Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3571
Peer-review started: December 24, 2022
First decision: February 20, 2023
Revised: March 5, 2023
Accepted: April 19, 2023
Article in press: April 19, 2023
Published online: May 26, 2023
Processing time: 152 Days and 8.6 Hours
Malignant melanoma (MM) has shown an increasing incidence worldwide, and a potential to metastasize to almost any part of the body. Clinically, MM with bone metastasis as the initial manifestation is extremely rare. Spinal metastatic MM can cause spinal cord or nerve root compression, resulting in severe pain and paralysis. Currently, the primary clinical treatments for MM are surgical resection in conjunction with chemotherapy, radiotherapy, and immunotherapy.
Here, we report the case of a 52-year-old male who presented to the clinic with progressive low back pain and limited nerve function. No primary lesion or spinal cord compression was detected from computed tomography and magnetic resonance imaging of the lumbar vertebrae and positron emission tomography scan. A lumbar puncture biopsy confirmed the diagnosis of lumbar spine metastatic MM. Following surgical resection, the patient’s quality of life improved, symptoms were relieved, and comprehensive treatment was initiated, which prevented recurrence.
Spinal metastatic MM is clinically rare, and may cause neurological symptoms, including paraplegia. Currently, the clinical treatment plan consists of surgical resection in combination with chemotherapy, radiotherapy, and immunotherapy.
Core Tip: The worldwide incidence of malignant melanoma (MM) is increasing every year. MM is very aggressive and has the potential to metastasize to any part of the body. However, symptoms of spinal metastasis are rare in their initial manifestation. Here, we performed total vertebral body resection, three-dimensional printed artificial vertebral body reconstruction, and comprehensive treatment, including targeted therapy and chemotherapy, which effectively controlled the condition.
- Citation: Guo ZX, Zhao XL, Zhao ZY, Zhu QY, Wang ZY, Xu M. Malignant melanoma resection and reconstruction with the first manifestation of lumbar metastasis: A case report. World J Clin Cases 2023; 11(15): 3571-3577
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3571.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3571
Malignant melanoma (MM) is a highly invasive malignant tumor with an increasing incidence and poor prognosis[1,2]. MM primarily originates in the skin and mucosa and can spread to any organ, most commonly the lymph nodes, lungs, and brain, but rarely the spine[3,4].
Initially, the patient presented with symptoms of aggravated lumbago, back pain, progressive numbness, and weakness of the lower limbs. A biopsy confirmed an isolated metastatic MM in the lumbar spine, but the primary lesion was not found. The symptoms improved after surgery, and his lower limb muscle strength recovered. The overall condition of the patient was good following continuous, comprehensive treatment.
A 52-year-old male presented to our hospital with low back pain for 1 mo and numbness and pain in the left thigh for 27 d.
One month ago, the patient experienced low back pain and discomfort, as well as obvious limitations in inactivity, whenever he suddenly turned around. The pain was constant, aggravated by activity, and occasionally worsened at night. He visited our hospital for treatment after experiencing numbness in the lateral side of his left thigh, pain, and weakness for 27 d.
The patient had diabetes and had been taking insulin injection, with good glycemic control. He denied any history of hypertension, coronary heart disease, tuberculosis, malaria, and other infectious diseases, other surgical histories, major trauma, blood transfusion history, and food or drug allergy.
The patient married at the appropriate age, had two daughters, and a healthy spouse and parents, and denied any family history of infectious or genetic diseases.
Consciousness is clear, walking into the ward, the waist does not touch an obvious mass, the skin color is normal, tenderness and percussion pain are positive, the pain radiates to the back of the left thigh, bilateral iliopsoas muscle strength level 5, both lower limb sensation and muscle strength are normal, bilateral dorsal foot and posterior tibial artery pulse can be palpatible, bilateral tendon reflex and Achilles tendon reflex are normal, bilateral patellar clonic and ankle clonic are negative.
Routine blood test results were as follows: White blood cells, 8 × 109 cells/L; hemoglobin, 145 g/L; and total platelet count, 438 × 109 cells/L. Biochemical analysis revealed the following: Total protein level, 71 g/L; albumin, 46.8 g/L; creatinine 61 μmol/L; C-reactive protein, 2.8 mg/L; D-dimer, 2.39 mg/L; and erythrocyte sedimentation rate, 16 mm. The urine and stool examination results were normal.
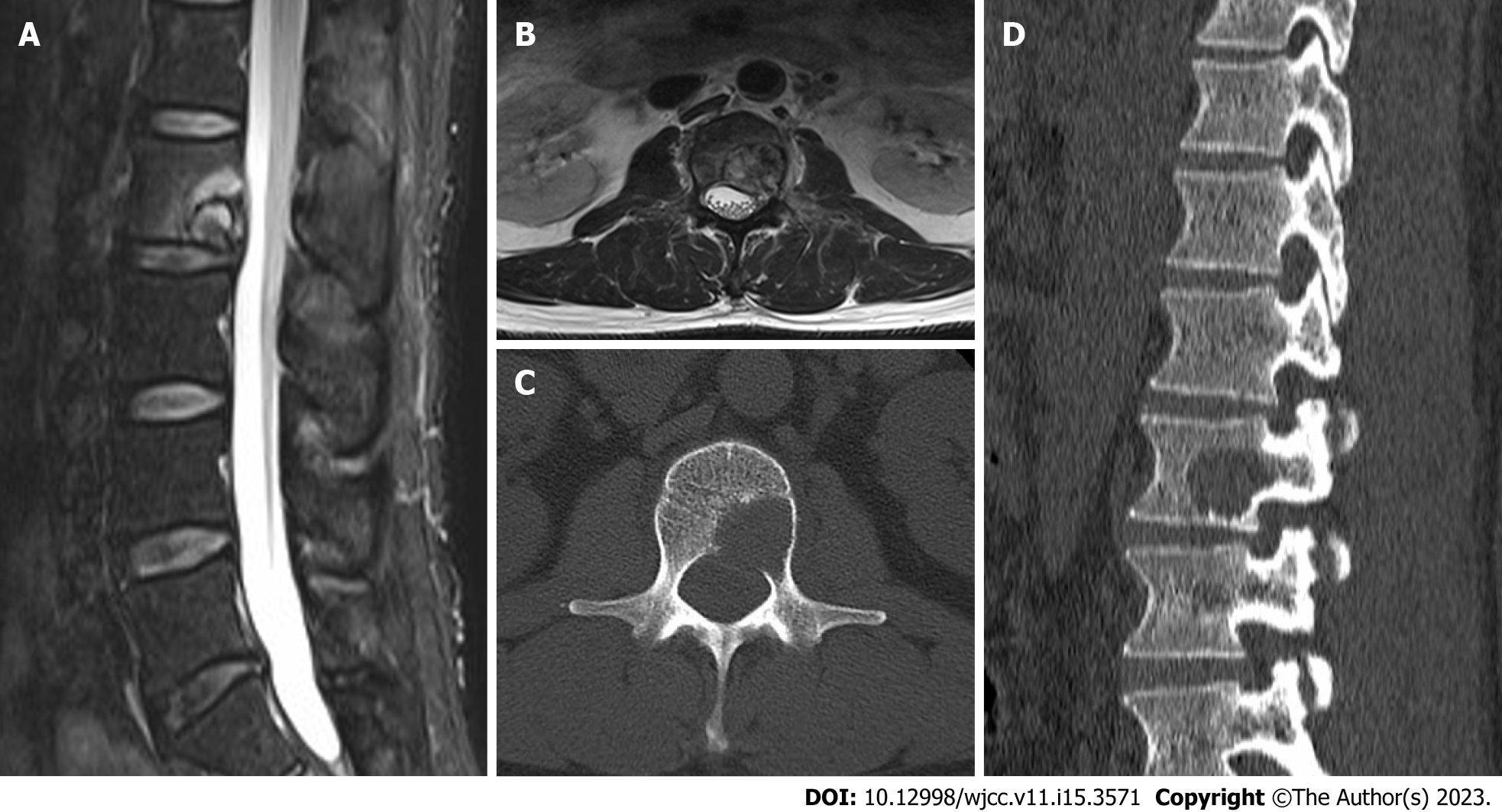
Magnetic resonance imaging (MRI) of lumbar spine showed that the lumbar 2 vertebral body was irregularly and slightly longer, short T1-weighted imaging (T1WI) and slightly longer and short T2-weighted imaging (T2WI) mixed signals, local protrusion to the left posterior, spinal canal and left intervertebral foramen compression changes, left pedicle involvement. Computed tomography (CT) findings of the lumbar vertebrae showed pathological changes in lumbar vertebra 2, bone destruction, involvement of the left vertebral arch, and pathological fractures. Combined positron emission tomography (PET) and CT (PET/CT): First, there was local bone destruction and increased metabolism in the lumbar vertebrae 2, small cavities in cervical 7, thoracic 3, 7, and normal metabolism in 8 vertebral bodies. Second, multiple slightly low-density nodular shadows with normal metabolism suggested that the liver was punctured and biopsied in the lumbar vertebrae 2. Therefore, it was recommended to combine enhanced CT and MRI examinations. Third, both lungs had multiple nodules but no increase in metabolism. Therefore, it was advisable to re-examine the anti-inflammatory treatment. Finally, PET/CT of the brain revealed no clear signs of abnormal metabolisms (Figure 1).
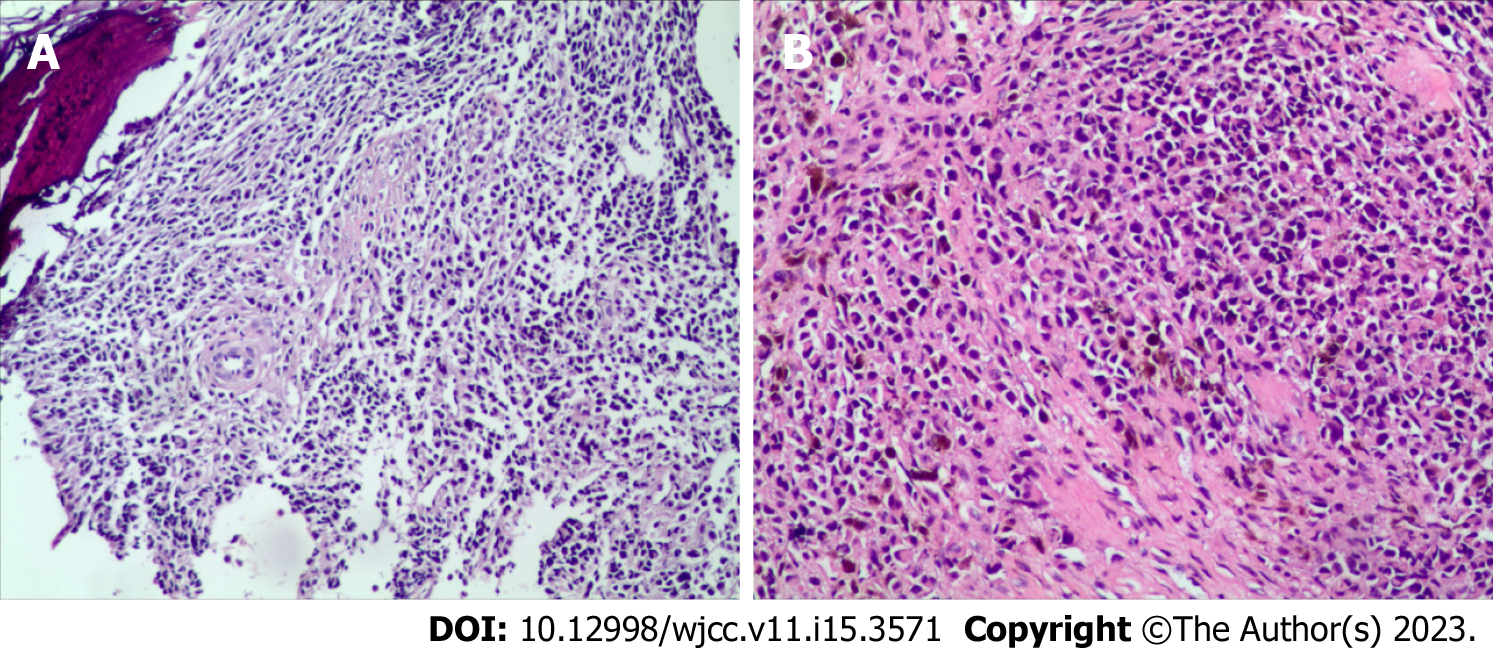
Lumbar puncture biopsy showed small round cell tumors in the trabecula, with small, medium, large and nucleoli. Combined with the immunohistochemical results, this finding was consistent with that of metastatic MM (Figure 2).
Immunohistochemistry results were as follows: HMB45(+), S100(+), CK(-), CK20(-), Villin(-), CD3(-), CD138(-), Kap(-), Lam(-), PLAP(-), alkp80(NKX2.2(+)), CD43(-), MPO(-), CD1a(-), CgA(-), Syn(-), CD56
Lumbar spine metastatic MM.
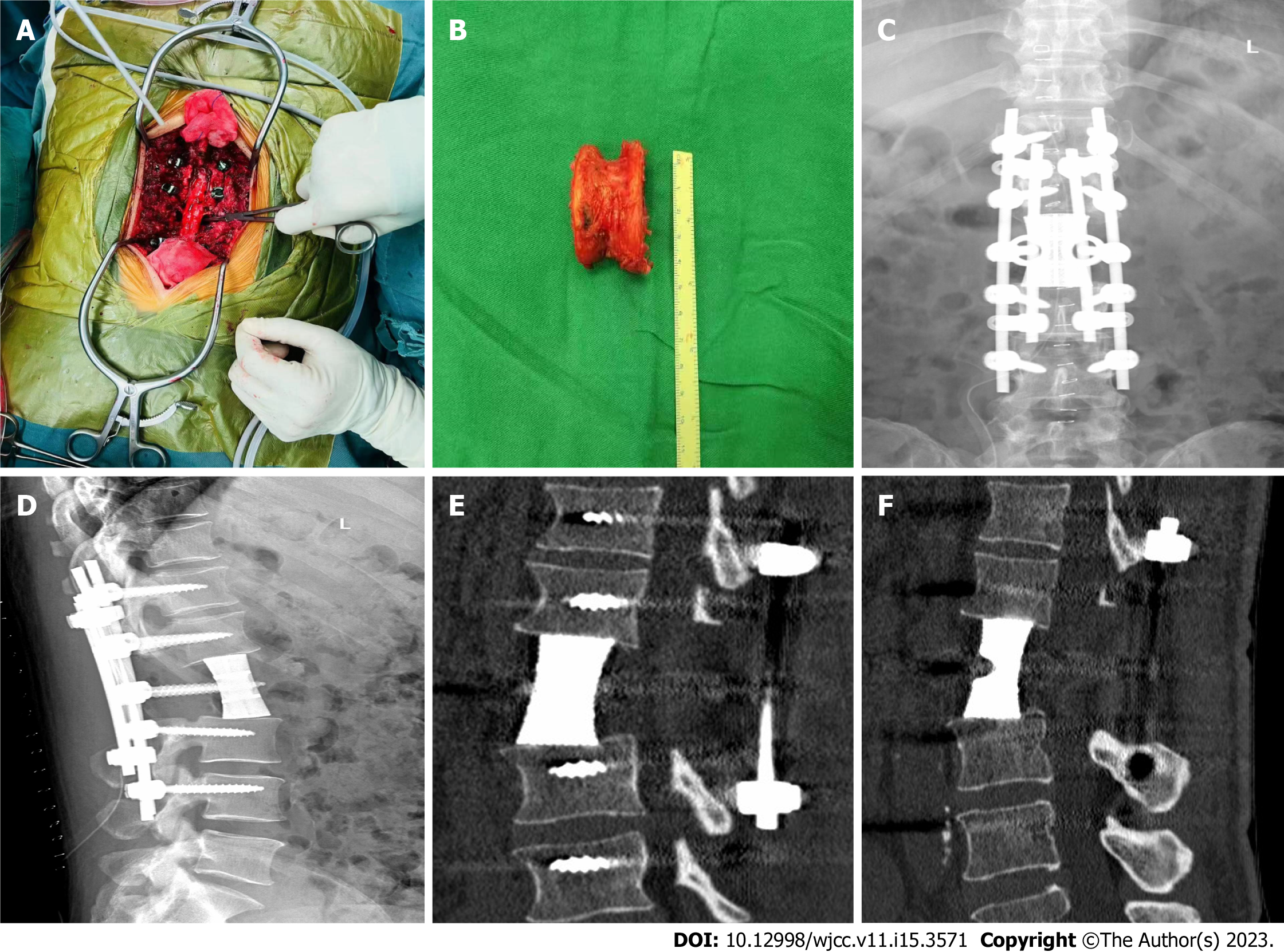
After admission, pertinent preoperative examinations were first improved, and after obtaining conclusive pathological findings, posterior lumbar tumor block resection, laminectomy, three-dimensional (3D) printed artificial vertebral body reconstruction, and nail-rod system internal fixation were performed (Figure 3).
The wound healed well 2 wk after the operation. The patient could move around on the ground with braces in good condition. He was transferred to the oncology department for comprehensive care, including chemotherapy regimens of albumin-paclitaxel, dacarbazine, nedaplatin, bevacizumab, and tereplizumab. Except for the side effects of chemotherapy, the patient is currently in good health.
MM is a highly malignant tumor caused by abnormal proliferation nerve spine melanocytes. This highly aggressive tumor primarily affects the skin mucosa and pigmented membranes. Lymphatic and blood metastases can occur in the early stages and are associated with a poor prognosis, with a 5-year survival rate of only 10%[5]. One study found that patients diagnosed with MM had a 24% higher risk of subsequent primary cancers, including breast cancer, prostate cancer and non-Hodgkin lymphoma, which may be due to genetic predisposition or environmental risk factors. However, most patients also develop MM in other sites, although the base may be thinner and less malignant[6]. MM can spread to any part of the body, but spinal metastasis is rare, with an incidence of only 2.4%[4]. However, MM with spinal metastasis as the first manifestation is even rarer[7]. If a patient’s biopsy reveals metastatic MM without primary lesions, metastatic MM of unknown origin is assumed. Several hypotheses have been proposed to explain the origin of MM with an unknown primary tumor, including spontaneous regression of the primary tumor and the presence of ectopic melanocytes in lymph nodes or internal organs. Although these hypotheses have only been tested in a few studies, preliminary evidence suggests a molecular basis for this disease[8]. Therefore, for patients who have been diagnosed with MM, we recommend lifelong follow-up and close clinical observation. This is especially important for the early detection of metastatic MM.
Imaging findings, in addition to performing a biopsy to confirm the pathology, are useful for the early detection of metastatic MM. Most melanoma bone metastases are osteolytic (87.5%), causing pathological fractures and soft tissue mass formation in approximately 22.5% and 12.5% of the patients[9]. MRI has significant advantages for the detection and diagnosis of spinal metastatic MM. Melanoma typically has a high signal intensity on T1WI and a low signal intensity on T2WI. According to Müller-Horvat et al[10], MRI has a higher detection rate for MM bone metastasis than CT, and could detect MM earlier in 14.6% of the patients. In addition, PET/CT showed that the patient had multiple slightly low-density nodular opacities in the liver. Therefore, combined contrast CT or MRI examination was recommended, but the patient refused further examination. Although cases of cystic liver metastases caused by MM are extremely rare, for patients with a relatively high degree of malignancy, further relevant liver tests can clarify the patient’s systemic condition and help develop appropriate treatment plans for them. Currently, few studies have described the radiographic findings of cystic liver metastases, and are mostly based on contrast-enhanced CT or contrast-enhanced MRI[11]. However, these techniques have low sensitivity and may not differentiate these metastases from other liver disorders[12]. Corvino et al[13] reported a case of rectal melanoma patient with cystic liver metastases detected using early liver contrast (CEUS). The technique supported a comprehensive and correct diagnosis, which led to an optimal treatment regimen for the patient. They believe that CEUS imaging can detect the liver-enhanced cyst wall and septum. In addition, the contrast agent enters the microvessels of the cyst wall and septum, and once destroyed, produces a high signal, which can be used to assess the internal structure of the cyst wall and cystic lesions. Using this approach, malignant lesions can be detected earlier, avoiding complications of invasive examinations and improving the accuracy of diagnosis.
Surgical resection is the first-line treatment option for neurological symptoms in spinal metastatic MM. Surgical resection and nerve decompression have been shown to improve neurological function, pain, and quality of life. With the improvement of surgical techniques, Tomita et al[14] proposed total en bloc spondylectomy, which can be used to treat primary spinal tumors and solitary spinal metastases for the following indications: (1) Primary lumbar malignant tumors in stage I and II in Enneking's surgical stage; (2) stage III benign aggressive tumor; and (3) isolated metastatic lumbar spine tumor, and expected survival time > 6 mo. Total vertebral body resection of spinal tumors is widely used by oncology surgeons because it ensures safe oncological boundaries and reduces tumor recurrence and metastasis.
In this report, patients with L2 vertebral metastatic MM were in a good general condition, with a Tomita score of 5 and a Tokuhashi score of 9, indicating an expected survival time > 6 mo, which met the indications for block resection. Here, we performed L2 total body resection and 3D-printed artificial vertebral body reconstruction surgery. The patient recovered well after surgery, and the numbness and muscle strength of both lower limbs were significantly improved. The rough surface and micropore design of 3D-printed prostheses not only increase osteoblast adhesion but also stimulate cell differentiation and promote bone growth. The porous structure allows tissue fluid to flow and promotes bone cell migration and proliferation[15]. Studies have shown that the best structures for bone integration have a pore size of 600–800 µm and porosity of 70%–80%, which are conducive to the recruitment of anti-inflammatory molecules, promotion of osteoblast differentiation, and cultivation of a microenvironment conducive to bone integration[16]. Three months after the operation, imaging revealed preliminary bone healing between the artificial vertebral body and adjacent vertebral body, indicating that the artificial vertebral body was biocompatible. Six months after the operation, a large number of bony connections were formed between the artificial and the adjacent vertebral bodies, and osseointegration occurred. This indicated that the artificial vertebral body could meet the requirements of interbody fusion and eventually achieve anterior column fusion.
Although surgical treatment can only improve the patients’ quality of life and relieve their pain symptoms, it does not appear to increase their survival rate. Currently, the clinical treatment for MM is based on surgical resection in conjunction with chemotherapy, radiotherapy, and immunotherapy. With advances in molecular biology technology and extensive research, drug therapy targeting key receptors and signal transduction kinases in the cancer process has been approved for clinical application, providing a new scheme for patients with MM. BRAF mutations account for 40%–50% of cases with MM. These gene mutations significantly increase the activity of the BRAF enzyme and continuously activate the signaling pathway of the targeted mitogen-activated protein kinase, leading to uncontrolled proliferation of tumor cells via various mechanisms, resulting in MM[17]. A multi-center controlled trial published in the New England Journal of Medicine used a BRAF inhibitor to treat MM, and the results were promising[18]. Furthermore, targeted drugs combined with immunotherapy are more likely to cause coordinated sensitization, play an early and powerful antitumor role, and achieve higher clinical efficacy compared with monotherapy. Experiments have shown that combining a BRAF/mitogen-activated protein kinase (MEK) inhibitor with a cytotoxic T lymphocyte antigen-4/programmed death-1 monoclonal antibody is more effective than either single-targeted therapy or immunotherapy[19]. Furthermore, whereas traditional radiotherapy and chemotherapy have low sensitivity and a poor curative effect, when combined with targeted therapy or immunotherapy, they have a stronger synergistic antitumor effect[20].
Indeed, immunotherapy for MM and targeted therapy with BRAF and MEK inhibitors have distinct advantages, but their potential side effects must also be considered. Increasing evidence suggests that the drugs used in MM therapy can produce more specific responses to MM cells and have less toxicity to organisms, leading to their wide use in various treatment schemes. However, there is currently no relevant, clear guideline for the treatment of patients with MM that demonstrates its curative effect. Therefore, medical professionals should collaborate and lead efforts to develop and apply MM therapy in clinical practice.
In this case report, we presented a rare case of MM with lumbar metastatic lesions as the first symptom. Diagnosing and treating such a rare case can be significantly challenging for surgeons. When pathology reveals that a patient has metastatic MM, surgical resection should be considered because it can effectively relieve symptoms and improve the patient’s quality of life. However, if the goal is to improve patient prognosis, surgery should be used with chemotherapy, vaccination, or targeted therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corvino A, Italy; Seledtsov V, Russia S-Editor: Liu JH L-Editor: A P-Editor: Chen YX
| 1. | Sacchetto L, Zanetti R, Comber H, Bouchardy C, Brewster DH, Broganelli P, Chirlaque MD, Coza D, Galceran J, Gavin A, Hackl M, Katalinic A, Larønningen S, Louwman MWJ, Morgan E, Robsahm TE, Sanchez MJ, Tryggvadóttir L, Tumino R, Van Eycken E, Vernon S, Zadnik V, Rosso S. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer. 2018;92:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 2. | Turner N, Ware O, Bosenberg M. Genetics of metastasis: melanoma and other cancers. Clin Exp Metastasis. 2018;35:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Gokaslan ZL, Aladag MA, Ellerhorst JA. Melanoma metastatic to the spine: a review of 133 cases. Melanoma Res. 2000;10:78-80. [PubMed] |
| 4. | Spiegel DA, Sampson JH, Richardson WJ, Friedman AH, Rossitch E, Hardaker WT Jr, Seigler HF. Metastatic melanoma to the spine. Demographics, risk factors, and prognosis in 114 patients. Spine (Phila Pa 1976). 1995;20:2141-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC Med. 2016;14:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Bradford PT, Freedman DM, Goldstein AM, Tucker MA. Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol. 2010;146:265-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Ku A, Henry A, Tunkel R, Lachmann E, Nagler W. Lumbosacral radiculopathy secondary to L5 metastatic melanoma of unknown primary. Arch Phys Med Rehabil. 1996;77:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Scott JF, Gerstenblith MR. Melanoma of Unknown Primary. In: Noncutaneous Melanoma [Internet]. Brisbane (AU): Codon Publications; 2018. [PubMed] |
| 9. | Patnana M, Bronstein Y, Szklaruk J, Bedi DG, Hwu WJ, Gershenwald JE, Prieto VG, Ng CS. Multimethod imaging, staging, and spectrum of manifestations of metastatic melanoma. Clin Radiol. 2011;66:224-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Müller-Horvat C, Radny P, Eigentler TK, Schäfer J, Pfannenberg C, Horger M, Khorchidi S, Nägele T, Garbe C, Claussen CD, Schlemmer HP. Prospective comparison of the impact on treatment decisions of whole-body magnetic resonance imaging and computed tomography in patients with metastatic malignant melanoma. Eur J Cancer. 2006;42:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Sugawara Y, Yamamoto J, Yamasaki S, Shimada K, Kosuge T, Sakamoto M. Cystic liver metastases from colorectal cancer. J Surg Oncol. 2000;74:148-152. [PubMed] [DOI] [Full Text] |
| 12. | Qian LJ, Zhu J, Zhuang ZG, Xia Q, Liu Q, Xu JR. Spectrum of multilocular cystic hepatic lesions: CT and MR imaging findings with pathologic correlation. Radiographics. 2013;33:1419-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Corvino A, Catalano O, Corvino F, Petrillo A. Rectal melanoma presenting as a solitary complex cystic liver lesion: role of contrast-specific low-MI real-time ultrasound imaging. J Ultrasound. 2016;19:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Tomita K, Kawahara N, Baba H, Tsuchiya H, Nagata S, Toribatake Y. Total en bloc spondylectomy for solitary spinal metastases. Int Orthop. 1994;18:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 271] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Yang J, Cai H, Lv J, Zhang K, Leng H, Wang Z, Liu Z. Biomechanical and histological evaluation of roughened surface titanium screws fabricated by electron beam melting. PLoS One. 2014;9:e96179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Olivares-Navarrete R, Hyzy SL, Slosar PJ, Schneider JM, Schwartz Z, Boyan BD. Implant materials generate different peri-implant inflammatory factors: poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine (Phila Pa 1976). 2015;40:399-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Ottaviano M, Giunta EF, Tortora M, Curvietto M, Attademo L, Bosso D, Cardalesi C, Rosanova M, De Placido P, Pietroluongo E, Riccio V, Mucci B, Parola S, Vitale MG, Palmieri G, Daniele B, Simeone E, On Behalf Of Scito Youth. BRAF Gene and Melanoma: Back to the Future. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat Rev Drug Discov. 2011;10:811-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Cohen-Inbar O, Shih HH, Xu Z, Schlesinger D, Sheehan JP. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg. 2017;127:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Fusi A, Dalgleish A. The importance for immunoregulation for long-term cancer control. Future Oncol. 2017;13:1619-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |