Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3552
Peer-review started: October 31, 2022
First decision: February 14, 2023
Revised: February 25, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: May 26, 2023
Processing time: 206 Days and 2.4 Hours
Immune-mediated necrotizing myopathy is a rare autoimmune myopathy characterized by muscle weakness and elevated serum creatine kinase, with unique skeletal muscle pathology and magnetic resonance imaging features.
In this paper, two patients are reported: One was positive for anti-signal recognition particle antibody, and the other was positive for anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibody.
The clinical characteristics and treatment of the two patients were analysed, and the literature was reviewed to improve the recognition, diagnosis, and treatment of this disease.
Core Tip: This paper describes two patients with different types of immune-mediated necrotizing myopathy, and compares the clinical features, imaging features, muscle pathology, and treatment of the two types in the discussion section, with an aim to further improve clinicians' understanding of the disease.
- Citation: Chen BH, Zhu XM, Xie L, Hu HQ. Immune-mediated necrotizing myopathy: Report of two cases. World J Clin Cases 2023; 11(15): 3552-3559
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3552.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3552
Immuno-mediated necrotizing myopathy (IMNM) is a muscle-specific autoimmune disease characterized by muscle weakness and elevated serum creatine kinase (CK). The 224th International Symposium of the European Neuromuscular Centre (ENMC) divided IMNM into three subtypes: Anti-SRP antibody-positive, anti-3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) antibody-positive, and antibody-negative necrotizing myopathy[1]. The clinical characteristics of different types of IMNMs are different. In this paper, we report one case positive for anti-SRP antibody and one case positive for anti-HMGCR antibody, and compare their characteristics.
Case 1: A 42-year-old woman was admitted to the hospital on November 18, 2020, due to the weakness of both lower limbs accompanied by walking instability for 3 mo and worsening for 1 mo.
Case 2: A 49-year-old woman was admitted to the hospital on January 20, 2021, with limb weakness for 10 years and aggravation for 2 years.
Case 1: Three months prior to admission, after wading, the patient developed weakness of both lower limbs, walking instability, and a sense of stepping on cotton. Two months prior, a computed tomography (CT) examination of the lumbar spine in another hospital showed lumber spine (L)4/5 and L5/sacrum (S)1 disc herniation and L4/5 spinal canal stenosis. The diagnosis and treatment were unknown, and the symptoms were not alleviated. One month prior, the weakness of both lower limbs worsened, walking was difficult, and climbing stairs was difficult. Cervical CT in another hospital showed cervical spine (C)4/5/6 disc herniation with spinal stenosis and dural sac compression. For further treatment, she was admitted to our department with myopathy.
Case 2: Ten years prior to admission, the patient was unable to hold heavy objects for no obvious reason, accompanied by muscle pain in the proximal upper limbs after exercise. Nearly 2 years prior, she could not lift her bilateral upper limbs, climbing stairs was difficult, and she was easily fatigued, squatting after the difficulty of getting up. For further treatment, she was admitted to our department with myopathy.
Neither patient had a history of past illness.
Neither patient had a personal or family history of similar diseases.
Case 1: Vital signs were stable, and cardiopulmonary and abdominal physical examinations were normal. Physical examination of the nervous system showed clear consciousness and normal advanced intelligence. Examination of the motor system revealed generally normal muscle volume, grade IV proximal muscle strength and grade V distal muscle strength of both upper limbs, grade III proximal muscle strength and grade IV distal muscle strength of both lower limbs, normal muscle tension of the extremities, slightly duck walk gait, and positive Gower sign. There were weakened tendon reflexes of the extremities and negative pathological signs of both lower limbs. The other cranial nerve and meningeal stimulation reflexes were normal.
Case 2: Vital signs were stable, and cardiopulmonary and abdominal physical examinations were normal. Physical examination of the nervous system showed clear consciousness and normal advanced intelligence. Examination of the motor system revealed approximately normal muscle volume, weak head lift, grade IV proximal and grade V distal upper limb muscle strength, grade III proximal and grade V distal lower limb muscle strength, normal muscle tone, normal ataxia movement, slightly duck walk gait, and positive Gower sign. There were weakened tendon reflexes of the extremities and negative pathological signs of both lower limbs. The other cranial nerve and meningeal stimulation reflexes were normal.
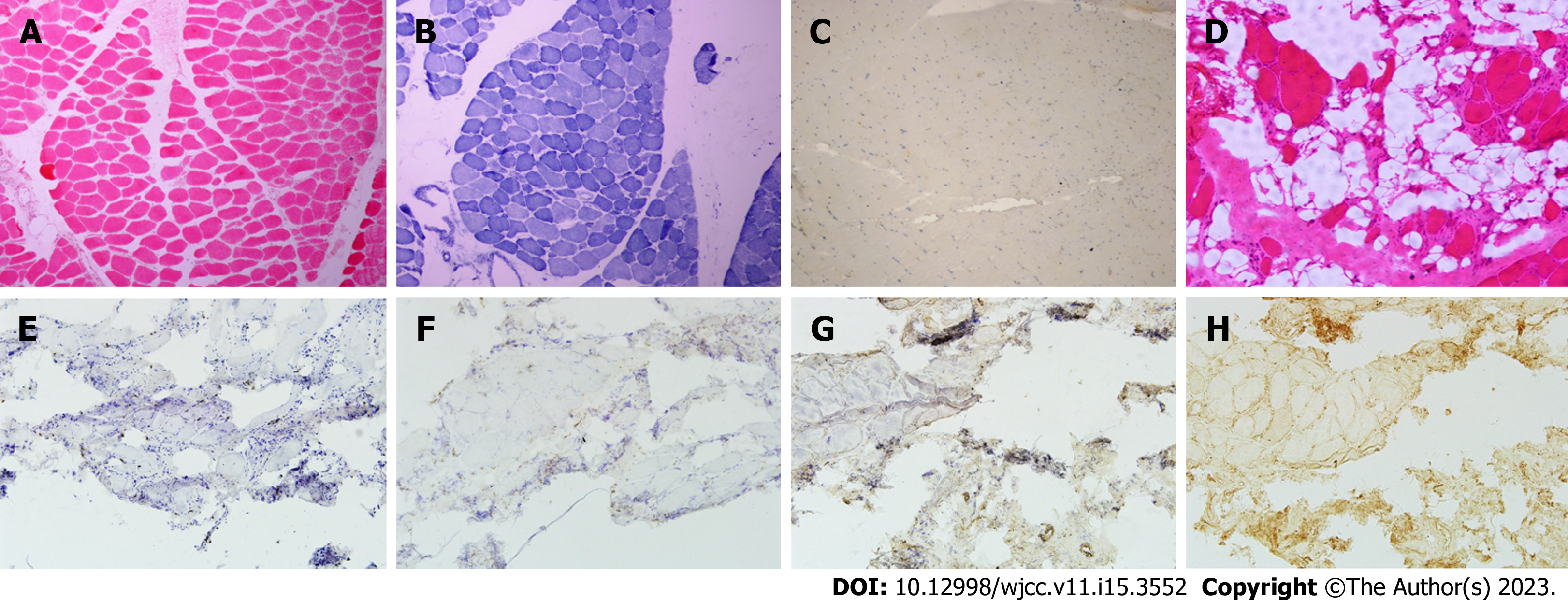
Case 1: The laboratory examination findings were as follows: Erythrocyte sedimentation rate 25 mm/h, alanine aminotransferase (ALT) 125 U/L, aspartate aminotransferase (AST) 101 U/L, CK 4915 U/L, lactate dehydrogenase (LDH) 751 U/L, CK-myocardial isoenzyme (CK-MB) 424 U/L, alpha-hydroxybutyrate dehydrogenase 508 U/L, and neuron-specific enolase 25.89 μg/L. The residual thyroid function, ferritin, vitamin B12, folic acid, erythropoietin immunoglobulin and light chain, complement, C-reactive protein, rheumatoid factor, antinuclear antibody spectrum, and tumour anti-nervous system antibody spectrum were normal. The myositis antibody spectrum was: Anti-SRP antibody (+++) and anti-Ro-52 antibody (++). Electromyography showed increased polyphasic waves of motor unit potential in the left biceps brachii and bilateral tibialis anterior muscles. On the pathological biopsy of the left anterior tibial muscle, hematoxylin-eosin staining showed multiple muscle bundles; the muscle fibre sizes were significantly different, including more small round muscle fibres and scattered necrotic and degenerative muscle fibres; and reduced coenzyme I - tetrazolium reductase (NADH) staining showed disorder of some myofibrillar meshwork, while modified gomori trichrome (MGT), oil red O (ORO), and periodic acid Schiff (PAS) staining showed no obvious abnormalities. Immunohistochemistry showed no significant abnormalities in cluster of differentiation (CD)8, CD20, CD68, major histocompatiblility complex (MHC)-1, or Dystrophin (Figure 1).
Case 2: The laboratory examination findings were as follows: ALT 60 U/L, AST 44 U/L, r-glutamyl transferase 51 U/L, CK 1153 U/L, CK-MB 71 U/L, LDH 311 U/L, high density lipoprotein cholesterol 0.95 mmol/L, and free thyroxine 15.16 pmol/L, and routine blood tests, coagulation, infectious disease, tumour markers, and anaemia markers were normal. Electromyography showed myogenic damage to the left biceps brachii and quadriceps femoris and myogenic damage to the right tibialis anterior muscle. Electrocardiogram, cardiac ultrasound, and abdominal ultrasound showed no obvious abnormalities. Thyroid ultrasonography showed thyroid nodules. Biopsy was performed on the left biceps brachii muscle. Histological examination showed a large number of vacuolated muscle fibres, scattered small groups of muscle fibres, significantly different sizes of muscle fibres, round shapes of muscle fibres, a large number of denaturetic muscle fibres, some regenerated muscle fibres, scattered hypertrophic muscle fibres, and significantly widened interstitial muscle, and no inflammatory cell infiltration was observed around the blood vessels. NADH staining showed that the two types of muscle fibres were poorly differentiated, and no special changes were observed in MGT, ORO, or PAS staining. Genetic testing did not detect pathogenic/suspected pathogenic variants clearly associated with the clinical phenotype. Immunohistochemical staining of muscle tissue showed that MHC-1 was expressed in many myofiber membranes, membrane attack complex was positively expressed in some myofiber membranes, and a small number of CD3- and CD68-positive cells infiltrated the endomysium (Figure 1). Myositis antibody spectrum detection showed anti-HMGCR antibody (+).
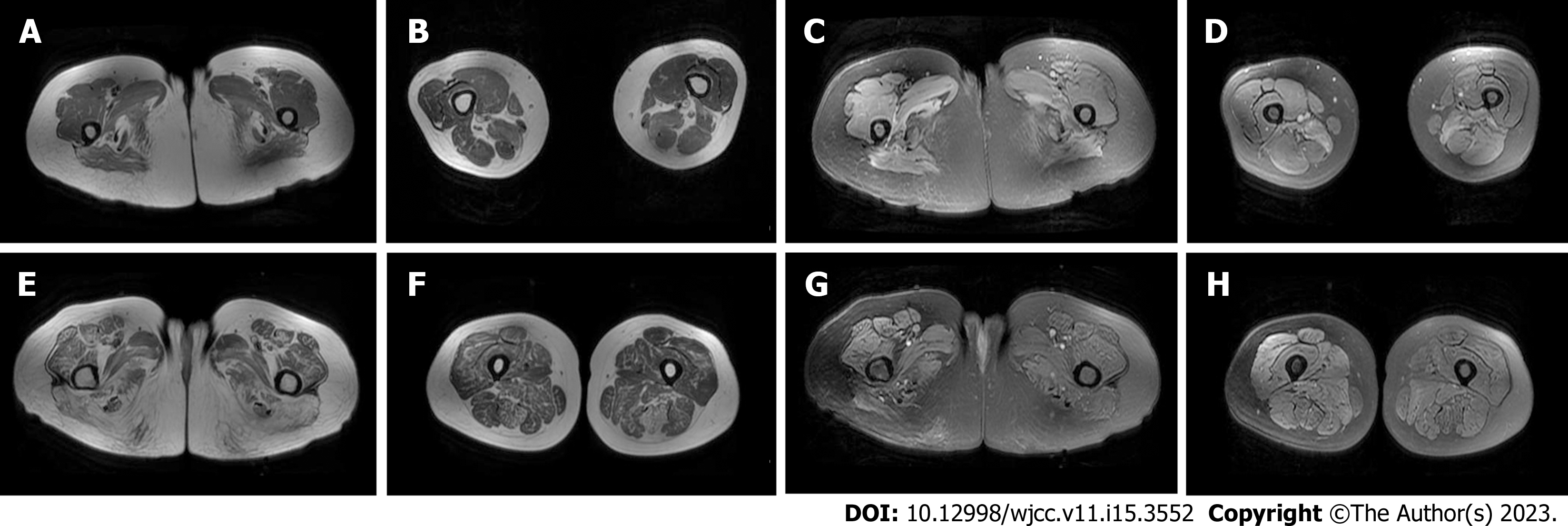
Case 1: Chest CT showed localized fibrous foci in both lungs. Magnetic resonance imaging (MRI) of both lower limbs showed abnormal changes in some muscles of both thighs and calves (Figure 2).
Case 2: Chest CT showed small benign nodules in the upper lobe of the right lung and localized fibrous foci in both lungs. MRI of both lower limbs and the lumbar vertebrae showed abnormal morphology and signal of bilateral psoas major muscles, erector spinae muscles, gluteus muscles, and bilateral quadriceps femoris muscles, as well as lumbar degeneration and L5 and S1 end laminitis (Figure 2).
Case 1: Anti-SRP antibody-positive necrotizing myopathy.
Case 2: Anti-HMGCR antibody-positive necrotizing myopathy.
Case 1: High-dose hormone shock therapy (500 mg, 240 mg, and 120 mg methylprednisolone intravenously for 3 d each, then oral prednisolone 60 mg, gradually reduced to a 30 mg maintenance dose).
Case 2: High-dose hormone shock therapy (500 mg, 240 mg, and 120 mg methylprednisolone intravenously for 3 d each, then oral prednisolone 60 mg, gradually reduced to a 30 mg maintenance dose).
After treatment, the patients' limb muscle strength improved.
The aetiology of IMNM is not completely clear, and it is believed to be related to genetic, environmental, tumour, and other factors[2,3]. IMNM is related to immunogenetic predisposition factors; for example, adult patients positive for anti-HMGCR antibodies are related to human leucocyte antigen-DRB1* 11:01, and the risk of positivity for anti-SRP antibodies is related to the HLA-DRB1* 08:03 allele[2]. Environmental risk factors are often related to statins and viral infections. Initially, necrotizing myopathy with positivity for anti-HMGCR antibody was considered to be related to statins. However, the studies by Alshehri et al[4] and Watanabe et al[3] showed that myopathy associated with HMGCR antibody should not be called "statin myopathy". The production of anti-HMGCR antibodies is not directly related to statin treatment. Pinal-Fernandez et al[2] studied IMNM and showed that the 54-kDa subunit and HMGCR protein of SRP had homologous regions with the proteins of varicella zoster virus and human papillomavirus type 58, respectively, and viral infection could lead to autoimmune abnormalities through molecular mimicry. Shimizu et al[5] also reported a case of anti-HMGCR antibody-positive myopathy after acute Epstein-Barr virus infection in 2020. Arouche-Delaperche et al[6] found in their study on the pathogenic mechanism of IMNM that anti-SRP- and anti-HMGCR-specific antibodies bind to intracellular antigens, induce muscle fibre atrophy through the complement pathway, and impair myoblast fusion to affect myocyte regeneration. In this process, macrophages release proinflammatory molecules, such as interleukin (IL)-6, tumor necrosis factor, and reactive oxygen species, and a decrease in two anti-inflammatory cytokines (IL-4 and IL-13) also inhibits myoblast fusion. Antigen-antibody binding can also trigger nonimmune factors, such as amyotrophic F-box protein and E3 ubiquitin-protein ligase TRIM63, to induce muscle atrophy[7].
More than two-thirds of IMNM patients have acute or subacute onset, and the main clinical manifestation is proximal limb weakness, but patients with anti-HMGCR antibody positivity progress more slowly and have less weakness than those with anti-SRP antibody positivity. The incidence of myalgia, dysphagia, interstitial pneumonia, pericarditis, and rash was higher in patients with positive anti-SRP antibody than in those with positive anti-HMGCR antibody. In patients with positive anti-SRP antibody, the neck and scapular girdle are more obviously affected than the lower limb muscle group, and winglike scapula may appear[8]. The risk of malignant tumours in patients positive for anti-SRP antibodies is not increased, while it is slightly increased in patients positive for anti-HMGCR antibodies[7]. The remaining patients have a slow onset, with clinical characteristics similar to those of limb-girdle muscular dystrophy[8]. Among 11 patients diagnosed with limb-girdle muscular dystrophy, Mohasse et all[9] detected 6 patients positive for anti-HMGCR antibodies. These patients usually had a very chronic disease course characterized by elevated CK levels without obvious symptoms of weakness. In this report, Case 1 had rapid disease progression and severe symptoms, while Case 2 had insidious-onset, slow progression, and mild weakness symptoms compared with SRP patients. Neither patient had extramuscular clinical manifestations.
The serological manifestations of IMNM patients are mainly increased CK, and CK levels are in direct proportion to the degree of muscle destruction[2]. Yang et al[10] showed that there was no statistically significant difference in the severity of muscle weakness or CK elevation in patients positive for anti-SRP antibody and anti-HMGCR antibody. Patients with IMNM usually undergo muscle MRI of the proximal segment of the lower limbs. The T1WI sequence can reflect the situation of fatty infiltration, and this part of muscle damage is irreversible. The short time of inversion recovery (STIR) sequence can reflect active muscle oedema associated with inflammation or muscle fibre necrosis, which can be reversed by treatment. The 12 patients positive for anti-SRP antibodies studied by Zheng et al[11] all had fatty infiltration and oedema. Oedema mainly occurred in the anterior external and posterior thigh muscles, while fatty infiltration mainly occurred in the medial and posterior thigh muscles. In the study by Mohassel et al[9], among the 6 patients positive for anti-HMGCR antibody, the T1WI sequence signal of thigh or calf muscles in patients with a short course of disease showed little change, while the T1WI sequence signal intensity of the paravertebral muscle, gluteus muscle, and adductor muscle in patients with a long course of disease was high, and the thin femoris muscle was relatively preserved. The STIR sequence signal could be inhomogeneous and patently enhanced. The two patients reported in this case underwent MRI examination. In Case 1, oedema was mainly in the posterior thigh muscle group, and fat infiltration was mainly in the medial thigh muscle group and the posterior thigh muscle group. In Case 2, oedema was found in the right lower extremity, mainly concentrated in the anterior external and posterior thigh muscles. Due to the long course of disease, the lower extremity muscles had different degrees of fat infiltration, the posterior muscles were more obvious, the quadriceps femoris and adductor magnus muscles were less severe, and the gracilis muscle was relatively preserved. In anti-SRP antibody-positive cases, muscle atrophy progresses faster due to inhibition of muscle regeneration, which tends to be more obvious than that in anti-HMGCR antibody-positive cases[12]. However, HMGCR may lead to more obvious fat infiltration observed clinically due to the lack of obvious symptoms and long course of disease. The chronic course of HMGCR needs to be differentiated from limb-girdle muscular dystrophy. In the former, skeletal muscle fat infiltration and oedema are common in the posterior thigh muscles, while in the latter, skeletal muscle fat infiltration is mainly seen in the anterior and posterior muscles, and muscle oedema is mainly seen in the anterior muscles[13].
The histological characteristics of patients positive for anti-SRP and anti-HMGCR antibodies were irregular, including enlarged or aggregated abnormal nuclei in nonnecrotic muscle fibres, necrosis and regeneration of the remaining muscle fibres in different stages, and little or no endomysial lymphocyte infiltration[3]. Patients positive for anti-SRP and anti-HMGCR antibodies usually had multifocal upregulation (approximately 50%) of MHC-1 and membrane attack complex (MAC) in the presence of sarcolemmal deposition (20%-50%) of nonnecrotic muscle fibres. MAC deposition was more common in nonnecrotic fibrosarcolemmas in patients with anti-HMGCR antibodies than in patients positive for anti-SRP antibodies[2]. In our patients, denatured and necrotic muscle fibres were mainly observed in Case 1, and no obvious abnormalities were observed by immunohistochemistry. In Case 2, a large number of necrotic and regenerated muscle fibres were observed, and the deposition of MHC-1 and MAC on the myocyte membrane was observed.
In the diagnosis of this disease, the detection of the myositis antibody profile is particularly important. Both anti-SRP and anti-HMGCR autoantibody titers are correlated with muscle strength and serum CK levels[7]. Anti-HMGCR autoantibodies are highly specific and have not been found in patients with other related diseases[9]. The specificity of anti-SRP antibody is inferior to that of anti-HMGCR antibody, which can also be detected in patients with systemic sclerosis and rheumatoid arthritis, but no myasthenia symptoms occur[14]. In IMNM, myositis-associated autoantibodies (MSA) can appear combined with anti-SRP antibodies, such as anti-Ro antibodies and anti-LA antibodies, to form overlapping myositis[15]. When combined with anti-RO-52 antibody, patients often present with interstitial lung disease[16]. Because of the different characteristics of different antibodies, patients positive for anti-HMGCR antibodies should be screened for cancer, while patients positive for anti-SRP antibodies should be screened for myocarditis. In this article, the first patient had typical clinical symptoms and laboratory tests and was clearly diagnosed with SRP-positive resistant necrotic myopathy by a myositis antibody spectrum test. In the second patient, the disease progressed slowly and had a long course. Muscle MRI indicated that fat infiltration and myopathic muscle atrophy were relatively serious. At first, it was suspected that the disease was limb-girdle muscular dystrophy. However, no abnormality was found in the genetic test of the patient. After improving the muscle immunohistochemistry, the patient was finally diagnosed with necrotizing myopathy with positivity for HMGCR antibody.
The consensus statement issued at the ENMC workshop[1] stated that IMNMs should be treated with both corticosteroids and immunosuppressants within 1 mo of the first presentation and proposed methotrexate as the initial immunosuppressant for IMNMs. If an adequate response is not observed within 6 mo of treatment, intravenous immunoglobulin may be added to the treatment of patients positive for anti-HMGCR antibodies. Allenbach et al[7] suggested that rituximab could be used instead of methotrexate in the treatment of anti-SRP antibody-positive patients, but rituximab had no obvious effect on anti-HMGCR antibody-positive patients. The study by Allenbach et al[7] on the prognosis of IMNM showed that after receiving immunotherapy, two-thirds of the patients positive for anti-HMGCR antibody over age 60 improved their symptoms within 4 years, and only half of the patients under age 50 recovered normal muscle strength, which was consistent with the results of a study by Tiniakou et al[17]. Younger patients with HMGCR-associated autoimmune myopathy have more severe disease and slower recovery. Patients positive for anti-SRP antibodies also have a poor prognosis, with only half achieving near full or full muscle strength after 4 years of immunotherapy. In the follow-up of patients, it is necessary to pay attention to CK levels, and it is not recommended to upgrade immunotherapy regimens for patients with normal CK[2]. MRI can also assess the activity of the disease. If there is continued STIR sequence hyperintensity, intensive immunotherapy is needed. IMNM requires long-term immunotherapy. During treatment, serum CK levels and muscle MRI should be reviewed to clarify further treatment plans.
In conclusion, after serum CK, muscle magnetic resonance, and muscle pathological examination of IMNM to identify the disease type, myositis antibody spectrum detection should be performed to confirm the diagnosis, immunotherapy should be started as soon as possible, patients should undergo immunotherapy for a long time, and the treatment plan should be adjusted according to changes in the patient’s condition during follow-up.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabaghi GG S-Editor: Cai YX L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Allenbach Y, Mammen AL, Benveniste O, Stenzel W; Immune-Mediated Necrotizing Myopathies Working Group. 224th ENMC International Workshop:: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14-16 October 2016. Neuromuscul Disord. 2018;28:87-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 355] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 2. | Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-Mediated Necrotizing Myopathy. Curr Rheumatol Rep. 2018;20:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 3. | Watanabe Y, Uruha A, Suzuki S, Nakahara J, Hamanaka K, Takayama K, Suzuki N, Nishino I. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry. 2016;87:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Alshehri A, Choksi R, Bucelli R, Pestronk A. Myopathy with anti-HMGCR antibodies: Perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm. 2015;2:e124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Shimizu T, Kondo Y, Kanazawa N, Kaneko A, Tominaga N, Nagai M, Iizuka T, Nishino I, Nishiyama K. Anti-HMGCR myopathy following acute Epstein-Barr virus infection. Muscle Nerve. 2020;61:E5-E8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Arouche-Delaperche L, Allenbach Y, Amelin D, Preusse C, Mouly V, Mauhin W, Tchoupou GD, Drouot L, Boyer O, Stenzel W, Butler-Browne G, Benveniste O. Pathogenic role of anti-signal recognition protein and anti-3-Hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: Myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann Neurol. 2017;81:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Allenbach Y, Benveniste O, Stenzel W, Boyer O. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol. 2020;16:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 8. | Kurashige T. Anti-HMGCR myopathy: clinical and histopathological features, and prognosis. Curr Opin Rheumatol. 2021;33:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Mohassel P, Landon-Cardinal O, Foley AR, Donkervoort S, Pak KS, Wahl C, Shebert RT, Harper A, Fequiere P, Meriggioli M, Toro C, Drachman D, Allenbach Y, Benveniste O, Béhin A, Eymard B, Lafôret P, Stojkovic T, Mammen AL, Bönnemann CG. Anti-HMGCR myopathy may resemble limb-girdle muscular dystrophy. Neurol Neuroimmunol Neuroinflamm. 2019;6:e523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Yang HX, Tian XL, Jiang W, Li WL, Liu QY, Peng QL, Wang GC, Lu X. [Clinical and pathological characteristics of immune mediated necrotizing myopathy]. Beijing Da Xue Xue Bao Yi Xue Ban. 2019;51:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Zheng Y, Liu L, Wang L, Xiao J, Wang Z, Lv H, Zhang W, Yuan Y. Magnetic resonance imaging changes of thigh muscles in myopathy with antibodies to signal recognition particle. Rheumatology (Oxford). 2015;54:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Saito Y, Nishino I. [Clinicopathological Features of Myositis and Necrotizing Myopathy: How to Distinguish between Myositis and Muscular Dystrophy on Muscle Pathology]. Brain Nerve. 2021;73:147-159. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Zhao YW, Wang YL, Wang ZX, Zhang Y, Yuan Y. [Clinical and imaging differences between limb-girdle muscular dystrophy type 2B and immune-mediated necrotizing myopathy]. Zhonghua Xiandai Shenjing Jibing Zazhi. 2020;9:773-778. [DOI] [Full Text] |
| 14. | Carvalho AAS, Silva VGD, Vieira TF, Delgado PO, Corazini R, Feder D, Fonseca FLA. Proposed cut-off for reactivity of anti-HMGCR and anti-SRP antibodies in patients statin-exposed and statin-unexposed. Medicine (Baltimore). 2018;97:e11858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Mammen AL. Autoimmune myopathies: autoantibodies, phenotypes and pathogenesis. Nat Rev Neurol. 2011;7:343-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Benveniste O, Stenzel W, Allenbach Y. Advances in serological diagnostics of inflammatory myopathies. Curr Opin Neurol. 2016;29:662-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Tiniakou E, Pinal-Fernandez I, Lloyd TE, Albayda J, Paik J, Werner JL, Parks CA, Casciola-Rosen L, Christopher-Stine L, Mammen AL. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford). 2017;56:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |