Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3643
Peer-review started: February 16, 2023
First decision: March 28, 2023
Revised: April 7, 2023
Accepted: April 19, 2023
Article in press: April 19, 2023
Published online: May 26, 2023
Processing time: 98 Days and 7.1 Hours
Chronic myelomonocytic leukemia (CMML), a rare clonal hematopoietic stem cell disorder characterized by myelodysplastic syndrome and myeloproliferative neoplasms, has a generally poor prognosis, and easily progresses to acute myeloid leukemia. The simultaneous incidence of hematologic malignancies and solid tumors is extremely low, and CMML coinciding with lung malignancies is even rarer. Here, we report a case of CMML, with ASXL1 and EZH2 gene mutations, combined with non-small cell lung cancer (lung squamous cell carcinoma).
A 63-year-old male, suffering from toothache accompanied by coughing, sputum, and bloody sputum for three months, was given a blood test after experiencing continuous bleeding resulting from a tooth extraction at a local hospital. Based on morphological results, the patient was diagnosed with CMML and bronchoscopy was performed in situ to confirm the diagnosis of squamous cell carcinoma in the lower lobe of the lung. After receiving azacitidine, programmed cell death protein 1, and platinum-based chemotherapy drugs, the patient developed severe myelosuppression and eventually fatal leukocyte stasis and dyspnea.
During the treatment and observation of CMML and be vigilant of the growth of multiple primary malignant tumors.
Core Tip: A 63-year-old male, with a long history of heavy smoking, was diagnosed with chronic myelomonocytic leukemia (CMML), with additional sex combs-like and enhancer of zeste homolog 2 gene mutations, as well as non-small cell lung cancer (squamous cell carcinoma). However, no such mutations were found in the lung cancer tissue. In CMML patients, pulmonary manifestations are non-specific, and the rare presence of malignant tumors in the lungs poses challenges in diagnosis. After receiving azacitidine, programmed cell death protein 1, and platinum-based chemotherapy drugs, the patient developed severe myelosuppression and eventually fatal leukocyte stasis and dyspnea. Therefore, during treatment and observation of CMML, medical practitioners should pay attention to the occurrence and evolution of solid tumors such as lung cancer, and be vigilant of the growth of multiple primary malignant tumors.
- Citation: Deng LJ, Dong Y, Li MM, Sun CG. Co-existing squamous cell carcinoma and chronic myelomonocytic leukemia with ASXL1 and EZH2 gene mutations: A case report. World J Clin Cases 2023; 11(15): 3643-3650
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3643.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3643
Chronic myelomonocytic leukemia (CMML), a rare clonal hematopoietic stem cell disorder characterized by myelodysplastic syndrome and myeloproliferative neoplasms, has generally poor prognosis and easily progresses to acute myeloid leukemia[1,2]. The simultaneous incidence of hematologic malignancies and solid tumors is extremely low[3], and CMML coinciding with lung malignancies is even rarer. Here, we report a case of CMML, with additional sex combs-like (ASXL1) and enhancer of zeste homolog 2 (EZH2) gene mutations, combined with non-small-cell lung cancer (lung squamous cell carcinoma).
A 63-year-old male, suffering from toothache accompanied by coughing, sputum, and bloody sputum for three months.
A 63-year-old male, suffering from toothache accompanied by coughing, sputum, and bloody sputum for three months, was given a blood test after experiencing continuous bleeding resulting from a tooth extraction at a local hospital. The results of the blood test included a white blood cell count of 111.34 × 109/L, monocyte count of 81.99 × 109/L, hemoglobin concentration 99 g/L, and platelet count of 150 × 109/L.
The patient was previously healthy.
The patient in this case had a long history of heavy smoking, had no history of other major diseases nor any significant family history of disease.
The rest of physical examination was normal.
The results of the blood test included a white blood cell count of 111.34 × 109/L, monocyte count of 81.99 × 109/L, hemoglobin concentration 99 g/L, and platelet count of 150 × 109/L. Flow cytometry showed that myeloid blasts and monocytes (with a predominantly mature phenotype) accounted for 1.31% and 52.6% of nucleated cells, respectively. Next-generation sequencing (NGS) showed ASXL1 gene mutation [exon12:c.1934 dupG:p.Gly646fs; variant allele frequency (VAF) = 33.72%], and EZH2 gene mutation (exon18:c.2084C>T:p.Ser695Leu; VAF = 49.73%; exonc.2077A>T:p.Asn693Tyr; VAF = 46.05%). No abnormal chromosomal results were observed.
A subsequent ultrasound revealed an enlarged spleen.
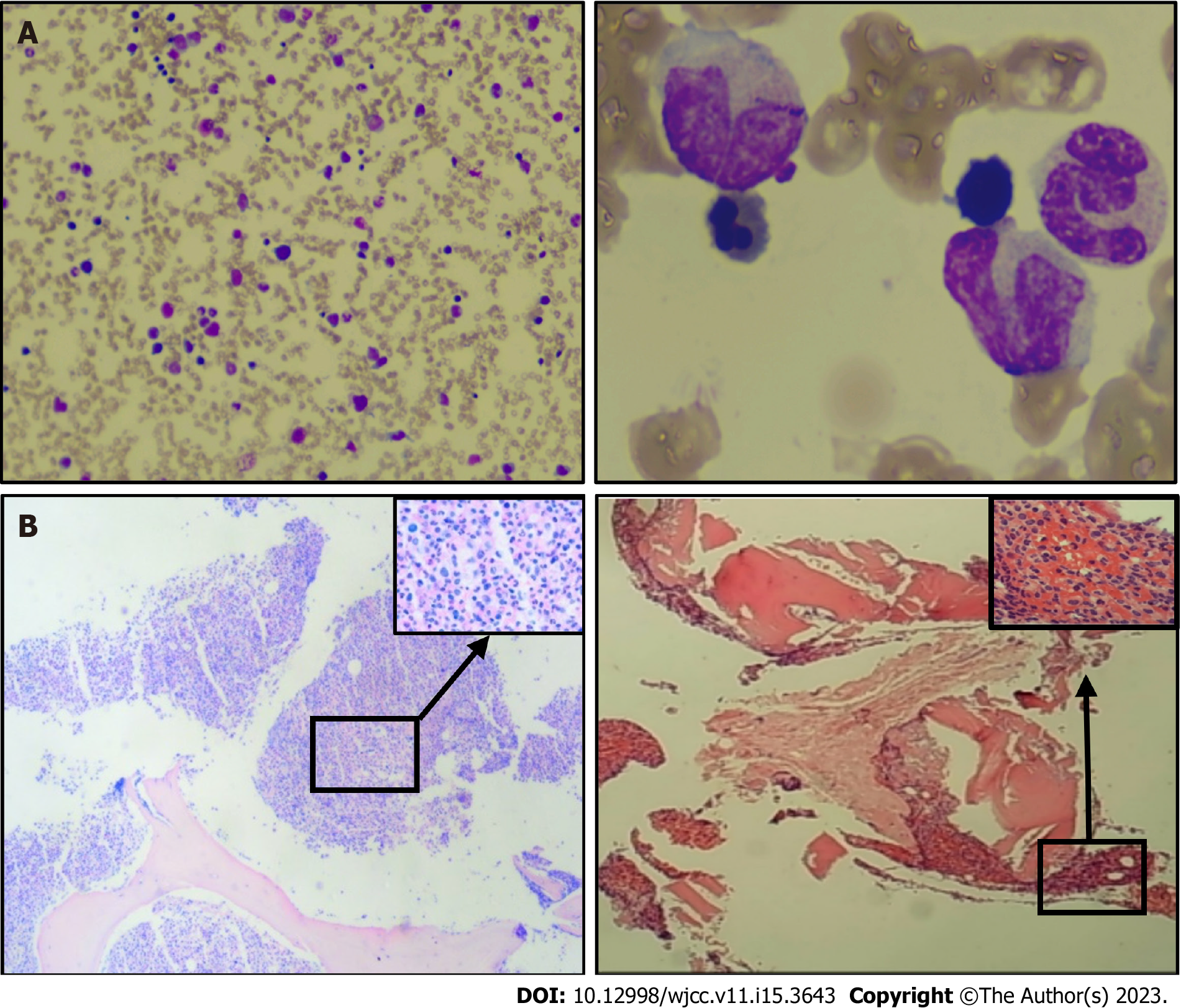
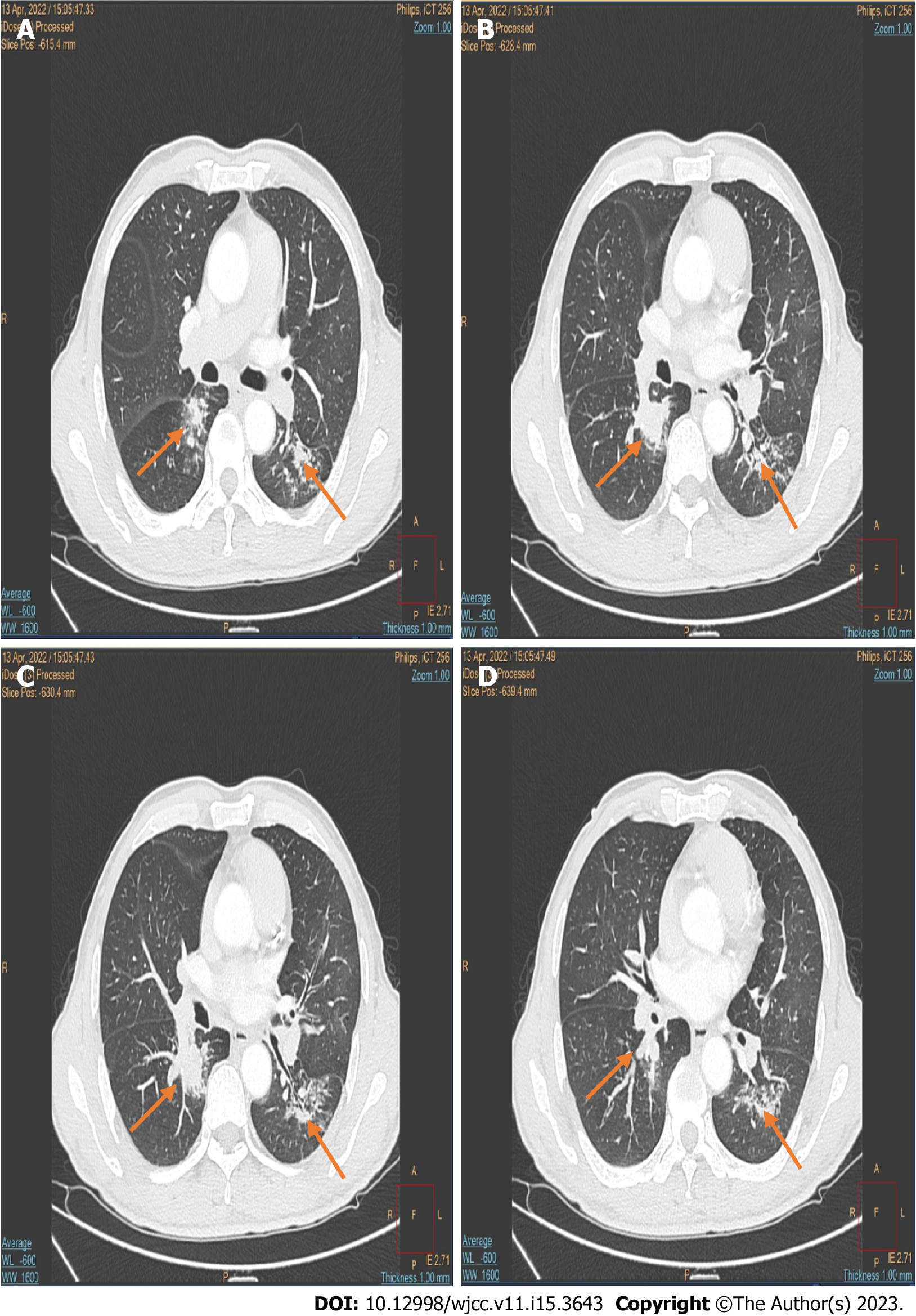
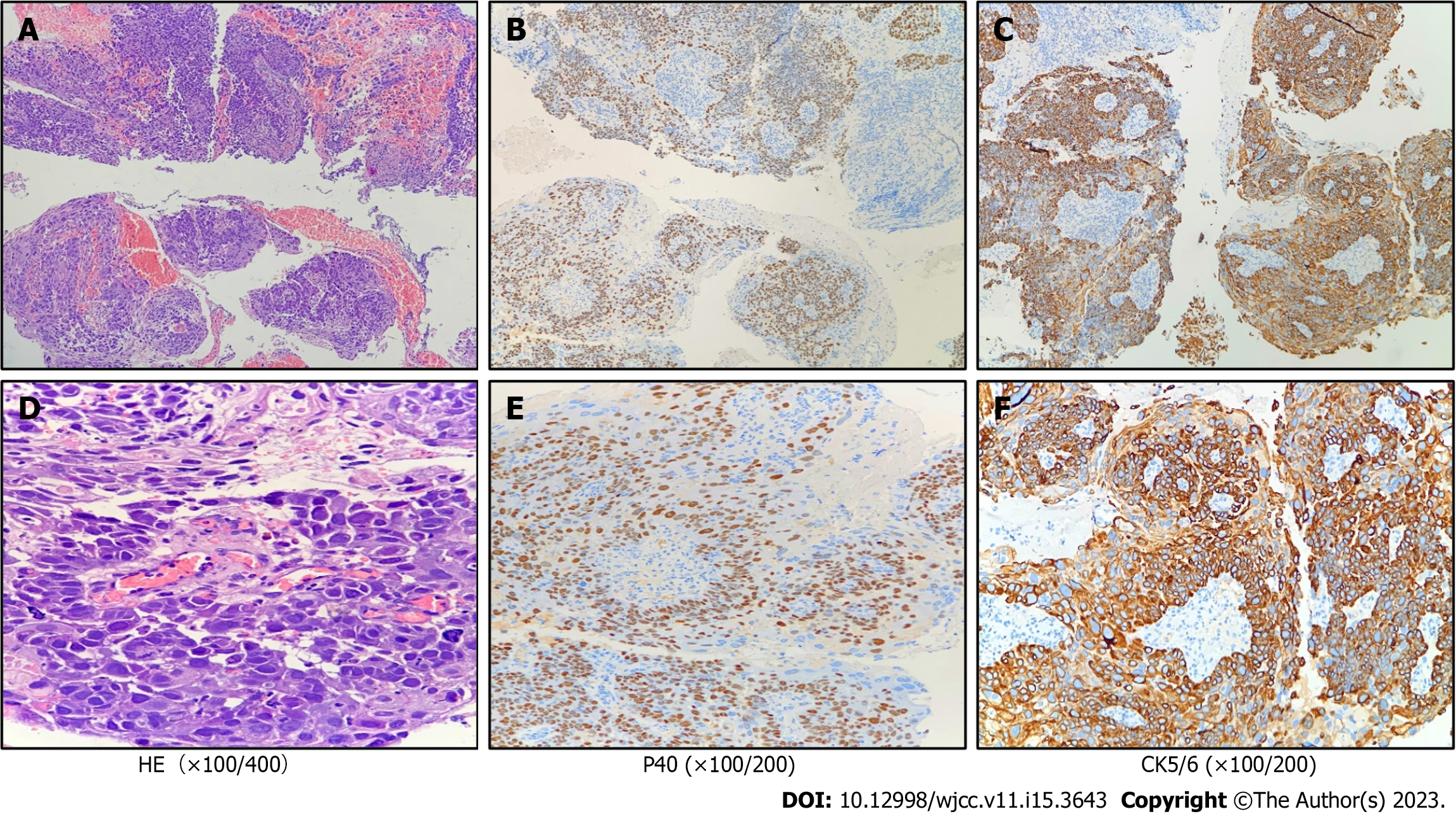
Bone marrow cell morphology examination of the patient showed: (1) An abnormal proliferation of monocytes, interspersed with occasional naïve monocytes; (2) that well-differentiated monocytes accounted for 45% of total monocytes; and (3) a positive alkaline phosphatase score of 80% with 270 points (Figure 1A). Additionally, erythroid hyperplasia was inhibited, three megakaryocytes were observed, and PLT was rare (Figure 1A). Based on these morphological results, the patient was diagnosed with CMML. Flow cytometry showed that myeloid blasts and monocytes (with a predominantly mature phenotype) accounted for 1.31% and 52.6% of nucleated cells, respectively, thus supporting the diagnosis of CMML. A bone marrow biopsy, utilizing hematoxylin-eosin/periodic-acid–Schiff staining, showed naïve-stage monocyte hypercellularity, with an increased granulocyte-to-nucleated red blood cell ratio (Figure 1B). As determined using reticulocyte staining (MF-0 grade), the granular lineage predominantly comprised intermediate (lower-stage) and visible monocytes (Figure 1B). NGS showed ASXL1 gene mutation [exon12:c.1934 dupG:p.Gly646fs; variant allele frequency (VAF) = 33.72%], and EZH2 gene mutation (exon18:c.2084C>T:p.Ser695Leu; VAF = 49.73%; exonc.2077A>T:p.Asn693Tyr; VAF = 46.05%). No abnormal chromosomal results were observed. The patient was finally diagnosed with CMML-0 with an MD Anderson Prognostic Score (MDAPS) of 3[4]. Lastly, the patient had a 50-year history of smoking approximately two to four packs of cigarettes per day, and a computed tomography scan revealed associated lung inflammation. As treatment, hydroxyurea was administered on an outpatient basis for the next three months. The patient came to our hospital to begin treatment with the demethylating drug, azacitidine (administered at a daily dosage of 100 mg on days 1–7). However, bloody sputum symptoms did not alleviate with the improvement of CMML, and enhanced computed tomography showed soft tissue density shadows in the dorsal segment of the right lower lobe of the lung, and inflammation in both lungs (Figure 2). A subsequent tuberculin test and T-cell test for tuberculosis infection both returned positive results. To rule out tuberculosis, the patient was transferred to the Chest Hospital, where bronchoscopy was performed in situ to confirm the diagnosis of squamous cell carcinoma in the lower lobe of the lung. Immunohistochemistry revealed the presence of TTF-1(-), NapsinA(-), CK5/6(+), P40(+), and CK7(-) (Figure 3). Subsequent NGS tests conducted on the lung biopsy tissue slices revealed negative epidermal growth factor receptor, anaplastic lymphoma kinase, ROS1, and KRAS genes. The final resulting diagnosis was lung squamous cell carcinoma (T2N2M0). To determine whether the ASXL1 and EZH2 genes were involved in lung tumorigenesis, lung cancer tissue sections were tested for their presence, with negative results.
After two courses of azacitidine treatment, leukocytes remained at normal levels, and partial remission was achieved. However, in response to subsequent lung tumor progression, the patient was administered with programmed cell death protein 1 (200 mg, q21) combined with DP (100 mg of docetaxel on day 1 and 30 mg of cisplatin on days 1–3), GP (1.2 g of gemcitabine on day 1, 8, and 30 mg of cisplatin on days 1–3), TP (200 mg of nab-paclitaxel on day 1 and 300 mg of carboplatin on day 1), and other chemotherapy regimens. Despite small doses of chemotherapy, the patient repeatedly suffered from grade IV myelosuppression, and multiple changes in chemotherapy regimens were similarly ineffective.
Six months later, after rapid lung tumor progression, 17% blasts were detected in the peripheral blood, with a white blood cell count of 122 × 109/L, a monocyte count of 43.4 × 109/L, hemoglobin concentration of 95 g/L, and platelet count of 71 × 109/L. Nine months after diagnosis, the patient developed leukocyte stasis and dyspnea. His condition deteriorated thereafter, and he died.
The global incidence of CMML is extremely low, occurring in approximately 0.3 per 100000 people[4]. Based on the MDAPS and CMML Prognostic Scoring System results, the median survival period in CMML is 5–72 mo[5,6]. Approximately 20% of CMML patients have some form of systemic inflammatory or autoimmune disease affecting other organ systems[7-9]. However, the relationship between CMML and solid tumors is unclear[10,11]. To our knowledge, this is the first reported case of CMML alongside squamous cell carcinoma. As such, there is currently no standard treatment plan for the co-occurrence of these associated malignant tumors.
In this case, we evaluated the patient’s lung condition and leukemic cell burden after lung cancer diagnosis. We gave priority to treatment with the demethylating drug azacitidine. Azacitidine is the preferred treatment for CMML, and there is increasing evidence that HMAs play an important role in the treatment of proliferative CMML[12]. Azacitidine and diazepam acetabine are the only lines of therapy approved by the US Food and Drug Administration for the treatment of CMML[13]. Unfortunately, the lung malignancy did not go into remission as CMML symptoms improved, but rather progressed. Mutation-targeting and immunologic agents are rapidly improving the treatment of advanced non-small cell lung cancer[14]; however, there is no standard treatment for lung squamous cell carcinoma, given the low rate of target mutations and its poor response to chemotherapy.
In patients with CMML, the precursors to normal blood cells in the bone marrow are either damaged or suppressed, and tolerance to myelotoxic drugs is reduced. This may be the main reason for the severe myelosuppression observed here. There is currently no evidence of azacitidine leading to the progression of lung malignancies. However, since the 1970s, studies have reported a risk of myelodysplastic syndrome and acute myeloid leukemia transformation linked to platinum drugs commonly used to treat solid tumors, such as cisplatin and carboplatin, with carboplatin posing a smaller carcinogenic risk than cisplatin[15-17]. Chronic myelomonocytic leukemia is at risk of transforming into acute myeloid leukemia. After three courses of platinum-based drug therapy, more blasts had appeared in the peripheral blood of the patient, suggesting that the rapid progression of the disease may be related to the use of platinum-based drugs and the presence of ASXL1 gene mutation may accelerate this progression. Currently, no biomarkers are available to predict treatment responses to demethylating agents in CMML. Nonetheless, the presence of the ASXL1 gene mutation in patients with CMML may reduce the benefits of demethylating-agent treatment. While another deduction is the complex mechanisms involved in multiple primary tumors, including genetic factors, gene abnormalities, or low immunity. These factors may result in accelerated evolution of malignant clone cells from earlier naïve cell clones. The pathogenesis of multiple primary malignant tumors is complex, the early “field cancerization” theory[18] revealed the occurrence of multiple primary malignant tumors in specific areas (such as in the respiratory tract). The patient reported here has a long history of heavy smoking, and studies have shown a direct relationship between smoking and the occurrence of cancers[19]. Alternatively, multiple primary malignant tumors may arise via extensive migration of cancerous cells, in which tumor progenitor cells carrying mutant genes migrate to new sites and continue to divide and grow[20].
With the development of molecular biology, the pathogenic mechanisms of multiple primary cancers have been revealed at the genetic level; these include BRCA1 and BRCA2 gene mutations, ALDH2 gene inactivation, microsatellite instability, DNA replication errors, and aneuploidy of chromosome 17[21,22].
With a positivity rate of 40%, ASXL1 gene mutation is thought to be an independent adverse prognostic factor affecting survival in CMML. In contrast, with a positivity rate of 5%, almost always occurring simultaneously with ASXL1 mutations, EZH2 gene mutations have no clear clinical impact on CMML. Both of these genes affect epigenetic regulation and histone modification[23,24]. Patients with two co-occurring mutations exhibited shorter survival periods and worse prognosis than those with a single mutation or without co-occurring mutations[25,26].
In this case, using NGS, we detected no mutations in ASXL1 or EZH2 in the patient’s lung squamous cell carcinoma tissue. Pulmonary manifestations in CMML patients are non-specific, and careful evaluation of leukemic involvement in the lungs is required to differentiate between infection, bleeding, leukemic infiltration, and the rare occurrence of pulmonary manifestations, presenting challenges in diagnosis. Computed tomography scan results are usually inconclusive, and bronchoscopy and bronchoalveolar lavage are typically recommended to exclude leukemic infiltration, bleeding, and infection. Lung biopsy[27] may be required for accurate diagnosis. The presence of this rare population should be given due consideration in clinical practice.
Although the simultaneous occurrence of CMML and lung malignancy is rare, two cases have so far been reported. Koopman et al[28] reported the case of a male patient with CMML exhibiting an IDH2 mutation; the patient was diagnosed with non-small cell lung cancer (adenocarcinoma) during the observation period. Adenocarcinoma and the same mutation were observed in a lung cancer biopsy, suggesting its origin in CMML. Kim et al[29] reported that a male patient with non-small cell lung cancer developed CMML within two years after receiving platinum-based chemotherapy; furthermore, their cytogenetic study revealed trisomy 8. Although our case also concerned an elderly male patient, it involved simultaneous CMML and lung cancer, with no evidence of CMML infiltrating the lung cancer tissue. Therefore, we emphasize the importance of: (1) Paying attention to the occurrence and evolution of solid tumors, such as lung cancer, when treating and monitoring CMML treatment; and (2) being vigilant of the presence of multiple primary malignancies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Papadopoulos VP, Greece; Sultana N, Bangladesh S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 6732] [Article Influence: 748.0] [Reference Citation Analysis (0)] |
| 2. | Guru Murthy GS, Dhakal I, Mehta P. Incidence and survival outcomes of chronic myelomonocytic leukemia in the United States. Leuk Lymphoma. 2017;58:1648-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Cui Y, Liu T, Zhou Y, Ji Y, Hou Y, Jin W, Feng Y. Five cases report of solid tumor synchronously with hematologic malignancy. Cancer Res Treat. 2012;44:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, Edwards BK, List AF. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 464] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 5. | Onida F, Kantarjian HM, Smith TL, Ball G, Keating MJ, Estey EH, Glassman AB, Albitar M, Kwari MI, Beran M. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 284] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Such E, Germing U, Malcovati L, Cervera J, Kuendgen A, Della Porta MG, Nomdedeu B, Arenillas L, Luño E, Xicoy B, Amigo ML, Valcarcel D, Nachtkamp K, Ambaglio I, Hildebrandt B, Lorenzo I, Cazzola M, Sanz G. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood. 2013;121:3005-3015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 7. | Zahid MF, Barraco D, Lasho TL, Finke C, Ketterling RP, Gangat N, Hanson CA, Tefferi A, Patnaik MM. Spectrum of autoimmune diseases and systemic inflammatory syndromes in patients with chronic myelomonocytic leukemia. Leuk Lymphoma. 2017;58:1488-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Grignano E, Mekinian A, Braun T, Liozon E, Hamidou M, Decaux O, Puéchal X, Kahn JE, Schoindre Y, Rossignol J, Lortholary O, Lioger B, Hermine O, Park S, Ades L, Montestruc F, Ricard L, Gardin C, Fenaux P, Fain O; GFM, SNFMI and CRI. Autoimmune and inflammatory diseases associated with chronic myelomonocytic leukemia: A series of 26 cases and literature review. Leuk Res. 2016;47:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Peker D, Padron E, Bennett JM, Zhang X, Horna P, Epling-Burnette PK, Lancet JE, Pinilla-Ibarz J, Moscinski L, List AF, Komrokji RS, Zhang L. A close association of autoimmune-mediated processes and autoimmune disorders with chronic myelomonocytic leukemia: observation from a single institution. Acta Haematol. 2015;133:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Sans-Sabrafen J, Woessner S, Besses C, Lafuente R, Florensa L, Buxó J. Association of chronic myelomonocytic leukemia and carcinoma: a possible paraneoplastic myelodysplasia. Am J Hematol. 1986;22:109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Stark AN, Thorogood J, Head C, Roberts BE, Scott CS. Prognostic factors and survival in chronic myelomonocytic leukaemia (CMML). Br J Cancer. 1987;56:59-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Liapis K, Kotsianidis I. Approaching First-Line Treatment in Patients With Advanced CMML: Hypomethylating Agents or Cytotoxic Treatment? Front Oncol. 2021;11:801524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Hunter AM, Zhang L, Padron E. Current Management and Recent Advances in the Treatment of Chronic Myelomonocytic Leukemia. Curr Treat Options Oncol. 2018;19:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Yu A, Huang E, Abe M, An K, Park SK, Park C. Cost-effectiveness analyses of targeted therapy and immunotherapy for advanced non-small cell lung cancer in the United States: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2021;21:381-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Kaldor JM, Day NE, Pettersson F, Clarke EA, Pedersen D, Mehnert W, Bell J, Høst H, Prior P, Karjalainen S. Leukemia following chemotherapy for ovarian cancer. N Engl J Med. 1990;322:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 170] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Bradley MO, Hsu IC, Harris CC. Relationship between sister chromatid exchange and mutagenicity, toxicity and DNA damage. Nature. 1979;282:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Zwelling LA, Bradley MO, Sharkey NA, Anderson T, Kohn KW. Mutagenicity, cytotoxicity and DNA crosslinking in V79 Chinese hamster cells treated with cis- and trans-Pt(II) diamminedichloride. Mutat Res. 1979;67:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 19. | Braisch U, Meyer M, Radespiel-Tröger M. Risk of tobacco-related multiple primary cancers in Bavaria, Germany. BMC Cancer. 2012;12:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Bedi GC, Westra WH, Gabrielson E, Koch W, Sidransky D. Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res. 1996;56:2484-2487. [PubMed] |
| 21. | Garrean S, Hering J, Saied A, Jani J, Espat NJ. Gastric adenocarcinoma arising from fundic gland polyps in a patient with familial adenomatous polyposis syndrome. Am Surg. 2008;74:79-83. [PubMed] |
| 22. | Hamm A, Veeck J, Bektas N, Wild PJ, Hartmann A, Heindrichs U, Kristiansen G, Werbowetski-Ogilvie T, Del Maestro R, Knuechel R, Dahl E. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: a systematic expression analysis. BMC Cancer. 2008;8:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Chan O, Renneville A, Padron E. Chronic myelomonocytic leukemia diagnosis and management. Leukemia. 2021;35:1552-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Palomo L, Acha P, Solé F. Genetic Aspects of Myelodysplastic/Myeloproliferative Neoplasms. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Patnaik MM, Vallapureddy R, Lasho TL, Hoversten KP, Finke CM, Ketterling R, Hanson C, Gangat N, Tefferi A. EZH2 mutations in chronic myelomonocytic leukemia cluster with ASXL1 mutations and their co-occurrence is prognostically detrimental. Blood Cancer J. 2018;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Castaño-Díez S, López-Guerra M, Bosch-Castañeda C, Bataller A, Charry P, Esteban D, Guijarro F, Jiménez-Vicente C, Castillo-Girón C, Cortes A, Martínez-Roca A, Triguero A, Álamo JR, Beà S, Costa D, Colomer D, Rozman M, Esteve J, Díaz-Beyá M. Real-World Data on Chronic Myelomonocytic Leukemia: Clinical and Molecular Characteristics, Treatment, Emerging Drugs, and Patient Outcomes. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Fayed M, Evans T, Abdulhaq H. Leukemic infiltration in the settings of acute respiratory failure. Oxf Med Case Reports. 2019;2019:482-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Koopman B, Hiddinga BI, Platteel I, Kluiver JL, Timens W, Mulder AB, van Doesum JA, Schuuring E, Diepstra A, van Kempen LC. Non-small-cell lung cancer infiltrated with chronic myelomonocytic leukaemia: a molecular diagnostic challenge to recognise mixed cancers in a single biopsy. Histopathology. 2021;78:1043-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Kim KB, Faderl S, Hwang CS, Khuri FR. Chronic myelomonocytic leukaemia after platinum-based therapy for non-small cell lung cancer: case report and review of the literature. J Clin Pharm Ther. 2006;31:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |