Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3592
Peer-review started: January 4, 2023
First decision: March 24, 2023
Revised: April 5, 2023
Accepted: April 21, 2023
Article in press: April 21, 2023
Published online: May 26, 2023
Processing time: 141 Days and 1.8 Hours
Nongestational ovarian choriocarcinoma (NGOC) is a rare but aggressive neoplasm with limited sensitivity to chemotherapy and a very poor prognosis. Few cases of NGOC have been reported, and there is limited information regarding its clinical features, treatment protocols, or prognosis.
A postmenopausal woman in her 5th decade of life visited our clinic because of abnormal vaginal bleeding and an abdominal mass. Although she had been menopausal for more than eight years and her last abortion occurred nine years ago, she had an increased level of serum β-human chorionic gonadotropin (β-hCG). Thus, an ovarian neoplasm of trophoblastic origin was suspected, and exploratory laparotomy was performed. Based on the patient’s clinical history and the histopathological examination and immunohistochemistry results obtained postoperatively, we concluded that she most likely had primary NGOC. Cytoreductive surgery was performed in combination with adjuvant chemotherapy comprising bleomycin, etoposide, and cisplatin. Serum β-hCG levels decreased to normal after two cycles, and there was no evidence of recurrence after four cycles of chemotherapy.
Even in postmenopausal women, ovarian choriocarcinoma should be considered in the initial differential diagnosis for an adnexal mass.
Core Tip: Ovarian choriocarcinoma (OC) typically affects teenagers and young women. However, this report presents a rare case of nongestational OC (NGOC) in a postmenopausal woman. Although DNA polymorphism analysis has been recommended for the confirmation of this disease, its applications are limited because of its high cost and technological deficiencies. Based on the long-time interval since her last pregnancy and menstruation and the histopathological examination and immunohistochemistry results, we suggested that the patient likely had primary NGOC. Early diagnosis and appropriate management are critical prognostic factors for this disease.
- Citation: Dai GL, Tang FR, Wang DQ. Primary ovarian choriocarcinoma occurring in a postmenopausal woman: A case report. World J Clin Cases 2023; 11(15): 3592-3598
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3592.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3592
Ovarian choriocarcinoma (OC), including intrauterine choriocarcinoma, can be classified as gestational or nongestational based on its origin. Gestational OC (GOC) develops during or after normal or molar gestation and can be considered primary or metastatic from elsewhere in the genital tract. Nongestational OC (NGOC) is a rare but extremely aggressive neoplasm that accounts for < 0.6% of all ovarian neoplasms[1]. NGOC develops independent of gestation and frequently affects adolescents and young women. However, rare cases have been diagnosed in postmenopausal women[2]. NGOC is predominantly considered to be differentiated from germ cell tumors[3] and most NGOCs are of mixed types associated with other malignant germ cell tumors, such as immature teratoma, yolk sac tumor, or dysgerminoma. Few cases of NGOC have been reported, and no consensus has been reached regarding its clinical features, treatment protocols, or prognosis. Here, we present a case of primary NGOC in a postmenopausal woman and discuss its clinical features and treatment, along with a brief literature review.
The patient complained of a 2-mo history of vaginal bleeding and 10-d history of a palpable abdominal mass.
Two months prior, the patient experienced spontaneous irregular vaginal bleeding that was minimal in amount and not associated with dizziness or weakness. Ten days prior, a palpable abdominal mass was detected.
The patient had experienced a normal vaginal delivery and four abortions. She had been menopausal for more than eight years, and her last abortion occurred nine years ago. Seven years ago, she underwent left oophorocystectomy, and postoperative histopathologic examination showed no signs of malignancy (however, the details were unclear).
The patient denied any family history of malignant tumors.
The patient’s vital signs were within the normal limits. Gynecological examination revealed a 12-cm, mildly tender, and mobile abdominopelvic mass.
The patient’s serum β-human chorionic gonadotropin (β-hCG) level sharply increased to 214405.5 mIU/mL (< 5.0 mIU/mL) and her carbohydrate antigen 125 level was elevated at 51.0 U/mL (< 35 U/mL). Carbohydrate antigen 19-9, alpha-fetoprotein (AFP), and carcinoembryonic antigen levels were within normal limits.
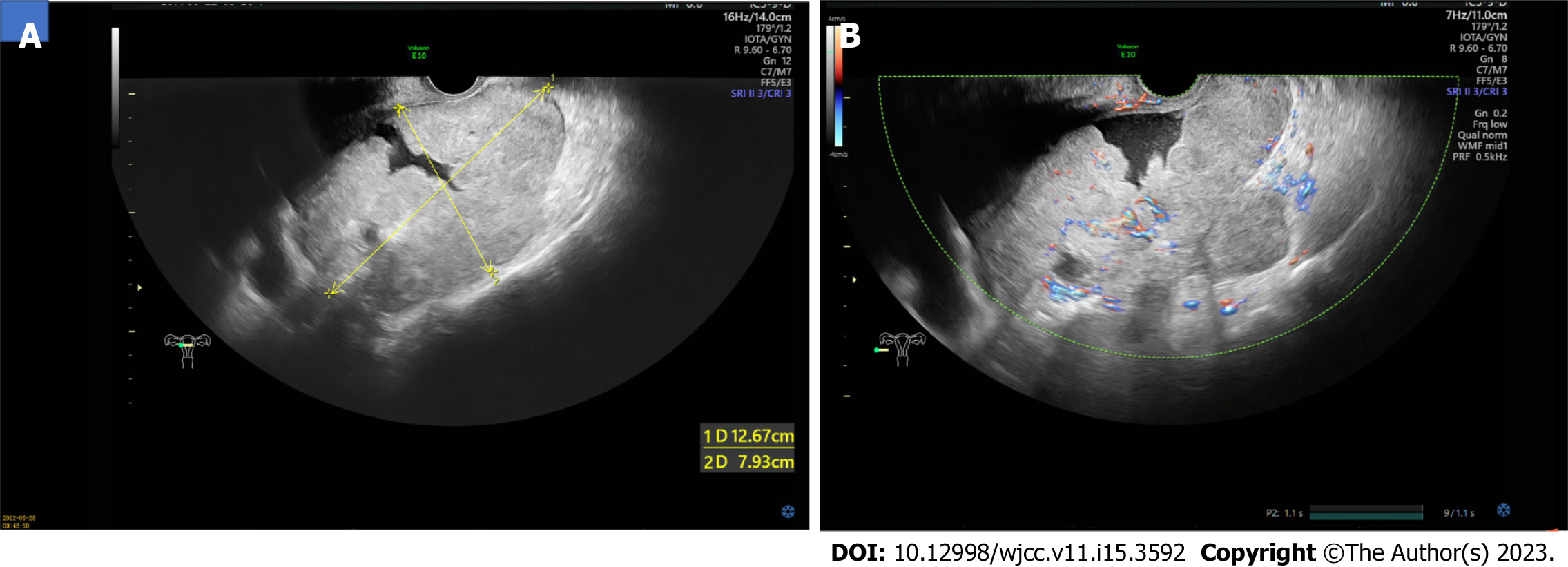
Ultrasonography revealed a 13 cm × 8 cm × 12 cm solid mass in the pelvis, posterior to the uterus, with an irregular shape and unclear boundaries (Figure 1A). Color Doppler examination revealed rich vascularization of the mass and surrounding tissues (Figure 1B). The patient was further evaluated using computed tomography (CT), which revealed an unclear boundary between the mass and surrounding tissues and showed the ovarian vascular pedicle sign. No evidence of intrauterine occupation or metastasis to other organs was observed. Further examination using magnetic resonance imaging (MRI) confirmed the absence of brain metastasis.
Based on the long-time interval since her last pregnancy and menstruation, absence of lesions in the uterine cavity, and exclusion of intrauterine and molar pregnancy, the patient was diagnosed with NGOC International Federation of Gynecology and Obstetrics stage IIIB by an experienced pathologist.
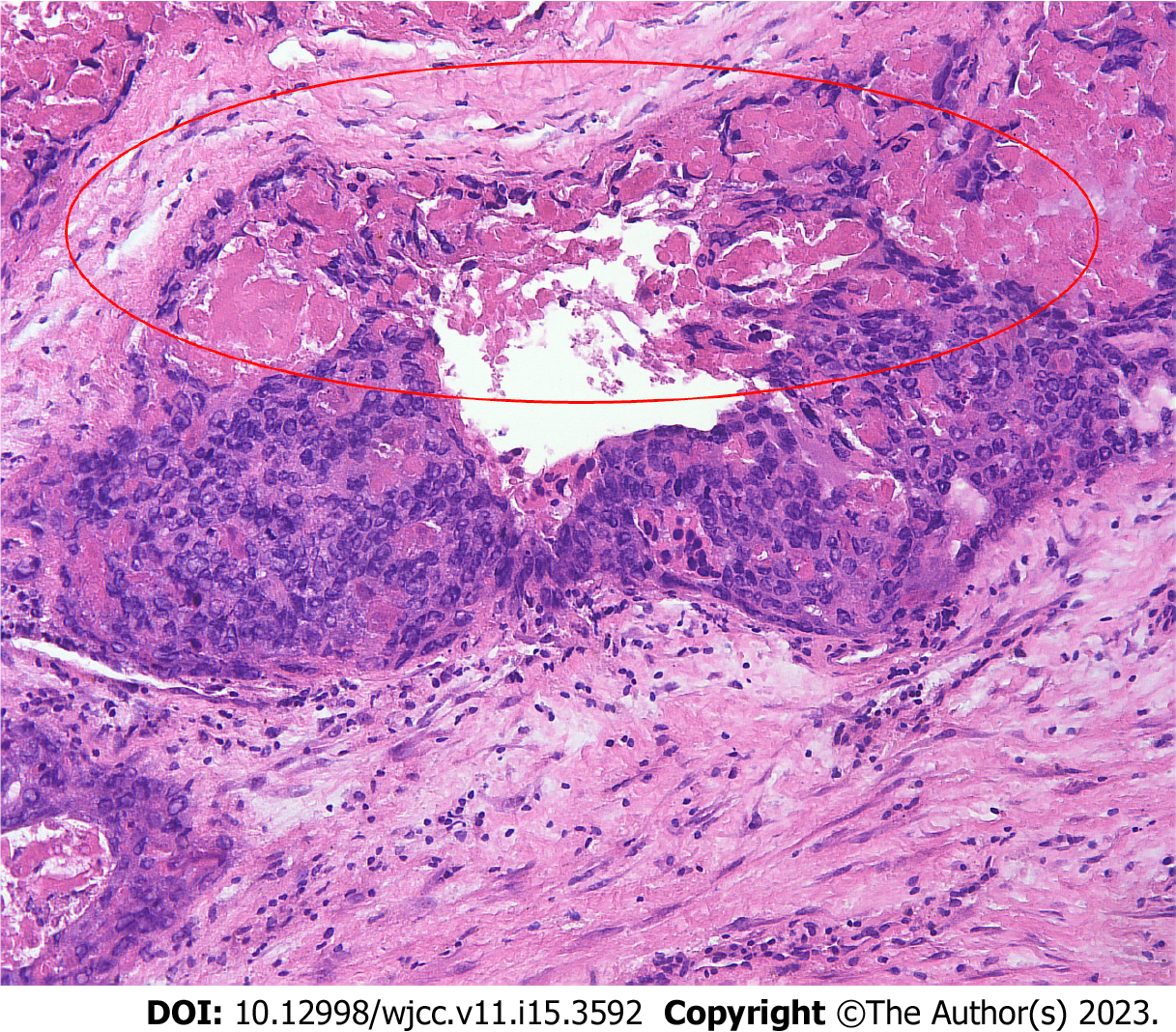
Exploratory laparotomy was performed for the suspected malignant ovarian tumor. Intraoperatively, a hemorrhagic, friable, necrotic, 12 cm × 12 cm mass, likely originating from the left adnexa, was detected, and the left ovary and fallopian tube could not be found. The mass wrapped densely around part of the sigmoid colon and rectum, germinated diffusely, and compressed the intestines severely. The right ovary and fallopian gland appeared normal. A tissue sample from the mass was immediately submitted for intraoperative frozen pathology, and a diagnosis of OC was made (Figure 2). Because of the difficulty in separating the mass and the affected bowel, a specialist in gastrointestinal surgery was invited, and rectotomy and colostomy were recommended. After written informed consent was obtained, cytoreductive surgery, including pelvic giant mass resection, total hysterectomy, right salpingo-oophorectomy, pelvic lymphadenectomy, para-aortic lymph node sampling, omentectomy, peritoneal biopsies, rectotomy, and colostomy, was performed. Optimal debulking was achieved with no macroscopic residual tumors (R0).
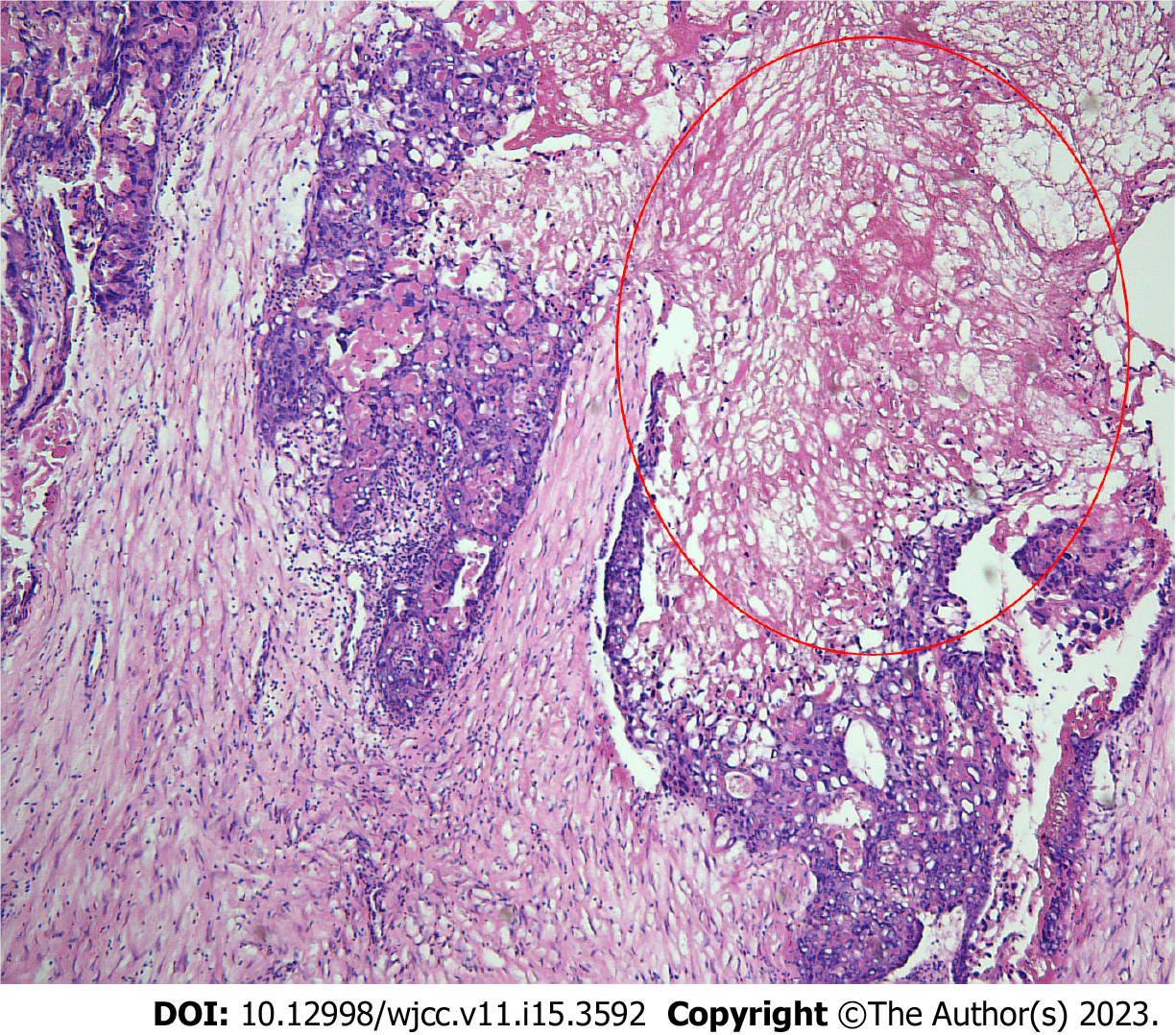
Postoperative immunohistochemical results indicated that the tumor was an OC with widespread necrosis. No germ cell elements were detected (i.e., the tumor was a pure OC) (Figure 3). The tumor infiltrated the left ovary, right ovarian serosa, serosal layer of the excised colon, and omentum. The tumor also invaded the vessels of the left ovary and omentum. The uterus, bilateral fallopian tubes, peritoneal biopsy samples, edges of the excised bowel and vagina, and excised lymph nodes showed no metastasis. The tissue sample from the mass was strongly positive for hCG and weakly positive for placental alkaline phosphatase (PLAP). Eighty-five percent of the tumor cells were strongly positive for Ki-67. Other immunohistochemical staining tests included pan-cytokeratin +++, cytokeratin 18 +++, cytokeratin 8 +++, α-inhibin ++, human placental lactogen (HPL)+, and P53 protein ++.
Postoperatively, the patient received a chemotherapy regimen of bleomycin, etoposide, and cisplatin (BEP), after chemotherapeutic contraindications were excluded.
Postoperatively, the serum β-hCG level sharply decreased to 796.7 mIU/mL. After the second cycle of chemotherapy, the serum β-HCG level further declined to < 2.0 mIU/mL, and there was no evidence of recurrence after four cycles of chemotherapy. The postoperative results of this patient who underwent cytoreductive surgery combined with BEP chemotherapy were satisfactory (Supplementary Table 1).
NGOC frequently affects teenagers and women of early reproductive age. Liu et al[4] found that its peak incidence occurs in adolescents aged 12–25 years[4] .However, a few cases of NGOC have been reported in the postmenopausal period. Due to the rarity of NGOC, there are still significant challenges in its clinical diagnosis and treatment. Thus, we present a rare case in which the tumor developed in a patient nine years after her last pregnancy and eight years after menopause and discuss its diagnosis and treatment.
Previous studies have revealed that NGOC develops independent of pregnancy and DNA polymorphism analysis can be used to confirm the absence of paternal genetics. However, the clinical applications of DNA polymorphism analysis are currently limited. Previous reproductive history plays an important role in distinguishing NGOC from OC. Cronin et al[5] have suggested that a diagnosis of NGOC is supported by the lack of sexual intercourse or pregnancy in the history. However, this postmenopausal patient had experienced a normal vaginal delivery and four abortions. She had been menopausal for more than eight years, and her last abortion occurred nine years ago. Therefore, further studies are required to determine whether a history of pregnancy or abortion is crucial in the diagnosis of NGOC.
NGOC typically presents with vaginal bleeding, abdominal pain, an adnexal mass on ultrasound, and positive pregnancy test results. Hemorrhage is the most common symptom and can lead to significant morbidity and mortality[6]. Due to the innate capability of trophoblastic cells to invade and erode vascular structures, hemorrhage can be found not only in the genital tract, but also in metastatic sites, such as the intestine[7], which can present with unexplained hematochezia, or the brain[8], which may cause catastrophic bleeding. Unilateral masses have been reported in most cases of NGOC[4]. NGOC is often not considered in the initial differential diagnosis of an adnexal mass because of its rarity and is often misdiagnosed as an ectopic pregnancy because of its atypical presentation.
The level of β-hCG can help exclude the possibility of an ectopic pregnancy, in which the level is rarely higher than 100000 mIU/mL. In contrast, the β-hCG level in NGOC is commonly 10–100 times higher[5]. In our report, the postmenopausal woman presented with vaginal bleeding, an abdominal mass, and a sharply elevated serum β-hCG level at 214405.5 mIU/mL. Although choriocarcinoma frequently occurs during or after normal or molar gestation during the reproductive age, the extreme elevation of the serum β-hCG level in this case suggested that choriocarcinoma cannot be excluded.
Ultrasound is the preferred imaging modality in gynecology. NGOC commonly presents as a richly vascular, echogenic, and non-homogeneous unilateral mass that yields non-specific findings[6]. CT can further evaluate the extent of the disease and reveal metastatic lesions located elsewhere[4]. Pre-operatively, the patient underwent CT, which revealed a huge mass likely originating from the left ovary and no evidence of metastasis to other organs. MRI ruled out cerebral metastasis.
Given the atypical signs and symptoms of OC, histopathological examination and immunohistochemistry are indispensable in confirming its diagnosis. Histopathologically, both GOC and NGOCs have an identical presentation, with abnormal trophoblastic hyperplasia and anaplasia, absence of chorionic villi, and the presence of hemorrhage and necrosis within the tumor tissue[9]. At present, it is almost impossible to distinguish between the two using histopathology, and only DNA polymorphism analysis can reveal whether the tumor contains a paternal genetic contribution, suggesting that it is related to pregnancy[10]. However, this method has limited applications because of its high cost and technological deficiencies. Meanwhile, the absence of immunohistochemical staining for CD30, PLAP, and AFP can preclude a mixed type of NGOC with other germ cell elements[11]. According to the criteria proposed by Saito, a diagnosis of NGOC can be confirmed by the absence of abnormalities in the uterine cavity, pathological confirmation of choriocarcinoma with a persistent elevation in β-HCG level, and exclusion of molar and intrauterine pregnancy[2].
Compared to GOCs, NGOCs are less sensitive to chemotherapy[5] and have worse prognosis. Because of the small number of cases, there is no standard therapy currently available. NGOC is thought to be a germ cell tumor that differentiates from the trophoblastic structure[2], and the basis for its therapeutic protocols is extrapolated from the treatment of germ cell tumors, which is vastly different from that of GOCs. Surgical resection combined with BEP chemotherapy, which is considered the gold standard for the treatment of germ cell tumors[12], is the most utilized regimen and has shown excellent outcomes. In addition, Liu et al[4] have reported that the etoposide–methotrexate–echinomycin/vincristine–cyclophosphamide (EMA/CO) protocol improves prognosis. Cytoreductive surgery is commonly recommended for NGOC[4]. Because the peak incidence of NGOC occurs among adolescents and women of reproductive age, fertility-preserving treatment should be considered in patients with early-stage cancer. Although complete cytoreduction (R0) has been reported to be an independent prognostic factor[13], it is still controversial whether patients with advanced disease can be offered fertility-preserving surgery. Inaba et al[14] have reported that patients with advanced disease, with the correct diagnosis, rational treatment, and strict surveillance of serum β-hCG, can often receive fertility-sparing treatment and achieve long-term survival.
In this case, the patient was a postmenopausal woman with a long time interval (9 years) since her last pregnancy. After cytoreductive surgery and adjuvant chemotherapy with the BEP regimen, her β-hCG level decreased to < 2.0 mIU/mL, with no signs of recurrence, and her prognosis improved. In highly malignant conditions with poor prognosis, early diagnosis and rational treatment are extremely important, especially for teenagers and women of reproductive age. Timely diagnosis and early intervention in the disease course can offer a reasonable opportunity for fertility preservation. When a suspicious adnexal mass is detected through ultrasound with an unusual elevation in the β-hCG level, laparoscopy should be considered to exclude choriocarcinoma. Differentiating between GOC and NGOC is important because of their distinctive clinical behaviors, different sensitivity levels to chemotherapy, and different prognoses[12]. Although DNA analysis has limited clinical applications, the sexual history of adolescents and length of time between menopause and tumor development can differentiate GOC and NGOC. Further studies are required to prove whether a history of pregnancy or abortion is crucial for the diagnosis of NGOC in postmenopausal patients. Once NGOC is suspected, CT or MRI should be performed to detect possible metastasis. In addition, unexplained bleeding with an accompanying increase in the level of β-hCG should be considered indicators of NGOC.
Despite its extreme rarity, NGOC should be included in the differential diagnoses of ectopic pregnancy or an adnexal mass in adolescent, reproductive, and even postmenopausal women. The early diagnosis of NGOC can facilitate effective treatment using surgery combined with appropriate chemotherapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chatterjee T, United States; Giménez-Bonafé P, Spain; Tolunay HE, Turkey S-Editor: Li L L-Editor: Wang TQ P-Editor: Zhang XD
| 1. | Vance RP, Geisinger KR. Pure nongestational choriocarcinoma of the ovary. Report of a case. Cancer. 1985;56:2321-2325. [PubMed] [DOI] [Full Text] |
| 2. | Shao Y, Xiang Y, Jiang F, Pan B, Wan X, Yang J, Feng F, Ren T, Zhao J. Clinical features of a Chinese female nongestational choriocarcinoma cohort: a retrospective study of 37 patients. Orphanet J Rare Dis. 2020;15:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Nogales FF, Dulcey I, Preda O. Germ cell tumors of the ovary: an update. Arch Pathol Lab Med. 2014;138:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Liu X, Zhang X, Pang Y, Ma Y, Liu P. Clinicopathological factors and prognosis analysis of 39 cases of non-gestational ovarian choriocarcinoma. Arch Gynecol Obstet. 2020;301:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Cronin S, Ahmed N, Craig AD, King S, Huang M, Chu CS, Mantia-Smaldone GM. Non-Gestational Ovarian Choriocarcinoma: A Rare Ovarian Cancer Subtype. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Anjum AS, Maqsood H, Younus S, Anjum S, Fatima M. A Rare Case of Non-Gestational Metastatic Ovarian Choriocarcinoma: Case Report and Literature Review With a Special Emphasis on Imaging. Cureus. 2021;13:e13121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kumar S, Raouf ZR, Saparamadu PAM, Burney IA. Massive Gastrointestinal Bleeding from Choriocarcinoma of the Ovary. Oman Med J. 2018;33:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Rao KV, Konar S, Gangadharan J, Vikas V, Sampath S. A pure non-gestational ovarian choriocarcinoma with delayed solitary brain metastases: Case report and review of the literature. J Neurosci Rural Pract. 2015;6:578-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Mizukawa M, Sato H, Nishikawa S, Kashimura A, Nishina H, Sakairi T. Spontaneous ovarian choriocarcinoma in a young ICR mouse. J Toxicol Pathol. 2021;34:123-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Exman P, Takahashi TK, Gattás GF, Cantagalli VD, Anton C, Nalesso F, Diz Mdel P. Primary ovary choriocarcinoma: individual DNA polymorphic analysis as a strategy to confirm diagnosis and treatment. Rare Tumors. 2013;5:89-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Gershenson DM. Update on malignant ovarian germ cell tumors. Cancer. 1993;71:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Nasioudis D, Chapman-Davis E, Frey MK, Caputo TA, Witkin SS, Holcomb K. Prognostic significance of residual disease in advanced stage malignant ovarian germ cell tumors. Int J Gynecol Cancer. 2019;29:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Inaba H, Kawasaki H, Hamazaki M, Okugawa T, Uchida K, Honzumi M, Komada Y, Ito M, Toyoda N, Sakurai M. A case of metastatic ovarian non-gestational choriocarcinoma: successful treatment with conservative type surgery and myeloablative chemotherapy. Pediatr Int. 2000;42:383-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |