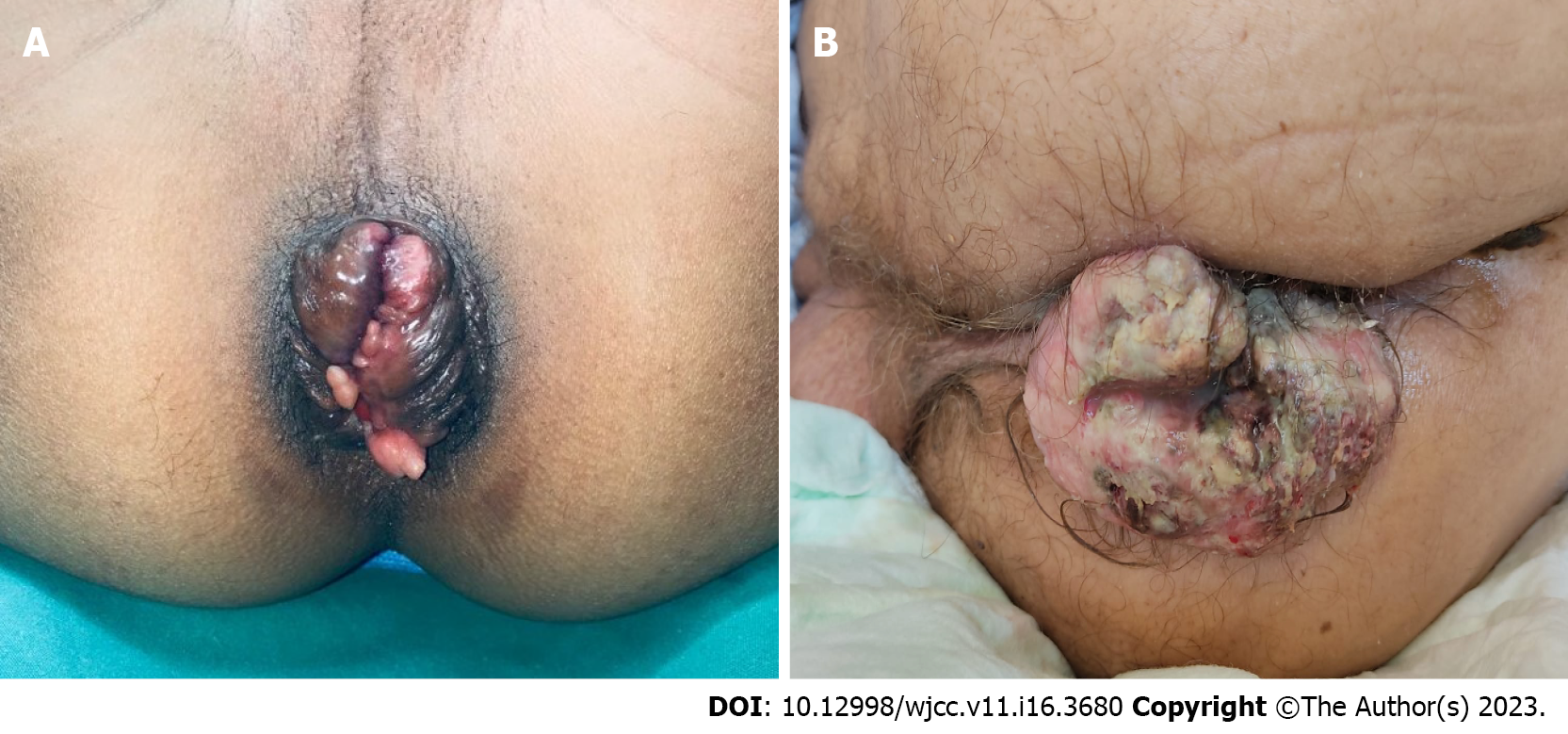
Other conditions similar to rectal prolapse include prolapsing hemorrhoids and a mass prolapsing out of the rectum (Figure 2). Concentric folds of the rectum cannot be observed in prolapsing hemorrhoids because the remaining muscular wall of the rectum remains in place. Occasionally, early prolapse may not be completely circumferential but can still be distinguished from hemorrhoids, because it lacks grooves between the columns of prolapsing tissue[14].
Constipation assessment is a critical component of examination. Constipation may result from rectal intussusception, which leads to narrowing of the bowel lumen; the subsequent blockage then deteriorates with excessive straining and colonic dysmotility[10,11]. Patients with extreme constipation suggestive of colonic inertia also require a workup for this condition. Different scoring systems, such as the Wexner Constipation Score or the Rome 4 Criteria, are used to evaluate such patients[20,21].
A redundant colon may also be a predisposing factor for constipation and rectal prolapse. Constipation and excess straining likely contribute to prolapse development, but can also be exacerbated by the prolapse itself[14]. Differentiating impaired rectal emptying from slow-transit constipation may be difficult, and transit studies (such as the Sitz Marker Study) are useful for differential diagnosis.
Fecal incontinence generally develops late in the clinical course of rectal prolapse. Several factors may contribute to the emergence of fecal incontinence; main factors comprise a patulous anus, continuous recto-anal inhibitory reflex with impaired recto-anal excitatory reflex, pudendal neuropathy, and external prolapse. Any kind of fecal incontinence (urge, passive, and mixed) may present with varying degrees of severity; in some cases, constipation may accompany incontinence[22]. Fecal incontinence is aggravated by recurrent prolapse, which creates stretch injuries in the sphincters[23]. Patient prognosis would improve if rectal prolapse is treated early. Although rectal prolapse repair does not directly correct sphincter dysfunction, it improves the symptoms of incontinence[24].
Fecal incontinence severity can be measured using different scoring systems, such as the Wexner/Cleveland Clinic Fecal Incontinence Score (CCFIS) and Fecal Incontinence Severity Index (FISI). The Wexner/CCFIS, which is the most well-known and most cited score, contains a five-item scale; each item is graded 0–4, and the total score is 20[1]. The FISI score qualifies the type and quantity of incontinence with regard to the number of episodes and generates a summary score in combination[25]. The FISI score is highly correlated with symptom severity and the Fecal Incontinence Quality of Life score. The Rapid Assessment Fecal Incontinence Score is a new scoring system that has been recently updated and validated; however, further research is required to assess its external validity[26].
The use of fluoroscopic defecography (FD) has gradually decreased over the years; however, compared with existing modalities, FD has a higher detection rate of pelvic floor anomalies and allows imaging in a more natural position[2]. A 2017 systematic review and meta-analysis revealed that compared with magnetic resonance defecography, traditional FD has higher detection rates for rectocele, rectal prolapse, rectoanal intussusception, and perineal descent, but not for enterocele[27]. However, fluoroscopy has the following disadvantages: Inability to demonstrate the intrapelvic interaction of the pelvic organs[28], radiation exposure, inability to visualize the pelvic soft tissue, and low sensitivity[29]. Moreover, evacuation proctography may reveal retro rectal intussusception in asymptomatic individuals; Palit et al[30] revealed that proctography revealed IRP in 20% of the healthy volunteers that they analyzed[30].
The Oxford radiological rectal prolapse grading system is used to categorize rectal prolapse[31]; it categorizes rectal prolapse into five levels: High rectal (Grade I - level above the rectocele), low rectal (Grade II - level of the rectocele but above the anal canal), high anal (Grade III - descending to the top of the anal canal), low anal (Grade IV - descending into the anal canal), and external ( Grade V - protrusion from the anal canal).
Dynamic magnetic resonance defecography (DMRD) allows the evaluation of concomitant pelvic floor disorders and enables clear demonstration of the pelvic anatomy. It provides information on both structural and functional abnormalities; this is extremely important, especially for patients who have undergone prior pelvic or perineal surgery[18]. DMRD can differentiate among mucosal, full-thickness rectorectal, and rectoanal intussusception. A small amount of rectal prolapse is normal and found in approximately 80% of the population[32]. DMRD also reveals associated anterior pelvic support defects, such as cystocele, rectocele, enterocele, and vaginal vault prolapse. In patients with multicompartment pelvic organ prolapse, urodynamic and urogynecological examinations should be performed before deciding whether concomitant surgical intervention is necessary[33]. The pelvic organ prolapse quantification system has been used for pelvic organ prolapse classification; it has a high correlation with DMRD findings[34]. Defecography may influence clinical decision-making and surgical approaches in 28%-41% of the cases[35,36].
DMRD must be used to evaluate the squeeze (Kegel), strain (Valsalva), and defecation (evacuation) phases for optimal reporting. For accurate visualization and grading of anterior and middle compartment prolapse, patients should be made to perform the Kegel exercise and evacuation first and the Valsalva maneuver thereafter[37]. The defecation phase should be repeated thrice for a proper diagnosis. Furthermore, radiologists must determine whether defecation has been achieved. Studies have revealed no significant differences in defecography findings between the supine and sitting positions[38,39].
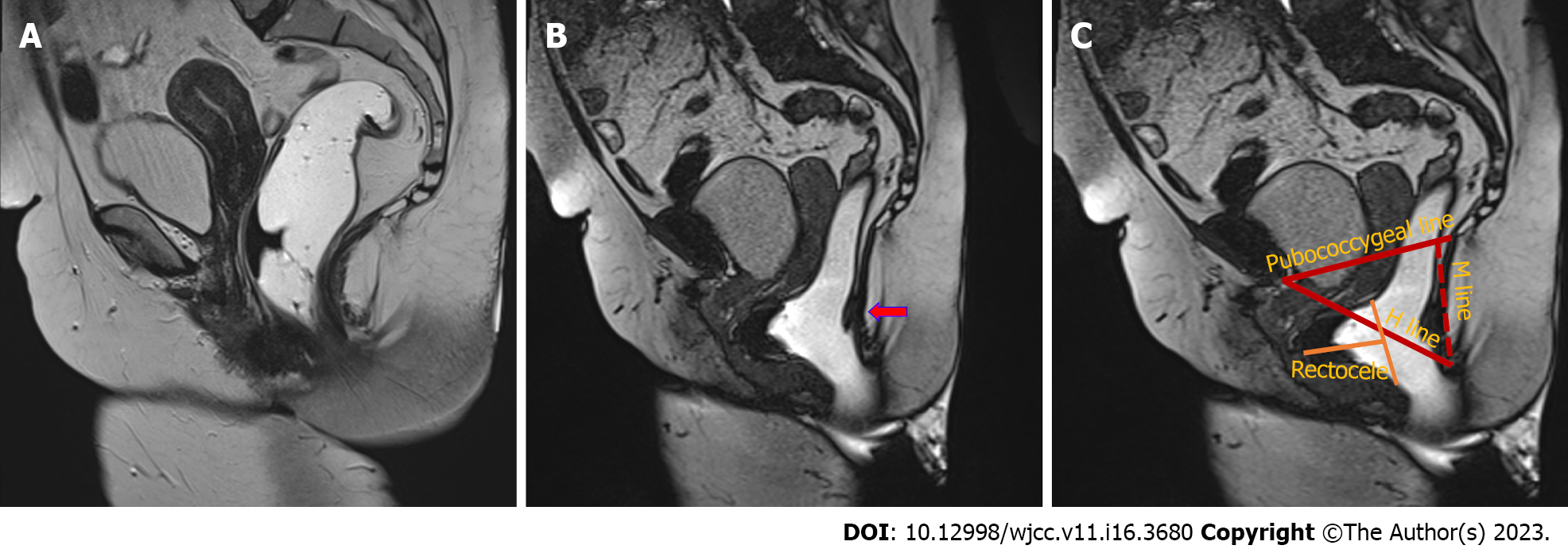
The 2019 recommendations of the European Society for Radiology outline the H-line, M-line, and organ prolapse system, which allows for consistent grading of various pelvic floor disorders[40]. The H-line is measured from the inferior pubic bone to the posterior anorectal junction; the M-line is drawn perpendicularly, connecting the pubococcygeal line to the posterior H-line. A pubococcygeal line is drawn from the inferior pubic bone to the final coccygeal point (Figure 3).
Patients with postoperative recurrence should also be evaluated using DMRD. DMRD can identify synthetic materials, especially polyvinylidene fluoride meshes, and evaluate their position, integrity, and associated complications (such as scarring, infections, fistula formation, and recurrent prolapse)[41,42].
Anal manometry provides valuable information about anal sphincter function, including the resting and squeeze pressures, length of the functional anal canal, recto-anal inhibitory reflex activity during rectal distension, rectal sensation, rectal compliance, and defecation function[2].
Manometric results indicative of anal hypotonia are frequently reported, and these include impaired maximal voluntary contraction in patients with rectal prolapse[43]; however, these findings rarely influence surgical planning, especially for external rectal prolapse[14]. Even then, anal manometry helps predict the postoperative patient prognosis. Patients with decreased anal pressures and slowed nerve conduction are more likely to have postoperative incontinence[44]. Glasgow et al[45] found that patients with a maximum squeeze pressure of > 60 mmHg had better postoperative continence, but the correlation between manometric findings and incontinence severity in them was low[45]. Therefore, postoperative functional evaluation using anal manometry may achieve more accurate findings. Manometry is more useful for addressing inconsistent data regarding pelvic floor function and evaluating continuous defecatory problems after surgery[2].
In patients with internal rectal prolapse, anorectal manometry and endoanal sonography confirm sphincter hypotonia and sphincter rupture, explain the origin of continence disorders, and identify dyssynergic defecation (anismus; a rare condition that requires physical therapy)[46].
Endoanal ultrasonography allows precise imaging of the sphincter complex, accurate recognition of occult anal sphincter defects[47], and mapping of the extent of sphincter injury. If a patient has a history of vaginal delivery, proctological/perianal surgery, or fecal continence impairment, sphincter integrity should be investigated using endoanal ultrasonography[23]. However, in patients with external rectal prolapse with no previous trauma, endoanal ultrasound is not necessary for the preoperative workup.
Before rectal prolapse surgery, neoplasms and inflammatory bowel disease should be ruled out. Furthermore, 10%-15% of the patients have solitary rectal ulcers, which are generally indicative of surgical treatment[14]. Abdominal and pelvic computed tomography is optional and useful for ruling out malignancy and other diseases.
Patients with internal rectal prolapse of Oxford grades I–III without incontinence, those with internal prolapse of Oxford grade IV with a high surgical risk, and those with minimally symptomatic external prolapse are candidates for conservative therapies[46]. Nonoperative management includes defecation training, use of stool softeners, and dietary changes. Patients should consume 30–40 g of fiber daily and perform at least 100 min of aerobic exercise weekly. Biofeedback therapy, which involves real-time training of pelvic muscle contraction and anal sphincter relaxation in coordination with rectal emptying, may also be beneficial. These treatments do not cure rectal prolapse, but may be useful for improving the quality of life. Surgery should be considered if conservative therapies fail after 2–3 mo[48].
Surgical treatment is indicated for internal prolapse of Oxford grades III–IV and symptomatic external rectal prolapse[48]. To date, many different surgical techniques have been described in literature; many of these have been completely abandoned or are rarely used in modern surgical treatment[4].
Surgical treatment options are generally divided into two categories, namely abdominal and perineal approaches. Choice of the optimum approach is usually dictated by the patient’s general condition (age, comorbidities, and bowel function) and the surgeon’s experience and preference[49]. Each approach has its own advantages and disadvantages.
Generally, frail older patients with comorbidities are better candidates for perineal operations because these procedures can be performed under locoregional anesthesia with lower perioperative morbidity and shorter hospital stays. Although some retrospective, low-powered studies suggest that, compared with abdominal procedures, these procedures have higher recurrence rates and worse functional outcomes[50], a recent Cochrane review found no difference between the two procedures[51]. Furthermore, the PROSPER trial did not find any differences in recurrence between abdominal and perineal approaches, especially since patients for whom a perineal procedure was elected were older and had worse physical status and bowel function than patients for whom an abdominal procedure was elected in this study[5]. A meta-analysis performed by Pellino et al[52] claimed that the recurrence rate might be higher with perineal approaches, which may be related to the fact that the patients are old and the follow-up periods are long; therefore, a clear result cannot be established[52].
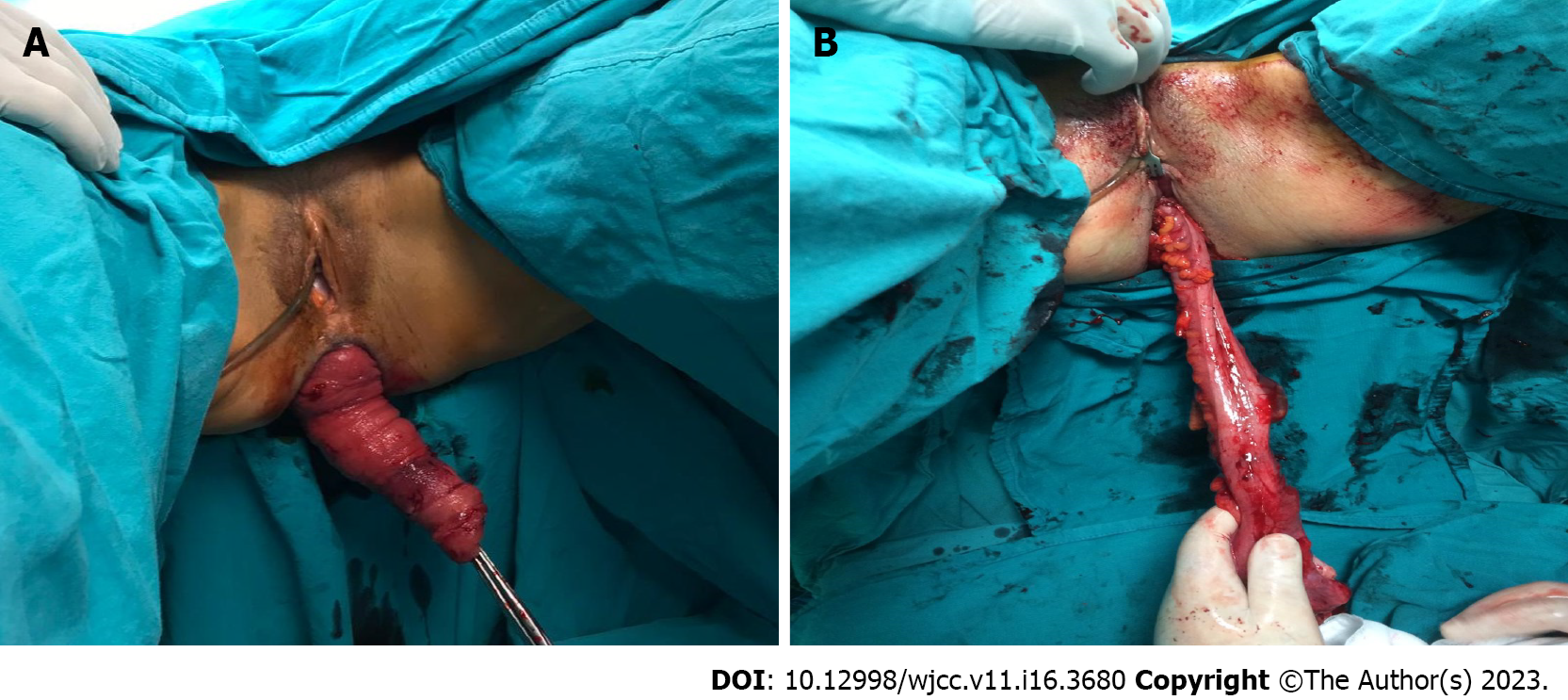
Among perineal interventions, the Delorme procedure (resection of the mucosa and plication of the rectal wall) is generally preferred for short-segment prolapse (< 5 cm long) and the Altemeier procedure (perineal proctosigmoidectomy; Figure 4) is reserved for long-segment prolapse; both technique achieve similar results in terms of reccurence[53]. Moreover, different randomized trials have suggested that these approaches have significant improvement from baseline quality of life[5,54].
Levatorplasty theoretically improves postoperative incontinence by restoring the anorectal anatomy, and can be performed if the surgeon prefers a perineal procedure; however, its benefit remains unclear[55]. Some studies have revealed significant improvements in incontinence scores and decreased recurrence rates with levatorplasty[56,57]. Furthermore, levatorplasty enables tension-free overlap repair and repair of any undetected sphincter damage in the upper part of the sphincter complex[7].
Stapled transanal rectal resection (STARR): Stapled transanal rectal resection is another transperineal approach that may be a good alternative, especially for patients with obesity who experience obstructive defecation, associated rectocele, rectal intussusception, and satisfactory sphincter performance[58]. As this reduces rectal compliance, patients with anal incontinence are not good candidates for this technique. The most frequent complication is urgency, and serious complications, such as staple line disruption, pelvic cellulitis, rectovaginal fistula, peritonitis, and stricture, have also been reported. However, the STARR procedure is safe and efficient in treating ODS symptoms and enhancing patients' quality of life[59].
Natural orifice transanal endoscopic rectopexy: This technique was introduced in 2019 as an alternative to the abdominal and perineal methods for complete rectal prolapse[60]. In this procedure, the colon is fixed to the abdomen and promontory using custom endoscopic devices without mesh. Although this method is unlikely to offer the same level of long-term durability as other approaches, it may be an effective choice for patients with frailty, particularly when mesh avoidance is preferred[61].
Abdominal procedures are generally preferred for patients fit to withstand surgery without age concerns[4] because of lower recurrence rates and better functional outcomes. Patients with severe endometriosis, history of severe adhesions, and peritonitis are unsuited for abdominal approaches.
Although the outcomes of open, laparoscopic, and robotic repairs are similar, minimally invasive approaches are more common because of faster recovery, lower morbidity, decreased postoperative pain, and lesser blood loss[62,63].
Abdominal surgeries vary according to the dissection plane and fixation technique. All of these different procedures aim to prevent prolapse by fixing the rectum and provide better functional outcomes.
The plane of rectal dissection is a controversial issue in rectal prolapse surgery. A pooled analysis of 532 patients was performed to determine the influence of the extent of rectal mobilization on the rate of recurrent rectal prolapse after abdominal rectopexy; both univariate and multivariate analyses suggested that circumferential mobilization was associated with a lower long-term recurrence rate[64]. However, during posterior and lateral mobilization, there is a risk of autonomic nerve plexus injury and worsening of preexisting constipation and the obstructive defecation syndrome. Speakman et al[65] found that division of the lateral ligaments was a risk factor for postoperative constipation and that the rectal electrical sensory thresholds were increased in patients who underwent ligament division[65].
The risk of sigmoidocele and enterocele development also increases with lateral dissection[7]. Several studies have suggested that avoiding complete rectal mobilization improves postoperative constipation and protects from de novo constipation with a similar rate of recurrence[66,67].
During posterior mesh rectopexy (Wells procedure), anterior dissection is avoided, and the dissection is performed through the lateral and posterior aspects. After attaching either a polyester or polypropylene mesh to the presacral fascia, it is loosely wrapped around the rectum (270˚)[68]. An overall improvement in continence (74%-100%) was reported with this technique, with conflicting results regarding constipation and de novo constipation in 5%-44% of the patients[12]. Other studies also revealed new-onset constipation after posterior mesh rectopexy in more than 50% of the patients[51,69].
In lateral mesh rectopexy (Orr–Loygue procedure), the rectum is mobilized circumferentially and the mesh is fixed to the anterolateral rectal wall and sacral promontory[57]. A significant reduction in incontinence scores was reported after 1 year[57]. However, as for posterior rectopexy, worsening of constipation has been reported in up to 27% of the patients[70].
In resection rectopexy (Frykman–Goldberg procedure), after complete rectal mobilization and sigmoid resection, the distal rectum is fixed to the presacral fascia using sutures[71]. As fixation performed by sutures this is the most preferred technique in the United States because of potential mesh complications and lawsuits[72,73]. This procedure is preferred in cases of proven slow-transit constipation, redundant sigmoid colon, and preexisting diverticular disease[18,74]. A Cochrane review conducted in 2015 suggested that bowel resection was associated with lower rates of constipation than rectopexy alone[51]. Smedberg et al[54] compared four surgical approaches (resection, suture, Altemeier, and Delorme) in a randomized clinical trial and found none to be superior; however, a 20% recurrence rate was observed. Laparoscopic resection rectopexy has higher complication rates than laparoscopic ventral mesh rectopexy (LVMR); however, it offers a better improvement in incontinence[75].
In suture rectopexy, the rectum is circumferentially mobilized, two or three sutures are placed on either side, and the lateral ligaments are fixed to the presacral fascia using non-absorbable sutures[76]. A systematic review suggested higher recurrence rates but lower operative times with suture rectopexy than with ventral mesh rectopexy[77]. As expected, owing to extensive mobilization of the rectum, a longer gastrointestinal transit time and worse functional outcomes in terms of constipation were reported in a randomized study[78].
Although a relatively new technique, ventral mesh rectopexy is the most common procedure for rectal prolapse in Europe[63]. D’ Hoore first described this technique in 2004; no rectal mobilization or lateral dissection is performed in the original technique, and the lateral ligaments are preserved[56]. The mesh is placed on the anterior rectal wall, and fixation to the sacral promontory is performed using sutures, staplers, or even surgical glue[79]. Reinforcement of the rectovaginal septum, correction of the enterocele, correction of genital prolapse by adding sacrocolpopexy, and preservation of the hypogastric and parasympathetic nerves are possible by this technique. Therefore, ventral mesh rectopexy may be the optimal treatment modality for patients with incontinence and concomitant anterior compartment disorders. Owing to the lower rate of postoperative constipation, lower recurrence rate, and avoidance of colonic anastomosis[74], it appears to be superior to the other abdominal techniques explained above in terms of functional outcomes[80].
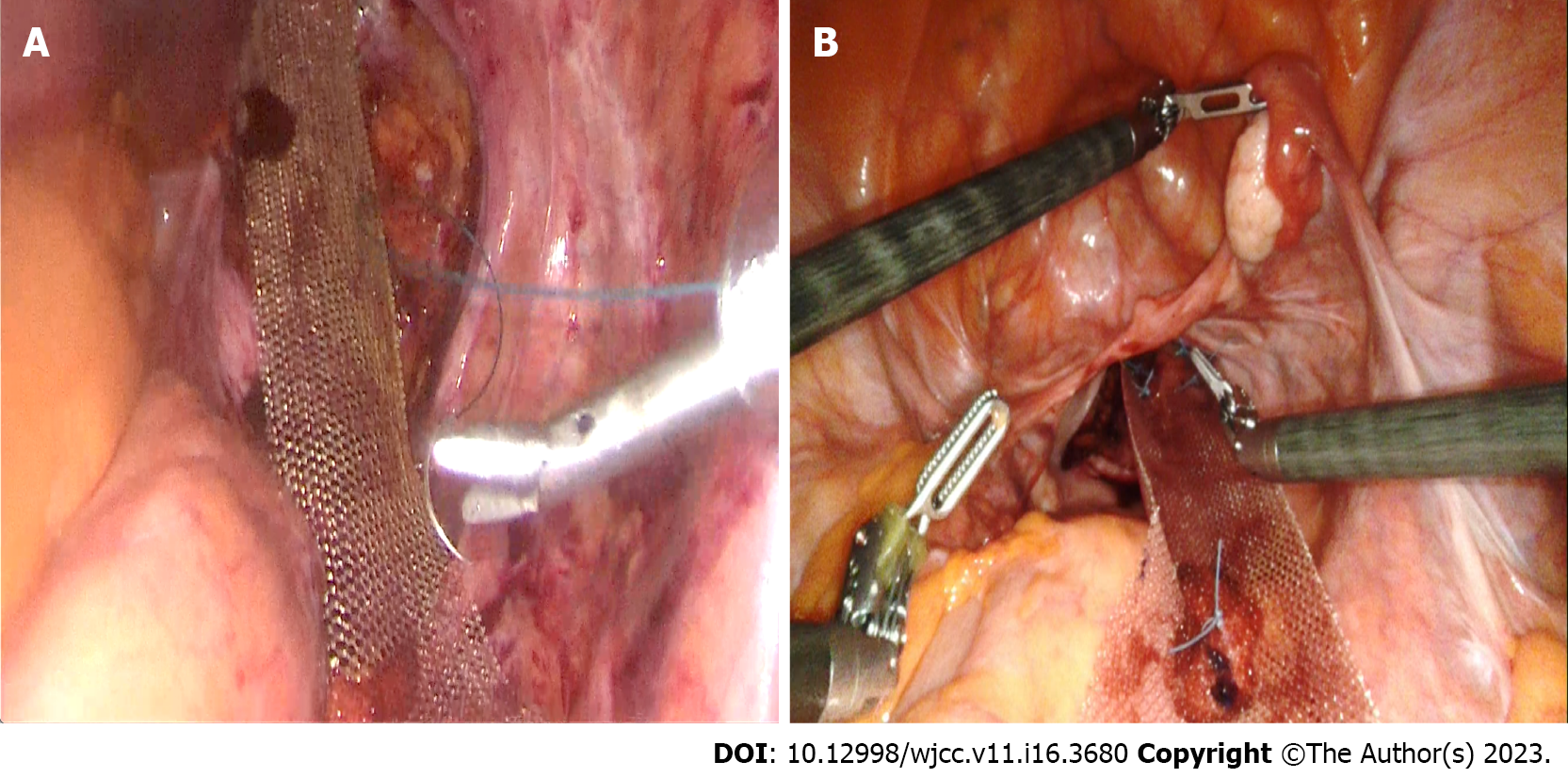
The laparoscopic and robotic approaches do not differ in terms of rates of conversion to open surgery[81]. However, in deep and narrow pelvises or in patients with morbid obesity, the robotic approach has eliminated the limitations of the laparoscopic approach; it enables meticulous dissection in deep and narrow spaces[82,83] (Figure 5).
Moreover, robots can ease the learning curve, a study revealed that almost 100 cases of the laparoscopic approach and only 20 cases of the robotic approach were required to gain proficiency[84].
A meta-analysis of eight studies suggested that robotic surgery is associated with significantly fewer complications than laparoscopic surgery[85]; furthermore, the recurrence rates do not differ significantly between the two (0%-20% vs 0%-26.7%)[86,87].
Robotic surgery is considered more expensive than other techniques; however, after adjusting for the cost of an improved health-related quality of life, the expenditure is almost comparable with that of laparoscopic surgery[88]. Although reinforcement of the rectovaginal septum is reportedly similar between the robotic and laparoscopic approaches, an improved quality of life has been attributed to a more precise mesh fixation[63].
Another contentious aspect of rectal prolapse surgery is the mesh type. Meshes can be divided into three categories: First generation (synthetic non-absorbable meshes made of polypropylene or expanded polytetrafluoroethylene), second generation (combinations of more than one synthetic material (such as polypropylene, polyester, or expanded polytetrafluoroethylene) and/or other materials (such as titanium, omega-3 fatty acids, poliglecaprone-25, and polyvinylidene fluoride), and third generation (biological prostheses). Compared with first-generation meshes, second-generation meshes are less susceptible to infection, adhesion, and recurrence. Furthermore, biological meshes provide a matrix for native cells to populate, which fills the hernia defect with connective tissue[89].
In 2008, the National Institute of Clinical Excellence conducted a review that revealed erosion rates of 0%, 7%, and 14% for biological meshes (xenografts), synthetic meshes, combined biological and synthetic meshes, respectively[90]. Even then, the European Society of Coloproctology guidelines on the use of mesh for rectal prolapse repair suggested that both mesh types were suitable for repair; however, this suggestion was based on low-quality data. The superiority of one mesh type over the other has not yet been demonstrated[91]. Biological grafts can be used in high-risk patients (diabetics; smokers; and those with previous pelvic radiation, inflammatory bowel disease, and intraoperative findings of a rectal or vaginal leak)[92,93], even though current data have not indicated any particular benefits.
After surgery, patients should be advised against lifting, engaging in sexual intercourse, and consuming laxatives for at least 6 weeks postoperatively. The functional outcomes may not improve promptly and may not resolve completely; patients should be informed of these possibilities. Pelvic floor physical therapy can be continued for patients who present with obstructive defecation or incontinence preoperatively[94].
Both patient-related (sex, body mass index, and prior history of prolapse repair)[95] and technical (inadequate anterior rectal dissection, inadequate fixation of the mesh to the anterior rectal wall or sacral promontory, and the mesh type)[84,96] factors may affect recurrence rates. In perineal approaches, stapled anastomosis, shorter specimen lengths, and severe pre-existing constipation are associated with an increased risk of recurrence[97]. Furthermore, Fu et al[98] found that a prolonged pudendal nerve terminal motor latency, which indicates denervation of the external anal sphincter, is predictive of recurrence.
Patients presenting with recurrence should undergo magnetic resonance imaging (MRI)-based evaluations for identifying the potential etiology. Most importantly, patients should wait for at least 6 months before undergoing a reoperation[94].
In patients with early recurrence of full-thickness rectal prolapse, the European Society of Coloproctology guidelines recommend reoperation to reattach the mesh to the sacral promontory. In case of erosion, location of the mesh erosion is important for treatment. Depending on the extent of the erosion, surgical removal of the mesh can be considered if a technically feasible, and diverting stoma should be considered. Reintervention presents a significant technical challenge and should only be performed at experienced centers[91].
A systematic literature review failed to develop an algorithm for treatment of recurrent rectal prolapse[99]. Steele et al[100] reported significantly more recurrences after a perineal procedure than after an abdominal procedure for recurrent external rectal prolapse[100]. Repetition of a perineal proctosigmoidectomy is possible for recurrence after a resection procedure but must be utilized with great caution because of the possibility of leaving an ischemic segment between anastomoses unless the previous anastomosis is resected[101,102]. In addition, the recurrence rate following redo perineal proctosigmoidectomy is higher than that after the primary procedure[103].
Studies have suggested that the efficacy of repeating LVMR for recurrent prolapse following a failed perineal or abdominal procedure is similar to that of primary LVMR[98,104]. Conversely, a prospective cohort study on 109 patients who underwent ventral rectopexy revealed 1-year, 3-year, and 5-year prolapse recurrence rates of 1.4%, 6.9%, and 9.7% for primary repairs and 13.9%, 25%, and 25% for recurrent prolapse repairs respectively. The time to recurrence was shorter in patients who underwent Ventral Rectopexy for recurrent prolapse[105]. Further studies are required to understand the effectiveness of LVMR in patients with a recurrence.
Management of rectal prolapse is complex. To select the appropriate surgical intervention, factors such as the patient's medical history, clinical symptoms, surgeon's experience, and hospital equipment must be considered. Precise preoperative planning would help choose the best option for the patient, including validated scoring systems and imaging modalities. Although robotic rectopexy is anticipated to eventually be deemed the gold standard, intriguing methods such as the NOTES technique continue to be developed. Close postoperative follow-up is crucial to monitor improvements in the quality of life, incomplete resolution of symptoms, or recurrence. More randomized controlled studies are still required to determine the best surgical treatment; however, close follow-up of quality of life and functional outcomes and proper management of patients will help achieve better results, regardless of the method chosen.
Dr. Ali Konan for permission to use photographs in Figure 3.
Provenance and peer review: Invited article; externally peer reviewed.
Peer-review model: Single blind.
Corresponding author's membership in professional societies: Turkish Colorectal Surgical Association, Turkish Surgical Association.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bustamante-Lopez LA, Brazil; Kumar S, India; Mehrvarz S, Iran S-Editor: Ma YJ L-Editor: A P-Editor: Fan JR
| 1. | Abou Khalil M, Boutros M. Quantitative and qualitative analysis of fecal incontinence. Ann Laparosc Endosc Surg. 2022;7:19. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Kwakye G, Maguire LH. Anorectal Physiology Testing for Prolapse-What Tests are Necessary? Clin Colon Rectal Surg. 2021;34:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | Gallo G, Trompetto M. Complete rectal prolapse: still a lot of work to do. Tech Coloproctol. 2019;23:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Bordeianou L, Paquette I, Johnson E, Holubar SD, Gaertner W, Feingold DL, Steele SR. Clinical Practice Guidelines for the Treatment of Rectal Prolapse. Dis Colon Rectum. 2017;60:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 5. | Senapati A, Gray RG, Middleton LJ, Harding J, Hills RK, Armitage NC, Buckley L, Northover JM; PROSPER Collaborative Group. PROSPER: a randomised comparison of surgical treatments for rectal prolapse. Colorectal Dis. 2013;15:858-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Jacobs LK, Lin YJ, Orkin BA. The best operation for rectal prolapse. Surg Clin North Am. 1997;77:49-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Kumar N, Kumar D. Fecal incontinence and rectal prolapse. Indian J Gastroenterol. 2019;38:465-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Küpfer CA, Goligher JC. One hundred consecutive cases of complete prolapse of the rectum treated by operation. Br J Surg. 1970;57:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 55] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Leal VM, Regadas FS, Regadas SM, Veras LR. Clinical and functional evaluation of patients with rectocele and mucosal prolapse treated with transanal repair of rectocele and rectal mucosectomy with a single circular stapler (TRREMS). Tech Coloproctol. 2010;14:329-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Kim DS, Tsang CB, Wong WD, Lowry AC, Goldberg SM, Madoff RD. Complete rectal prolapse: evolution of management and results. Dis Colon Rectum. 1999;42:460-6; discussion 466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Madoff RD, Mellgren A. One hundred years of rectal prolapse surgery. Dis Colon Rectum. 1999;42:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Tsunoda A. Surgical Treatment of Rectal Prolapse in the Laparoscopic Era; A Review of the Literature. J Anus Rectum Colon. 2020;4:89-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Hawkins AT, Olariu AG, Savitt LR, Gingipally S, Wakamatsu MM, Pulliam S, Weinstein MM, Bordeianou L. Impact of Rising Grades of Internal Rectal Intussusception on Fecal Continence and Symptoms of Constipation. Dis Colon Rectum. 2016;59:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Cannon JA. Evaluation, Diagnosis, and Medical Management of Rectal Prolapse. Clin Colon Rectal Surg. 2017;30:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Bloemendaal AL, Buchs NC, Prapasrivorakul S, Cunningham C, Jones OM, Hompes R, Lindsey I. High-grade internal rectal prolapse: Does it explain so-called "idiopathic" faecal incontinence? Int J Surg. 2016;25:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Hatch Q, Steele SR. Rectal prolapse and intussusception. Gastroenterol Clin North Am. 2013;42:837-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Faccioli N, Comai A, Mainardi P, Perandini S, Moore F, Pozzi-Mucelli R. Defecography: a practical approach. Diagn Interv Radiol. 2010;16:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Gachabayov M, Bendl R, Flusberg M, Grimes CL, Altomare DF, Ozuner G, Longo A, Bergamaschi R. Rectal prolapse and pelvic descent. Curr Probl Surg. 2021;58:100952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wijffels NA, Jones OM, Cunningham C, Bemelman WA, Lindsey I. What are the symptoms of internal rectal prolapse? Colorectal Dis. 2013;15:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 851] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 21. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1036] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 22. | Gallo G, Martellucci J, Pellino G, Ghiselli R, Infantino A, Pucciani F, Trompetto M. Consensus Statement of the Italian Society of Colorectal Surgery (SICCR): management and treatment of complete rectal prolapse. Tech Coloproctol. 2018;22:919-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Woods R, Voyvodic F, Schloithe AC, Sage MR, Wattchow DA. Anal sphincter tears in patients with rectal prolapse and faecal incontinence. Colorectal Dis. 2003;5:544-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Hiltunen KM, Matikainen M. Improvement of continence after abdominal rectopexy for rectal prolapse. Int J Colorectal Dis. 1992;7:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, Lowry AC. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 582] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | de la Portilla F, Ramallo I, Maestre MV, Roig JV, Devesa M, Padillo FJ. Validation of a Novel Fecal Incontinence Scale: The Rapid Assessment Fecal Incontinence Score (RAFIS). J Clin Gastroenterol. 2021;55:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Barfield LR. Perineal Approaches to Rectal Prolapse. Clin Colon Rectal Surg. 2017;30:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Fitzgerald J, Richter LA. The Role of MRI in the Diagnosis of Pelvic Floor Disorders. Curr Urol Rep. 2020;21:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Tomšič MV, Podkrajšek M. Dynamic MR imaging of pelvic floor dysfunction. Appl Radiol. 2017;46:21-27. |
| 30. | Palit S, Bhan C, Lunniss PJ, Boyle DJ, Gladman MA, Knowles CH, Scott SM. Evacuation proctography: a reappraisal of normal variability. Colorectal Dis. 2014;16:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Lindsey I. Internal rectal prolapse. In: Lindsey I, Nugent K, Dixon T, editors. Pelvic Floor Disorders for the Colorectal Surgeon (Oxford, 2010; online edn, Oxford Academic, 1 May 2013). Oxford: Oxford University Press, 2010: 93-102. |
| 32. | El Sayed RF, Alt CD, Maccioni F, Meissnitzer M, Masselli G, Manganaro L, Vinci V, Weishaupt D; ESUR and ESGAR Pelvic Floor Working Group. Magnetic resonance imaging of pelvic floor dysfunction - joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017;27:2067-2085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Lim M, Sagar PM, Gonsalves S, Thekkinkattil D, Landon C. Surgical management of pelvic organ prolapse in females: functional outcome of mesh sacrocolpopexy and rectopexy as a combined procedure. Dis Colon Rectum. 2007;50:1412-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Pollock GR, Twiss CO, Chartier S, Vedantham S, Funk J, Arif Tiwari H. Comparison of magnetic resonance defecography grading with POP-Q staging and Baden-Walker grading in the evaluation of female pelvic organ prolapse. Abdom Radiol (NY). 2021;46:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Groenendijk AG, Birnie E, de Blok S, Adriaanse AH, Ankum WM, Roovers JP, Bonsel GJ. Clinical-decision taking in primary pelvic organ prolapse; the effects of diagnostic tests on treatment selection in comparison with a consensus meeting. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Kaufman HS, Buller JL, Thompson JR, Pannu HK, DeMeester SL, Genadry RR, Bluemke DA, Jones B, Rychcik JL, Cundiff GW. Dynamic pelvic magnetic resonance imaging and cystocolpoproctography alter surgical management of pelvic floor disorders. Dis Colon Rectum. 2001;44:1575-1583; discussion 1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Khatri G, Kumar NM, Xi Y, Smith W, Bacsu C, Bailey AA, Zimmern PE, Pedrosa I. Defecation versus pre- and post-defecation Valsalva maneuvers for dynamic MR assessment of pelvic floor dysfunction. Abdom Radiol (NY). 2021;46:1362-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Iacobellis F, Brillantino A, Renzi A, Monaco L, Serra N, Feragalli B, Iacomino A, Brunese L, Cappabianca S. MR Imaging in Diagnosis of Pelvic Floor Descent: Supine versus Sitting Position. Gastroenterol Res Pract. 2016;2016:6594152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Pannu HK, Scatarige JC, Eng J. Comparison of supine magnetic resonance imaging with and without rectal contrast to fluoroscopic cystocolpoproctography for the diagnosis of pelvic organ prolapse. J Comput Assist Tomogr. 2009;33:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Zayed R. MRI of pelvic floor dysfunction: a practical review. ECR 2019/C-1978 conference. [DOI] [Full Text] |
| 41. | Khatri G, Carmel ME, Bailey AA, Foreman MR, Brewington CC, Zimmern PE, Pedrosa I. Postoperative Imaging after Surgical Repair for Pelvic Floor Dysfunction. Radiographics. 2016;36:1233-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Laitakari KE, Mäkelä-Kaikkonen JK, Pääkkö E, Ohtonen P, Rautio TT. A prospective pilot study on MRI visibility of iron oxide-impregnated polyvinylidene fluoride mesh after ventral rectopexy. Tech Coloproctol. 2019;23:633-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Tsunoda A, Takahashi T, Matsuda S, Kusanagi H. Predictive Factors for Recurrence of External Rectal Prolapse after Laparoscopic Ventral Rectopexy. J Anus Rectum Colon. 2021;5:376-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 44. | Birnbaum EH, Stamm L, Rafferty JF, Fry RD, Kodner IJ, Fleshman JW. Pudendal nerve terminal motor latency influences surgical outcome in treatment of rectal prolapse. Dis Colon Rectum. 1996;39:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Glasgow SC, Birnbaum EH, Kodner IJ, Fleshman JW, Dietz DW. Preoperative anal manometry predicts continence after perineal proctectomy for rectal prolapse. Dis Colon Rectum. 2006;49:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Cariou de Vergie L, Venara A, Duchalais E, Frampas E, Lehur PA. Internal rectal prolapse: Definition, assessment and management in 2016. J Visc Surg. 2017;154:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Faltin DL, Boulvain M, Irion O, Bretones S, Stan C, Weil A. Diagnosis of anal sphincter tears by postpartum endosonography to predict fecal incontinence. Obstet Gynecol. 2000;95:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | van der Schans EM, Paulides TJC, Wijffels NA, Consten ECJ. Management of patients with rectal prolapse: the 2017 Dutch guidelines. Tech Coloproctol. 2018;22:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Alwahid M, Knight SR, Wadhawan H, Campbell KL, Ziyaie D, Koch SMP. Perineal rectosigmoidectomy for rectal prolapse-the preferred procedure for the unfit elderly patient? 10 years experience from a UK tertiary centre. Tech Coloproctol. 2019;23:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Riansuwan W, Hull TL, Bast J, Hammel JP, Church JM. Comparison of perineal operations with abdominal operations for full-thickness rectal prolapse. World J Surg. 2010;34:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Tou S, Brown SR, Nelson RL. Surgery for complete (full-thickness) rectal prolapse in adults. Cochrane Database Syst Rev. 2015;2015:CD001758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Pellino G, Fuschillo G, Simillis C, Selvaggi L, Signoriello G, Vinci D, Kontovounisios C, Selvaggi F, Sciaudone G. Abdominal versus perineal approach for external rectal prolapse: systematic review with meta-analysis. BJS Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Emile SH, Elfeki H, Shalaby M, Sakr A, Sileri P, Wexner SD. Perineal resectional procedures for the treatment of complete rectal prolapse: A systematic review of the literature. Int J Surg. 2017;46:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Smedberg J, Graf W, Pekkari K, Hjern F. Comparison of four surgical approaches for rectal prolapse: multicentre randomized clinical trial. BJS Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Ris F, Colin JF, Chilcott M, Remue C, Jamart J, Kartheuser A. Altemeier's procedure for rectal prolapse: analysis of long-term outcome in 60 patients. Colorectal Dis. 2012;14:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | D'Hoore A, Cadoni R, Penninckx F. Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg. 2004;91:1500-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 317] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 57. | Loygue J, Nordlinger B, Cunci O, Malafosse M, Huguet C, Parc R. Rectopexy to the promontory for the treatment of rectal prolapse. Report of 257 cases. Dis Colon Rectum. 1984;27:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Guttadauro A, Chiarelli M, Maternini M, Baini M, Pecora N, Gabrielli F. Value and limits of stapled transanal rectal repair for obstructed defecation syndrome: 10 years-experience with 450 cases. Asian J Surg. 2018;41:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Ripamonti L, Guttadauro A, Lo Bianco G, Rennis M, Maternini M, Cioffi G, Chiarelli M, De Simone M, Cioffi U, Gabrielli F. Stapled Transanal Rectal Resection (Starr) in the Treatment of Obstructed Defecation: A Systematic Review. Front Surg. 2022;9:790287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Chandra A, Rajan P, Dangi A, Kumar N, Kumar S, Gupta V, Rungta S, Pai A, Rajashekhara M, Patel R. Natural Orifice Transanal Endoscopic Rectopexy: A Novel Option for Rectal Prolapse. Dis Colon Rectum. 2020;63:e523-e528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Sylla P. A Viable Option for Frail Patients? Natural Orifice Transluminal Endoscopic Surgery Rectopexy for Rectal Prolapse Using Transanal Fixation. Dis Colon Rectum. 2023;66:3-5. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 62. | Faucheron JL, Trilling B, Girard E. Robotic ventral mesh rectopexy for rectal prolapse: a few years until this becomes the gold standard. Tech Coloproctol. 2019;23:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Formisano G, Ferraro L, Salaj A, Giuratrabocchetta S, Pisani Ceretti A, Opocher E, Bianchi PP. Update on Robotic Rectal Prolapse Treatment. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Bishawi M, Foppa C, Tou S, Bergamaschi R; Rectal Prolapse Recurrence Study Group. Recurrence of rectal prolapse following rectopexy: a pooled analysis of 532 patients. Colorectal Dis. 2016;18:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Speakman CT, Madden MV, Nicholls RJ, Kamm MA. Lateral ligament division during rectopexy causes constipation but prevents recurrence: results of a prospective randomized study. Br J Surg. 1991;78:1431-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 153] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Boons P, Collinson R, Cunningham C, Lindsey I. Laparoscopic ventral rectopexy for external rectal prolapse improves constipation and avoids de novo constipation. Colorectal Dis. 2010;12:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 67. | Samaranayake CB, Luo C, Plank AW, Merrie AE, Plank LD, Bissett IP. Systematic review on ventral rectopexy for rectal prolapse and intussusception. Colorectal Dis. 2010;12:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 68. | Zbar AP. Mesh Rectopexy: The Wells Technique. Rectal Prolapse: In: Springer, 2008: 113-120. |
| 69. | Scaglia M, Fasth S, Hallgren T, Nordgren S, Öresland T, Hultén L. Abdominal rectopexy for rectal prolapse: Influence of surgical technique on functional outcome. Dis Colon Rectum. 1994;37:805-813. [RCA] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Lechaux D, Trebuchet G, Siproudhis L, Campion JP. Laparoscopic rectopexy for full-thickness rectal prolapse: a single-institution retrospective study evaluating surgical outcome. Surg Endosc. 2005;19:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Frykman HM. Abdominal proctopexy and primary sigmoid resection for rectal procidentia. Am J Surg. 1955;90:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Formijne Jonkers HA, Draaisma WA, Wexner SD, Broeders IA, Bemelman WA, Lindsey I, Consten EC. Evaluation and surgical treatment of rectal prolapse: an international survey. Colorectal Dis. 2013;15:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Balla A, Quaresima S, Smolarek S, Shalaby M, Missori G, Sileri P. Synthetic Versus Biological Mesh-Related Erosion After Laparoscopic Ventral Mesh Rectopexy: A Systematic Review. Ann Coloproctol. 2017;33:46-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Joubert K, Laryea JA. Abdominal Approaches to Rectal Prolapse. Clin Colon Rectal Surg. 2017;30:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Formijne Jonkers HA, Maya A, Draaisma WA, Bemelman WA, Broeders IA, Consten EC, Wexner SD. Laparoscopic resection rectopexy versus laparoscopic ventral rectopexy for complete rectal prolapse. Tech Coloproctol. 2014;18:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Muir EG. The surgical treatment of severe rectal prolapse. Proc R Soc Med. 1959;52 (Suppl):104-105. [PubMed] |
| 77. | Hajibandeh S, Hajibandeh S, Arun C, Adeyemo A, McIlroy B, Peravali R. Meta-analysis of laparoscopic mesh rectopexy versus posterior sutured rectopexy for management of complete rectal prolapse. Int J Colorectal Dis. 2021;36:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 78. | Hidaka J, Elfeki H, Duelund-Jakobsen J, Laurberg S, Lundby L. Functional Outcome after Laparoscopic Posterior Sutured Rectopexy Versus Ventral Mesh Rectopexy for Rectal Prolapse: Six-year Follow-up of a Double-blind, Randomized Single-center Study. EClinicalMedicine. 2019;16:18-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Leventoglu S, Mentes B, Balci B, Yildiz A. Surgical techniques for rectal prolapse. Gastroenterol Insights. 2021;12:310-318. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Hamahata Y, Akagi K, Maeda T, Nemoto K, Koike J. Management of Pelvic Organ Prolapse (POP) and Rectal Prolapse. J Anus Rectum Colon. 2022;6:83-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 81. | Vogler SA. Rectal Prolapse. Dis Colon Rectum. 2017;60:1132-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 82. | Faucheron JL, Trilling B, Barbois S, Sage PY, Waroquet PA, Reche F. Day case robotic ventral rectopexy compared with day case laparoscopic ventral rectopexy: a prospective study. Tech Coloproctol. 2016;20:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Ramage L, Georgiou P, Tekkis P, Tan E. Is robotic ventral mesh rectopexy better than laparoscopy in the treatment of rectal prolapse and obstructed defecation? A meta-analysis. Tech Coloproctol. 2015;19:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Mackenzie H, Dixon AR. Proficiency gain curve and predictors of outcome for laparoscopic ventral mesh rectopexy. Surgery. 2014;156:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 85. | Bao X, Wang H, Song W, Chen Y, Luo Y. Meta-analysis on current status, efficacy, and safety of laparoscopic and robotic ventral mesh rectopexy for rectal prolapse treatment: can robotic surgery become the gold standard? Int J Colorectal Dis. 2021;36:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Albayati S, Chen P, Morgan MJ, Toh JWT. Robotic vs. laparoscopic ventral mesh rectopexy for external rectal prolapse and rectal intussusception: a systematic review. Tech Coloproctol. 2019;23:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 87. | van Iersel JJ, Paulides TJ, Verheijen PM, Lumley JW, Broeders IA, Consten EC. Current status of laparoscopic and robotic ventral mesh rectopexy for external and internal rectal prolapse. World J Gastroenterol. 2016;22:4977-4987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 88. | Mäkelä-Kaikkonen J, Rautio T, Ohinmaa A, Koivurova S, Ohtonen P, Sintonen H, Mäkelä J. Cost-analysis and quality of life after laparoscopic and robotic ventral mesh rectopexy for posterior compartment prolapse: a randomized trial. Tech Coloproctol. 2019;23:461-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 89. | Gavlin A, Kierans AS, Chen J, Song C, Guniganti P, Mazzariol FS. Imaging and Treatment of Complications of Abdominal and Pelvic Mesh Repair. Radiographics. 2020;40:432-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Jia X, Glazener C, Mowatt G, Jenkinson D, Fraser C, Bain C, Burr J. Systematic review of the efficacy and safety of using mesh in surgery for uterine or vaginal vault prolapse. Int Urogynecol J. 2010;21:1413-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 91. | Maeda Y, Espin-Basany E, Gorissen K, Kim M, Lehur PA, Lundby L, Negoi I, Norcic G, O'Connell PR, Rautio T, van Geluwe B, van Ramshorst GH, Warwick A, Vaizey CJ. European Society of Coloproctology guidance on the use of mesh in the pelvis in colorectal surgery. Colorectal Dis. 2021;23:2228-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Evans C, Stevenson AR, Sileri P, Mercer-Jones MA, Dixon AR, Cunningham C, Jones OM, Lindsey I. A Multicenter Collaboration to Assess the Safety of Laparoscopic Ventral Rectopexy. Dis Colon Rectum. 2015;58:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 93. | Mercer-Jones MA, D'Hoore A, Dixon AR, Lehur P, Lindsey I, Mellgren A, Stevenson AR. Consensus on ventral rectopexy: report of a panel of experts. Colorectal Dis. 2014;16:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 94. | Loh KC, Umanskiy K. Ventral Rectopexy. Clin Colon Rectal Surg. 2021;34:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Tsunoda A, Takahashi T, Matsuda S, Oka N, Kusanagi H. Midterm functional outcome after laparoscopic ventral rectopexy for external rectal prolapse. Asian J Endosc Surg. 2020;13:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Badrek-Al Amoudi AH, Greenslade GL, Dixon AR. How to deal with complications after laparoscopic ventral mesh rectopexy: lessons learnt from a tertiary referral centre. Colorectal Dis. 2013;15:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Kim M, Reibetanz J, Schlegel N, Krajinovic K, Köstler H, Germer CT, Isbert C. Recurrence after perineal rectosigmoidectomy: when and why? Colorectal Dis. 2014;16:920-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Fu CW, Stevenson AR. Risk Factors for Recurrence After Laparoscopic Ventral Rectopexy. Dis Colon Rectum. 2017;60:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Hotouras A, Ribas Y, Zakeri S, Bhan C, Wexner SD, Chan CL, Murphy J. A systematic review of the literature on the surgical management of recurrent rectal prolapse. Colorectal Dis. 2015;17:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 100. | Steele SR, Goetz LH, Minami S, Madoff RD, Mellgren AF, Parker SC. Management of recurrent rectal prolapse: surgical approach influences outcome. Dis Colon Rectum. 2006;49:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Chung JS, Ju JK, Kwak HD. Comparison of abdominal and perineal approach for recurrent rectal prolapse. Ann Surg Treat Res. 2023;104:150-155. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 102. | Fengler SA, Pearl RK, Prasad ML, Orsay CP, Cintron JR, Hambrick E, Abcarian H. Management of recurrent rectal prolapse. Dis Colon Rectum. 1997;40:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Ding JH, Canedo J, Lee SH, Kalaskar SN, Rosen L, Wexner SD. Perineal rectosigmoidectomy for primary and recurrent rectal prolapse: are the results comparable the second time? Dis Colon Rectum. 2012;55:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Randall J, Smyth E, McCarthy K, Dixon AR. Outcome of laparoscopic ventral mesh rectopexy for external rectal prolapse. Colorectal Dis. 2014;16:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 105. | Gurland B, E Carvalho MEC, Ridgeway B, Paraiso MFR, Hull T, Zutshi M. Should we offer ventral rectopexy to patients with recurrent external rectal prolapse? Int J Colorectal Dis. 2017;32:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |