Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3651
Peer-review started: February 21, 2023
First decision: April 10, 2023
Revised: April 13, 2023
Accepted: April 19, 2023
Article in press: April 19, 2023
Published online: May 26, 2023
Processing time: 91 Days and 23.1 Hours
Electromagnetic navigational bronchoscopy (ENB) is an emerging diagnostic tool that enables practitioners to biopsy peripheral lung tissues that were previously only accessible under computed tomography (CT) guidance. However, few studies have investigated ENB use in children. Here, we report a case of a 10-year-old girl with peripheral lung lesions who complained of a 7-d persistent fever. She was diagnosed with Streptococcus parasanguinis infection based on findings obtained using ENB-guided transbronchial lung biopsy (TBLB).
A 10-year-old girl presented with constitutional symptoms of cough and fever of 7 days’ duration. Chest CT scans detected peripheral lung lesions and no endobronchial lesions. TBLB performed under the guidance of an ENB Lungpro navigation system was safe, well-tolerated, and effective for biopsying peripheral lung lesions. Examination of biopsied samples indicated the patient had a pulmonary Streptococcus parasanguinis infection, which was treated with antibiotics instead of more invasive treatment interventions. The patient’s symptoms resolved after she received a 3-wk course of oral linezolid. Comparisons of pre-treatment and post-treatment CT scans revealed absorption of some lung lesions within 7 mo of hospital discharge.
ENB-guided TBLB biopsying of peripheral lung lesions in this child is a safe, well-tolerated, and effective alternative to conventional interventions.
Core Tip: Electromagnetic navigational bronchoscopy-guided transbronchial lung biopsy is used to diagnose and treat pulmonary disorders in adults worldwide, but very few were reported in children. Moreover, diagnosing patients with peripheral lung lesions is still challenging, due to difficulties related to existing biopsy procedures. Here we present a case study conducted in China that demonstrated that electromagnetic navigational bronchoscopy-guided transbronchial lung biopsy is safe and effective when used to diagnose pediatric patients afflicted with peripheral pulmonary lesion-inducing disorders. Ultimately, clinicians adopting this procedure will be able to diagnose pulmonary disease cases without subjecting patients to invasive and risky surgical interventions.
- Citation: Meng FZ, Chen QH, Gao M, Zeng L, Lin JR, Zheng JY. Diagnosis based on electromagnetic navigational bronchoscopy-guided biopsied peripheral lung lesions in a 10-year-old girl: A case report. World J Clin Cases 2023; 11(15): 3651-3657
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3651.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3651
With the development of auxiliary navigation equipment, such as the Lungpro navigation system, electromagnetic navigational bronchoscopy (ENB) has been increasingly applied in pulmonary clinical practice. Importantly, ENB can be used to guide transbronchial lung biopsying (TBLB) of peripheral lung tissues that were previously only accessible by computed tomography (CT)-guided conventional bronchoscopy. To perform this function, ENB systems are used to virtually plan the lung bronchoscopic path in advance and to navigationally guide the bronchoscope in real time to ensure accurate localization of the disease focus during the biopsy. Although ENB has been shown to improve success rates of TBLB in adults with pulmonary diseases[1], this technology has been infrequently used to diagnose pediatric pulmonary diseases. In fact, a search of medical literature[2,3] revealed only one report of a Chinese pediatric patient with pulmonary lesions who was successfully diagnosed using ENB-guided TBLB, which permitted physicians to safely obtain biopsied tissues to distinguish between lung infection and lung tumor metastasis when diagnosing the patient[3]. Here, we report the clinical application of ENB for diagnosing a second Chinese pediatric patient with pulmonary lesions.
A 7-d history of fever and cough.
A ten-year old girl presented to our hospital with a fever and cough of 7 days’ duration. Her temperature peaked at 39.2 °C. She had been initially admitted to a local hospital, where she was diagnosed with community-acquired pneumonia and treated with intravenous antibiotics that did not resolve her fever and cough. Due to lingering symptoms, she sought further treatment at our hospital.
Her medical history was unremarkable.
The patient had no relevant personal or family history.
At admission, the patient’s weight was 34.0 kg and her vital signs were as follows: Body temperature, 37.0 °C; blood pressure, 90/62 mmHg; heart rate, 130 beats/min; respiratory rate, 20 breaths/min; oxygen saturation, 98% in room air. At the time of admission, moderate and fine moist rales were heard in both lungs with no wheezing observed.
Infection index white blood cell count, 17.52 × 109/L; platelet count, 256 × 109/L; erythrocyte sedimentation rate, 30 mm/h; C-reactive protein, 60.65 mg/L (0.00-5.00 mg/L) (December 16, 2021). Immune function was normal. Liver function: Alanine aminotransferase, 29 U/L (0-40 U/L); aspartate aminotransferase, 33U/L (0-40 U/L). A positive culture result for Streptococcus parasanguiniswas obtained, with no fungal growth detected.
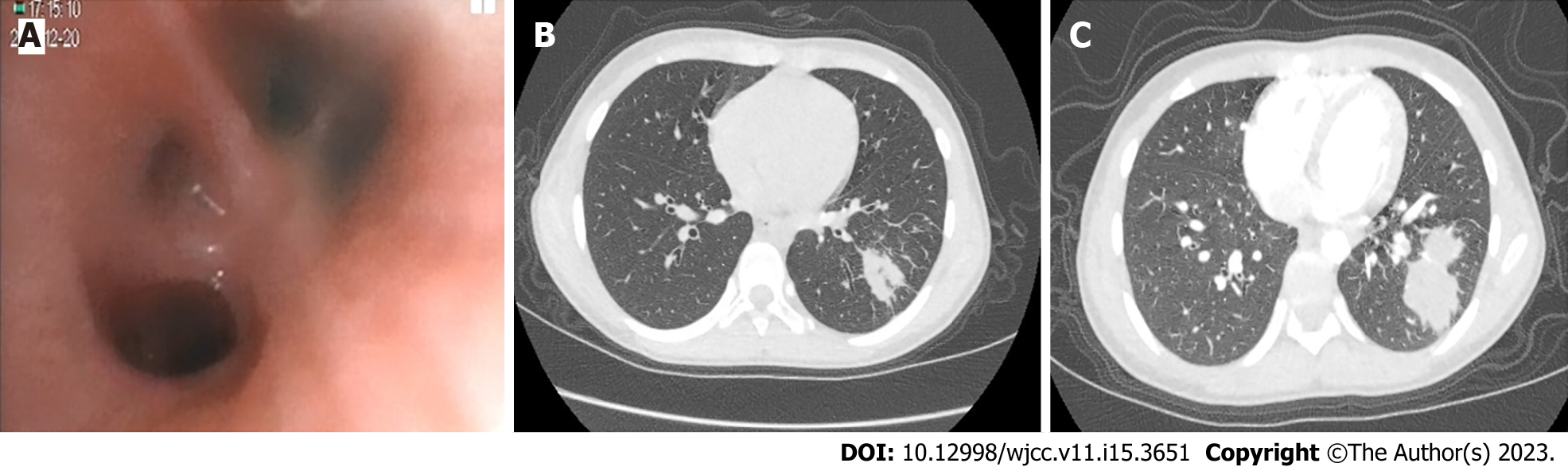
During her first stay at our hospital, the patient underwent electronic lung bronchoscopy, which detected endobronchial inflammation in the left upper lobe proper segment and left lower lobe basal segment (Figure 1A). Results of CT retesting obtained 7 d later (Figure 1B) revealed growing, dense masses within lung tissues, as evidenced by darker shadows on the scan coinciding with the position of the outer basal segment of the lower lobe of the left lung that indicated the presence of multiple cystic translucent lesions. Moreover, various lesions of different sizes, multilocular thin-walled air-filled cavities, and patchy and highly dense shadows were observed that lacked clear boundaries and had approximate dimensions of 72 mm × 60 mm × 42 mm. The child was subsequently discharged with oral amoxicillin clavulanate potassium and advised to undergo an enhanced pulmonary CT scan 2 wk after discharge. The CT results revealed that the previously noted multiple cystic lesions within the lower left lung lobe and the dense mass within the basal segment outside that lobe remained unchanged in appearance. In contrast, the inflammatory lesions within the middle and lower lobes of the right lung were larger than before (Figure 1C). Due to the fact that the pathological nature of the peripheral lung lesion could not be determined from these findings, the patient underwent a TBLB procedure to ensure that appropriate treatment would be administered to treat the disorder.
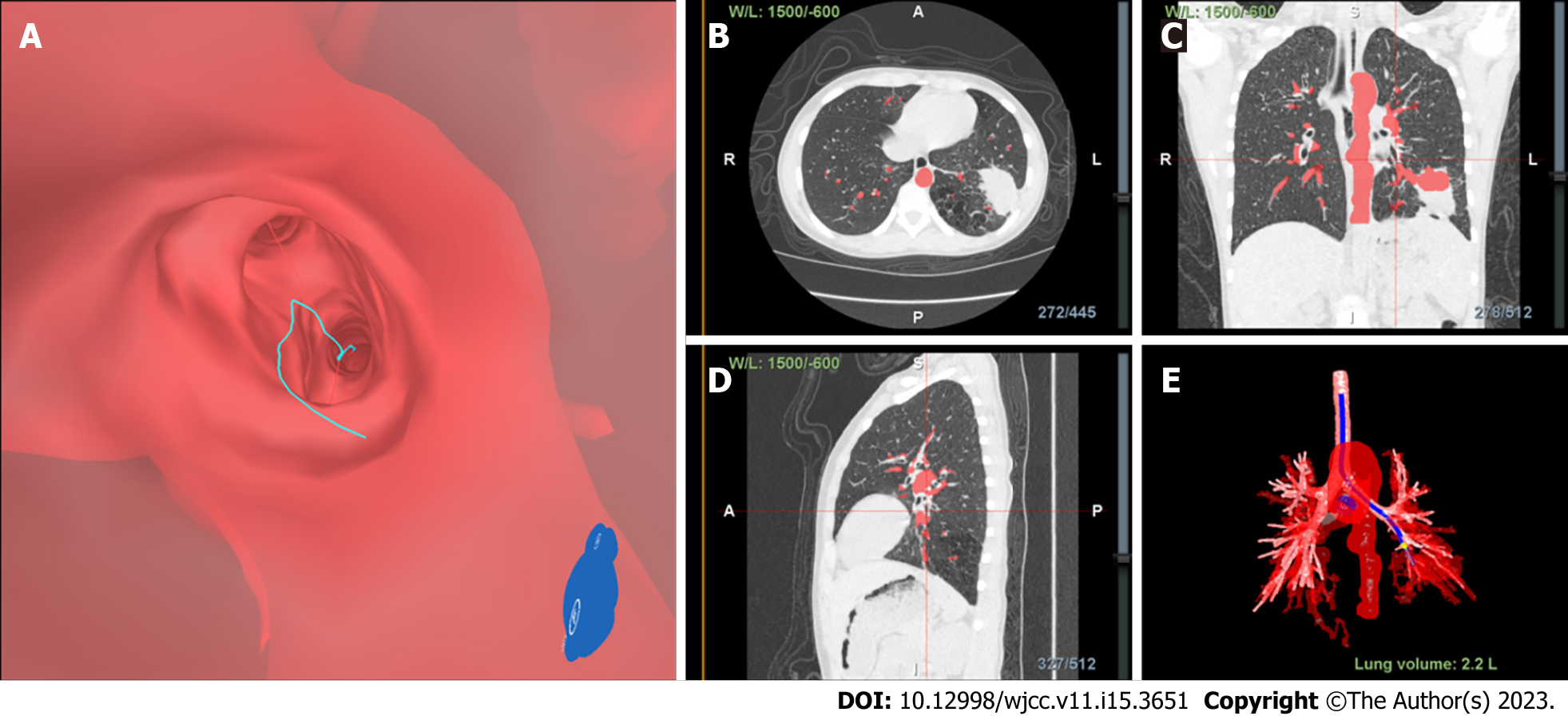
The entire procedure was conducted using the following steps. Prior to TBLB, inspiratory and expiratory CT images were uploaded to the Lungpro system (LungPoint VBN, version 3,4, Broncus Medical Inc, CA, United States), which was used to construct a virtual airway leading to the above-mentioned peripheral lesions. Next, picture archiving and communication system-based integration was conducted to build a virtual three-dimensional image of the patient’s lungs. Then, a bronchial centerline was used as a frame of reference to help the bronchoscope operator plan a path to the airway wall puncture point or focus point.
After the patient was prepared for the procedure, she was administered local anesthesia and intravenous midazolam, a mild sedative. Endobronchial occlusion was then performed using a bronchoscope (BF-P260, Olympus Ltd.). The patient was placed in the supine position and was administered oxygen through a nasal catheter. Using a nasal point of entry, the optimal navigational path to pulmonary lesions was selected, and the reconstructed three-dimensional image was superimposed onto the image as viewed under the bronchoscope. During real-time navigation, the position of the bronchoscope and the distance from the pleura and the focus were displayed.
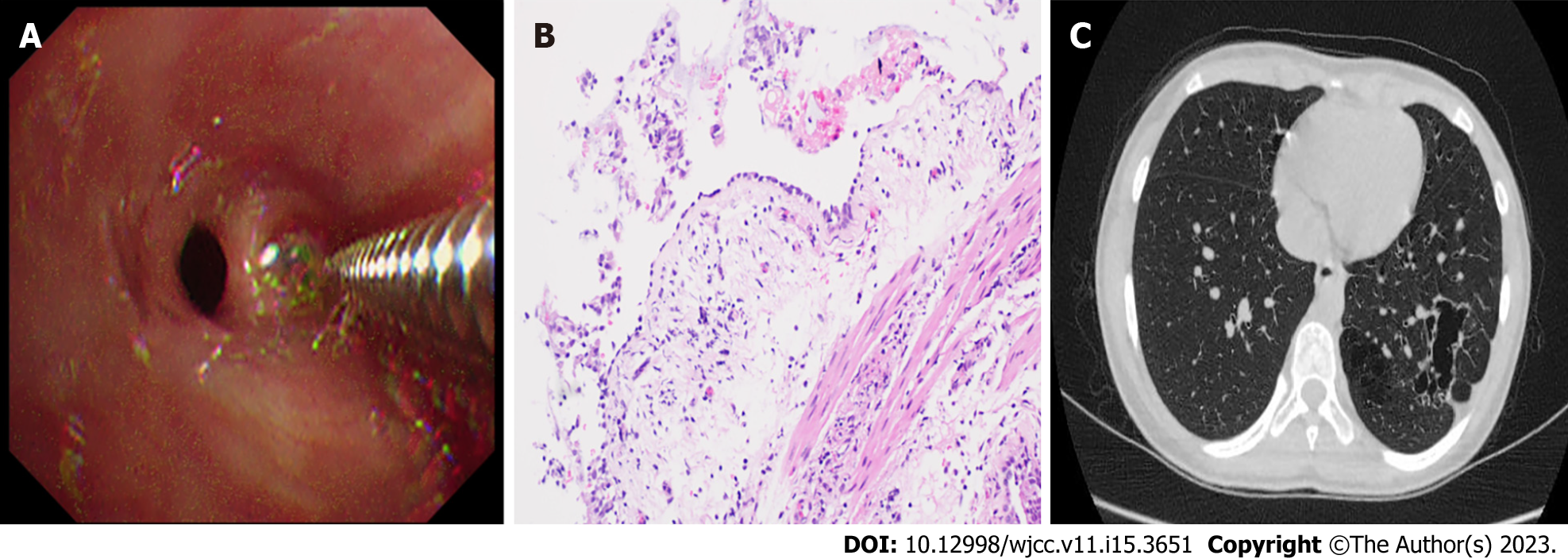
Finally, Lungpro-guided TBLB was performed using a bronchoscope with a working channel diameter of 2.8 mm (Figure 2, Figure 3A). Biopsy samples taken from the posterior basal segment of the left lower lobe were sent to the hospital pathology department for microscopic examination (Figure 3B). The patient experienced no complications after the procedure.
Streptococcus parasanguinis infection of the lung.
During the patient’s first stay at our hospital, her fever decreased after intravenous antibiotic therapy (intravenous amoxicillin clavulanate potassium, oral azithromycin) and anti-inflammatory therapy (intravenous methylprednisolone). Results of ENB-guided TBLB performance (which utilized the Lungpro navigation system) indicated lung infection with Streptococcus parasanguinis. Based on this finding, the patient was treated with a 3-wk course of oral linezolid.
The patient’s symptoms disappeared after a 3-wk oral linezolid treatment, after which the patient’s health status was monitored through regular follow-up visits. A second chest CT scan obtained at seven months post-treatment revealed increased transparency of the basal segment of the left lower lobe, multiple lesions of unequal size, and multilocular thin-walled air-filled cavities. A comparison of the first and second chest CT scans suggested that some lesions were absorbed before the 7-mo follow-up visit (Figure 3C).
Modern imaging techniques have led to increased detection of peripheral lung lesions, of which the vast majority often require tissue biopsies for diagnosis. Barber et al[4] retrospectively analyzed etiologies of 88 pediatric cases with pulmonary nodules. They found that most nodules were caused by infections, while etiologies of the others included autoimmune diseases, hematologic diseases, vasculitis, and Crohn's disease. Nevertheless, diagnosing patients with peripheral lung lesions is still challenging due to difficulties related to existing biopsy procedures. The child described in this case report presented with cough and fever symptoms and rales. Chest high-resolution computed tomography (HRCT) imaging revealed a dense mass, as evidenced by a dark area on the HRCT scan after the child had received regular antibiotic therapy. However, the lesion was located within the anterior segment of the left lower lobe in an area close to the anterior artery and thus would be inaccessible by conventional electronic bronchoscopy and require CT-guided percutaneous lung puncture treatment. As a major disadvantage, this procedure can lead to severe lung tissue damage, increasing the risk of intraoperative and postoperative bleeding and pneumothorax and leaving local skin wounds. To avoid these issues, we performed ENB-guided TBLB to obtain an accurate diagnosis for our patient.
Several bronchoscopic approaches are currently adopted to biopsy tissues in order to obtain diagnostic tissue samples, with pros and cons associated with each approach. Importantly, Broncho
CT is currently considered the gold standard imaging technique for guiding bronchoscopic biopsies of pulmonary lesions, due to its effectiveness as a diagnostic tool and ability to target a bronchoscope to a selected cytologic sampling site accurately. Nevertheless, CT-guided transthoracic biopsy has several disadvantages, such as exposure to ionizing radiation, the need to move the patient to a radiology unit, and prolonged execution time. Moreover, this modality requires an accessible path for biopsy needle insertion and is associated with increased risks of pneumothorax, significant hemorrhaging, and respiratory failure.
Alternatively, radial EBUS is a current method to precisely locate peripheral lung lesions that are not visible by fluoroscopy, while it also provides clues regarding their internal structures. However, the effective use of this method requires careful patient selection, since the ultrasound wave does not easily penetrate aerated pulmonary parenchyma. Therefore, EBUS is used to locate peripheral pulmonary nodules that are not separated by areas of aerated pulmonary parenchyma, which would induce excessive acoustic impedance that would reflect the ultrasound wave. In addition, ultrasound is technically applicable for imaging peripheral pulmonary nodules that adhere to visceral pleura, detecting direct pleural infiltration, and detecting atelectasis involving subpleural lung parenchyma. Nonetheless, EBUS cannot target specific types of peripheral lesions that may be targeted using a navigational system.
TBLB, an important method used to clinically diagnose pulmonary lesion-associated disorders, offers advantages over conventional bronchoscopic biopsy methods that provide low diagnostic yields due to inaccurate targeting of lesions with biopsy forceps[9]. However, during TBLB operation, misalignments (offsets) between actual and virtual bronchoscopic images can occur when the bronchoscope is rotated as it advances to the target site, which can lead to lesion-targeting errors. Furthermore, rapid realignment of the virtual bronchoscopic image to actual bronchial branch points is not easily accomplished after the bronchoscope moves unexpectedly (e.g., when the patient moves or coughs)[10]. To address these issues[10,11], the Lungpro system was recently developed to serve as a virtual navigation device that can display virtual bronchoscopic images adjacent to actual video images (Video 1), thus enabling the operator to overlay an electronic pathway onto the endoscopic image and maintain their alignment in real-time. In practice, the identified lesion shape is initially overlaid onto the airway wall near the target location (as indicated on the aligned actual and virtual bronchoscopic images) to provide further biopsy site localization guidance. Importantly, this method can be applied to quickly and accurately guide the bronchoscope to the focus without puncturing blood vessels, thus making it possible to more effectively biopsy lung lesions present outside the airway[10,12]. Notably, when this procedure was used to biopsy our patient, no adverse events, such as pneumothorax or hemorrhage, occurred during or after the procedure.
Ultimately, the biopsy results obtained for our pediatric patient suggested that the child’s lungs were infected with S. parasanguinis, a gram-positive, non-motile, catalase-negative organism belonging to the sanguinis group of viridans streptococci. Although this organism is not viewed as an important human pathogen, it has been isolated from human blood, urine, and throat samples[13], is a primary colonizer of teeth that supports biofilm formation on tooth surfaces[14], and is a common cause of native and prosthetic valve endocarditis. S. parasanguinis-induced endocarditis has been shown to begin with bacterial colonization on heart valve surfaces that is followed by biofilm formation that leads to colonization in valves with small surface aggregates of bacteria. These aggregates grow into rounded bacterial colonies that promote the deposition of a layer of fibrin and platelets on their surfaces that ultimately supports biofilm formation. This biofilm then shields the bacteria from antibiotics, thus making their eradication difficult despite the fact that the organisms are sensitive to vancomycin, linezolid, daptomycin, quinupristin–dalfopristin, clindamycin, and levofloxacin[15]. Notably, an analysis of 50 cancer patients with streptococcal infections revealed that 5 patients harbored S. parasanguinis with intermediate resistance to penicillin and azithromycin and susceptibility to vancomycin, linezolid, quinupristin–dalfopristin, and ceftriaxone[16]. These results were consistent with S. parasanguinis antibiotic responsiveness as observed in the current case study. In our patient, inflammatory lesions increased in size after treatment with amoxicillin clavulanate potassium and subsequently shrank significantly after 3 wk of oral linezolid treatment. Our results thus highlight the bacteriological and therapeutic challenges associated with pneumonia caused by Streptococcus parasanguinis infection.
Worldwide, ENB-guided TBLB is used to diagnose and treat pulmonary disorders in adults but has been infrequently performed in children as its effectiveness and safety in pediatric patients remains unknown. Meanwhile, ENB technology is expensive, and ENB systems are not available in all countries of regions. Here we present a case study conducted in China that demonstrated that ENB-guided transbronchial lung biopsy is safe and effective when used to diagnose pediatric patients afflicted with peripheral pulmonary lesion-inducing disorders. Ultimately, clinicians adopting this procedure may help diagnose pulmonary disease cases without subjecting patients to invasive and risky surgical interventions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kwiecień I, Poland; Zeng C, United States S-Editor: Ma YJ L-Editor: A P-Editor: Yu HG
| 1. | Tamiya M, Okamoto N, Sasada S, Shiroyama T, Morishita N, Suzuki H, Yoshida E, Hirashima T, Kawahara K, Kawase I. Diagnostic yield of combined bronchoscopy and endobronchial ultrasonography, under LungPoint guidance for small peripheral pulmonary lesions. Respirology. 2013;18:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Piccione J, Hysinger EB, Vicencio AG. Pediatric advanced diagnostic and interventional bronchoscopy. Semin Pediatr Surg. 2021;30:151065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Yang H, Turgon E, Pan Y, Wen X, Zhang X, Shen Y, Wang F. Pilot study of archimedes virtual bronchoscopic navigation system-guided biopsy to diagnose lung nodules in children. Front Pediatr. 2022;10:1053289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Barber A, Passarelli P, Dworsky ZD, Gatcliffe C, Ryu J, Lesser DJ. Clinical implications of pulmonary nodules detected in children. PediatrPulmonol. 2021;56:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Ishida T, Asano F, Yamazaki K, Shinagawa N, Oizumi S, Moriya H, Munakata M, Nishimura M; Virtual Navigation in Japan Trial Group. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax. 2011;66:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Kalanjeri S, Holladay RC, Gildea TR. State-of-the-Art Modalities for Peripheral Lung Nodule Biopsy. Clin Chest Med. 2018;39:125-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 470] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 9. | Han Y, Kim HJ, Kong KA, Kim SJ, Lee SH, Ryu YJ, Lee JH, Kim Y, Shim SS, Chang JH. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS One. 2018;13:e0191590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Eberhardt R, Kahn N, Gompelmann D, Schumann M, Heussel CP, Herth FJ. LungPoint--a new approach to peripheral lesions. J Thorac Oncol. 2010;5:1559-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Merritt SA, Gibbs JD, Yu KC, Patel V, Rai L, Cornish DC, Bascom R, Higgins WE. Image-guided bronchoscopy for peripheral lung lesions: a phantom study. Chest. 2008;134:1017-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Silvestri GA, Herth FJ, Keast T, Rai L, Gibbs J, Wibowo H, Sterman DH. Feasibility and safety of bronchoscopictransparenchymal nodule access in canines: a new real-time image-guided approach to lung lesions. Chest. 2014;145:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Fernández-Garayzábal JF, Fernández E, Las Heras A, Pascual C, Collins MD, Domínguez L. Streptococcus parasanguinis: new pathogen associated with asymptomatic mastitis in sheep. Emerg Infect Dis. 1998;4:645-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Chen Q, Wu H, Fives-Taylor PM. Investigating the role of secA2 in secretion and glycosylation of a fimbrial adhesin in Streptococcus parasanguis FW213. Mol Microbiol. 2004;53:843-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Sader HS, Streit JM, Fritsche TR, Jones RN. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin Surveillance Programme (2002-2004). Clin Microbiol Infect. 2006;12:844-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Sadjadi SA, Ali H. Streptococcus parasanguis peritonitis: report of a case and review of the literature. Perit Dial Int. 2011;31:603-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |