Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3533
Peer-review started: October 13, 2022
First decision: January 30, 2023
Revised: March 6, 2023
Accepted: April 13, 2023
Article in press: April 13, 2023
Published online: May 26, 2023
Processing time: 191 Days and 2 Hours
Adult neuronal ceroid lipofuscinosis (ANCL) can be caused by compound heterozygous recessive mutations in CLN6. The main clinical features of the disease are neurodegeneration, progressive motor dysfunction, seizures, cognitive decline, ataxia, vision loss and premature death.
A 37-year-old female presented to our clinic with a 3-year history of limb weak
There is presently no effective treatment for ANCL. However, early diagnosis and symptomatic treatment are possible.
Core Tip: Adult neuronal ceroid lipofuscinosis (NCL) is a rare neurodegenerative disease that can be caused by mutations in the CLN6 gene. Our patient experienced limb weakness and unstable walking. Whole exome sequencing and Sanger sequencing revealed that the patient had a recessive compound heterozygous mutation in CLN6. The mutation sites are novel and contribute to the knowledge of mutations causing NCL. Although there is no curative treatment for NCL, early diagnosis and symp
- Citation: Wang XQ, Chen CB, Zhao WJ, Fu GB, Zhai Y. Rare adult neuronal ceroid lipofuscinosis associated with CLN6 gene mutations: A case report. World J Clin Cases 2023; 11(15): 3533-3541
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3533.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3533
Neuronal ceroid lipofuscinosis (NCL) is a group of genetically heterogeneous neurodegenerative diseases. NCL is primarily caused by a genetic defect in the processing of proteases by lysosomes, resulting in intralysosomal storage of ceroid lipofuscin, which affects nerve function. The disease is more common in children and rare in adults. The age of onset is typically before 30 years. The incidence of the disease varies by region. In European and American countries, the incidence is 0.1-7.0/100000[1].
The main clinical features of the disease are neurodegeneration, progressive motor dysfunction, seizures, cognitive decline, ataxia, vision loss and premature death[2]. NCL is divided into four categories according to clinical manifestations and age of onset, as follows: Infantile NCL (6-24 months old); late infantile NCL (2-4-years-old); juvenile NCL (5-10-year-old); and adult NCL (ANCL) (over 18-years-old)[3]. Thirteen different NCL subtypes have been found to be associated with mutations in 13 different genes (CLN1-CLN8 and CLN10-CLN14)[4]. Each gene mutation leads to a specific subtype of the disease, and the protein products of these genes (CLN1-CLN14) differ in their function and intracellular localization. NCL-related proteins are localized to lysosomes (CLN1, CLN2, CLN3, CLN5, CLN7, CLN10, CLN12, CLN13), the endoplasmic reticulum (CLN6, CLN8) or the cytosol associated with vesicle membranes (CLN4, CLN14). Some of the proteins are lysosomal soluble proteins [e.g., CLN1 (palmitoyl protein thioesterase 1), CLN2 (tripeptidyl peptidase 1), CLN5, CLN10 (cathepsin D), CLN13 (cathepsin F)], and others have been proposed as lysosomaltransmembrane proteins (e.g., CLN3, CLN7, CLN12)[5,6].
The CLN6 subtype of ANCL is a primarily autosomal recessive neurodegenerative disorder. CLN6 encodes an endoplasmic reticulum nonglycosylated transmembrane protein involved in lysosomal acidification. Mutations in CLN6 have been linked to late infantile NCL, juvenile NCL and ANCL (also known as Kufs disease)[2]. Individuals affected by this disease have two identical (homozygous) or two different (compound heterozygous) CLN6 mutant alleles. Due to the lack of information on the physiological role of CLN6, the pathogenesis of the disease is currently unclear[7].
A rare form of ANCL is caused by variants in CLN6, with symptoms normally presenting in adulthood after the age of 30 years. The typical symptoms include ataxia, epilepsy and progressive cognitive function decline, and usually without vision loss. Adults with this disorder usually do not live more than 10 years after diagnosis[2]. We present here the first case of CLN6 subtype ANCL with novel mutations in CLN6 in a Chinese patient.
A 37-year-old female was admitted to the hospital on May 12, 2021 due to limb weakness and walking instability for 3 years.
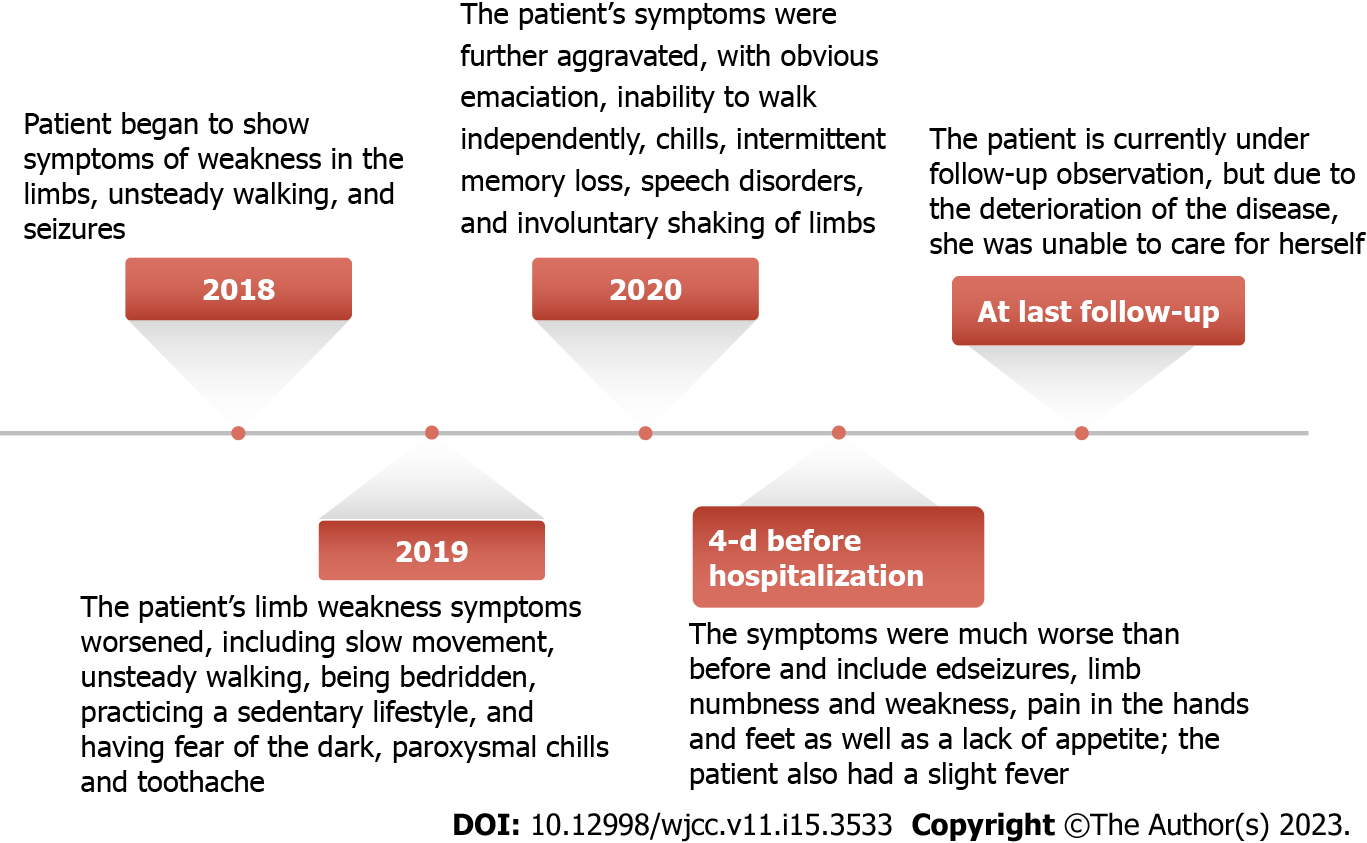
Three years prior to admission, the patient began to develop limb weakness and walking instability without obvious inducement. She reported an episode in which she became clouded in mind and fell to the ground, which was accompanied by limb stiffness, convulsions and upturned eyelid. The symptoms were alleviated after 3-5 min, and she did not experience dizziness, headache, nausea or vomiting. Atonic-clonic seizure was diagnosed.
Two years prior to admission, the limb weakness worsened. The patient reported slow movement, unstable walking, bedridden status, fear of the dark, paroxysmal chills and toothache. There was no memory loss or unconsciousness disorder. The family members were sent to another hospital for hospitalization. At that time, “brain atrophy” was considered.
One year prior to admission, the patient’s symptoms again worsened, with obvious emaciation, chills, intermittent memory loss, speech disorder, and the inability to walk independently. Ataxia and involuntary limb shaking were present. She was admitted to another hospital, but her symptoms did not improve significantly by the time she was discharged.
Four days prior to admission, her symptoms had worsened significantly (Figure 1). The patient reported numbness in the left lower limb below the knee and the right upper limb fingers and wrist joint, recurrent seizures, hand and foot pain, decreased appetite and a high temperature of 37.8 °C. The patient did not experience nausea, vomiting or incontinence.
The patient denied having a history of hypertension, diabetes, heart disease, infectious disease and food and drug allergies. The patient’s vaccination history was unknown.
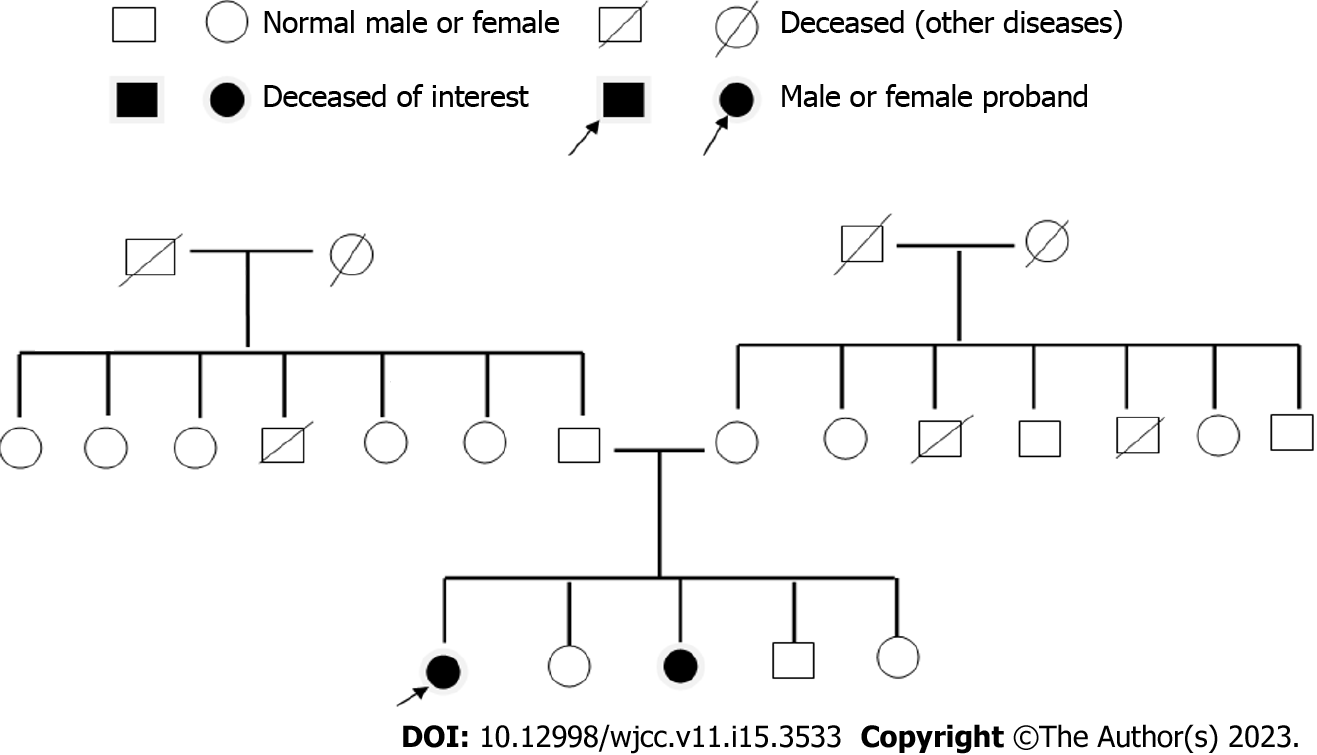
The patient was a life-long resident of the area and denied any long-term exposure to radioactivity, poisons or drugs. She was legally married and had a daughter. Both her husband and daughter were in good health. The patient and her family denied any disease-related family history. However, her sister had a similar history of walking instability, photophobia, seizures and poor memory.
Physical examination of the patient showed poor orientation to the surrounding environment, poor memory and a decline in comprehension capacity and numeracy. She had tremor of tongue and high limb muscle tension, and the limb muscle strength was level 4. Superficial sense hypoesthesia of the left lower extremity below the knee and the right upper extremity finger and wrist joints and paresthesia of deep sensation and combined sensation accompanied by involuntary tremors in the extremities were observed. We noted bilateral ankle clonus (+), detection of ataxia (+) and left side pathological sign (+).
Routine blood work revealed white blood cell count of 8.09 × 109/L (normal range: 3.5-9.5 × 109/L), neutrophil percentage of 76.8% (normal range: 50%-70%), hemoglobin of 144 g/L (normal range: 113-151 g/L) and platelets of 221 × 109/L (normal range: 100-300 × 109/L). No abnormalities were found for hypersensitive C-reactive protein, calcitonin, blood culture, bacteria, acid-fast bacilli, fungi and ink stain. No abnormalities were found in the routine stool and urine tests. Potassium levels were 3.34 mmol/L (normal range: 3.5-5.3 mmol/L). Liver and kidney function, heart function and blood lipids were normal. Tumor indexes and immune indexes were not abnormal. No abnormality was found in thyroid function, rheumatism, tuberculosis antibody and the antinuclear antibody spectrum. The patient was negative for hepatitis B, hepatitis C, human immunodeficiency virus and syphilis. Blood coagulation function, trace elements, N-terminal brain natriuretic peptide and troponin were normal. The intracranial pressure was 110 mmH2O. Routine examination of cerebrospinal fluid showed it to be colorless and clear, with normal cell counts and biochemistry.
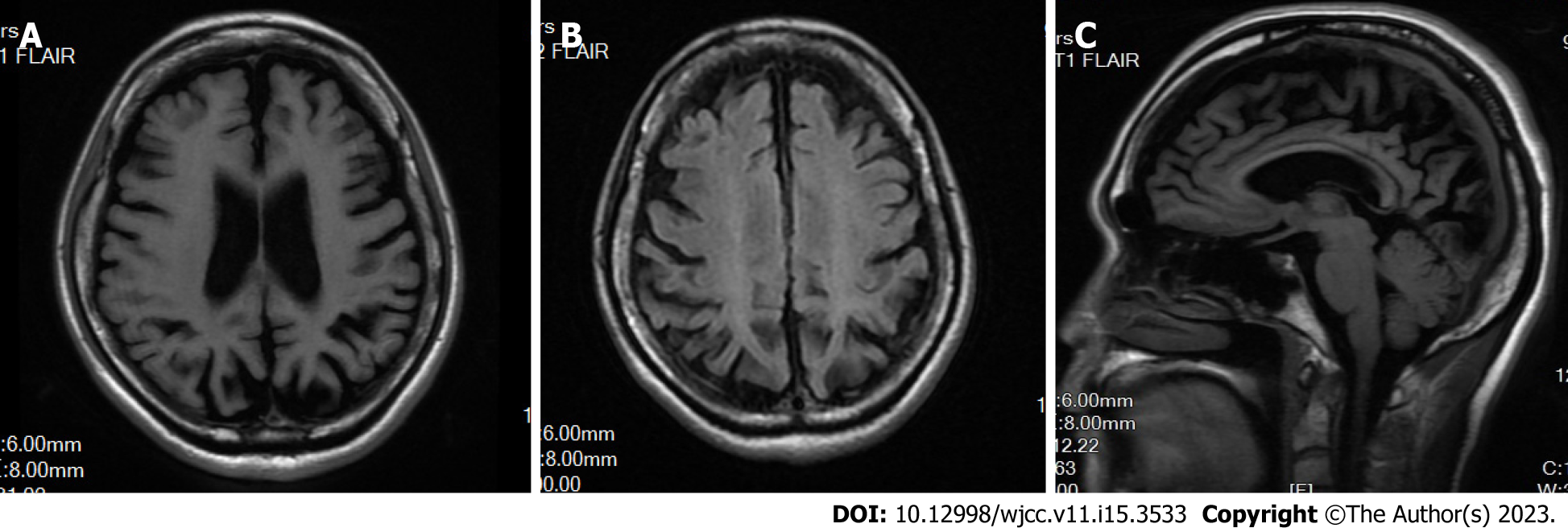
Brain magnetic resonance imaging (MRI) and magnetic resonance angiography revealed leukoaraiosis and brain atrophy. No cerebral infarction was observed. Cerebral atherosclerosis was considered, and no obvious vascular stenosis was observed (Figure 2).
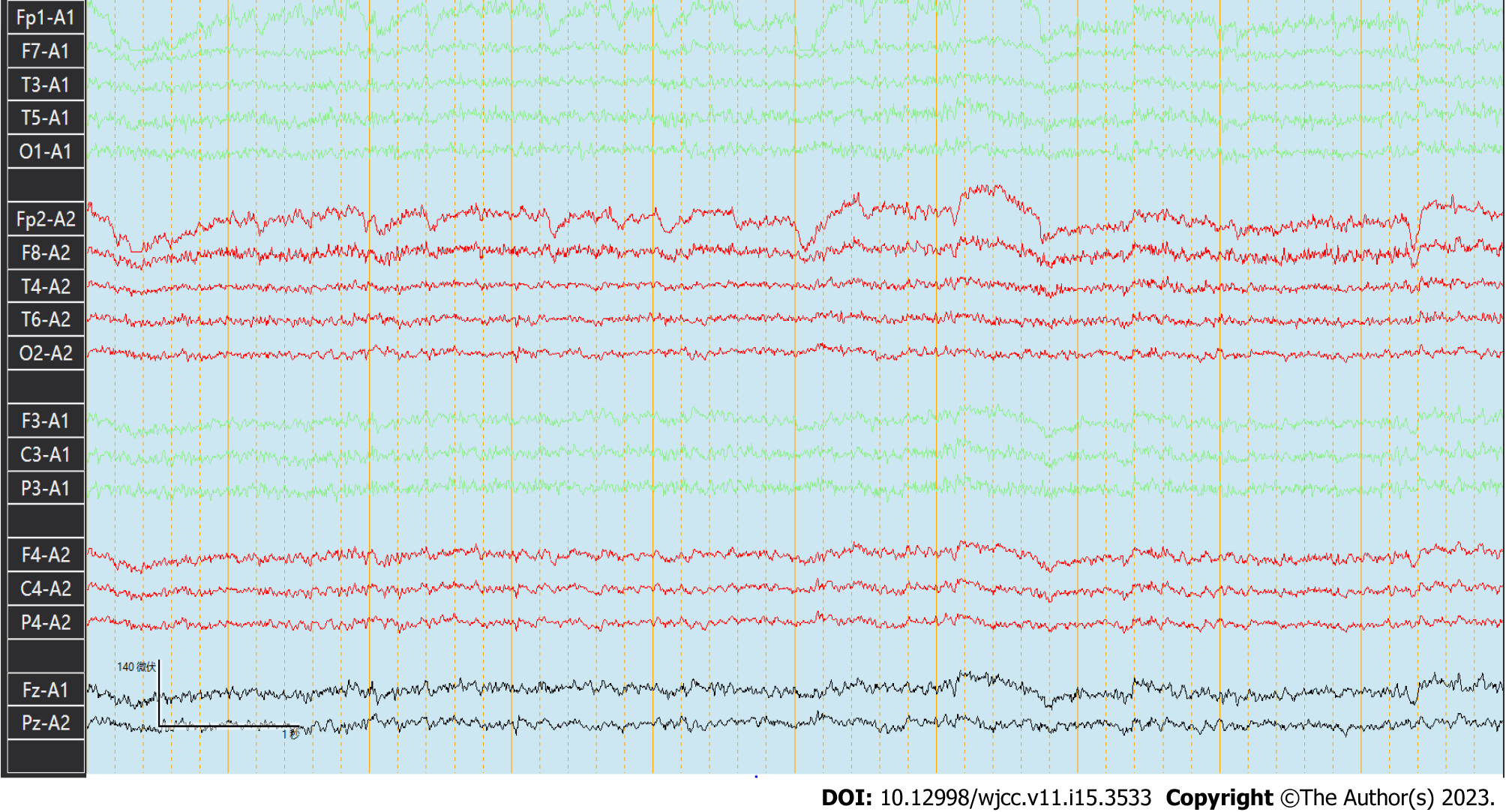
The basic rhythmic activity on electroencephalogram was the mid-potential 8-9c/s alpha wave and poor amplitude adjustment. Both sides were approximately equivalent. The visual response existed, and increased fast waves in both hemispheres were recorded without an obvious spike and slow wave (Figure 3).
Electromyography revealed normal nerve conduction in the extremities and abnormal F waves and sympathetic skin response in both lower extremities. Somatosensory evoked potential test revealed poor waveform differentiation and bilateral asymmetry as well as event-related evoked potential latency delay and potential instability. Abdominal ultrasound, cardiac ultrasound and neck vascular ultrasound showed no abnormalities.
After consultation with experts from the neurology, neuroelectrophysiology, pediatrics, imaging and oncology departments, genetic examination was arranged with the patient and her family to determine the genetic basis for the symptoms.
The patient’s younger sister also had similar symptoms of unsteady walking and seizures that were induced by light stimuli. A genetic family map was constructed (Figure 4) on the basis of clinical symptoms, laboratory examinations and imaging examinations of patients to exclude other related diseases.
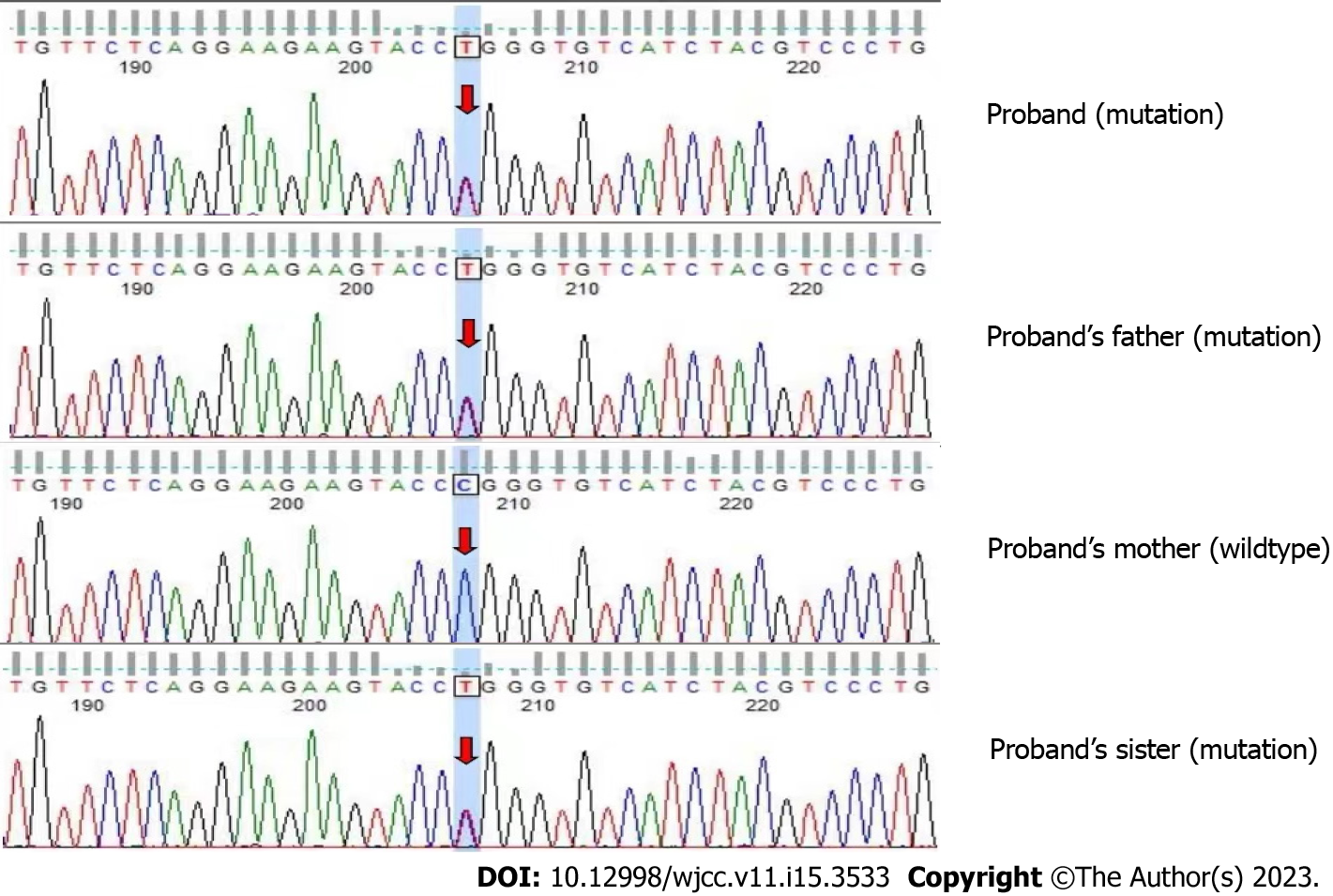
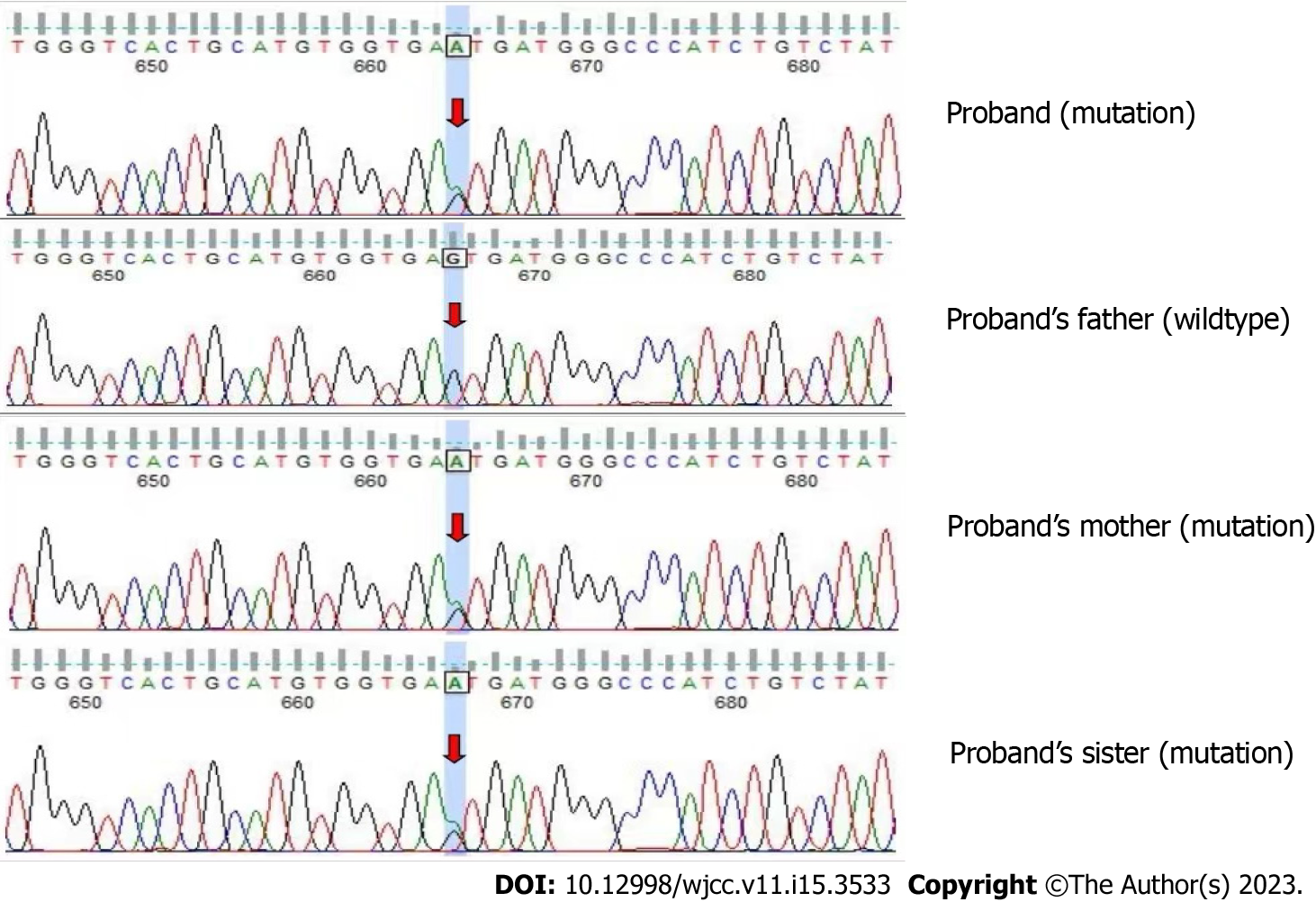
The patient and her immediate family members consented to peripheral blood collection for gene detection and analysis. Whole exome sequencing revealed the following CLN6 gene mutation in the patient and the patient’s father and sister: Exon 7 c.872C>T (p.Pro291Leu). This mutation causes amino acid 291 of the encoded protein to change from a proline to a leucine, which is a missense mutation (Figure 5), and results in impaired protein function. Another mutation in the CLN6 gene was identified in the patient and the patient’s mother and sister: Intron 5 c.542+5G>A(p.?) (Figure 6). The recessive compound heterozygous mutations in the proband were considered to be pathogenic based on the clinical and laboratory findings. Consistent with autosomal recessive inheritance, it is a recessive compound heterozygous mutation.
The final diagnosis was NCL, CLN6 type, based on the patient’s clinical manifestations, imaging examination, genetic test and other examination results as well as the consultation of multidisciplinary experts.
The patient received antiepileptic drugs for symptomatic relief and to improve her cognitive function.
The patient is currently under follow-up observation. At the last follow-up, the patient’s condition had deteriorated. Unfortunately, she was unable to care for herself.
NCL occurs in the presence of two deleterious mutation alleles. All known genes are on autosomal chromosomes, and most are inherited in a recessive manner. Different gene mutations lead to different forms of NCL, and the mutation often determines the age of onset, symptoms and the rate of disease progression, which is generally fatal[8]. However, there are also individual NCL gene mutations that are autosomal dominant, such as ANCL due to mutations in CLN4/DNAJC5[9]. The CLN6 gene is located on chromosome 15q21-23, contains seven exons and encodes a protein with seven transmembrane domains, an N-terminal cytoplasmic domain and a lumen C-terminal. This protein is involved in endoplasmic reticulum-to-Golgi transfer of lysosomal enzymes, causing clinical symptoms[10-13].
The first report of ANCL was in 1987, by Martin et al[14]. The initial symptoms were more common in people in their 30s, but the age of onset ranged from teenage to over 50-years-old. Two phenotypes were reported: Kufs type A and Kufs type B. Kufs type A presents with intractable epilepsy, dementia and myoclonus with no visual impairment. Kufs type B presents as abnormal behavior, dyskinesia, dementia, ataxia, extrapyramidal symptoms and symptoms of brainstem involvement. Although the current clinical manifestations of ANCL do not include visual abnormalities and retinal atrophy, NCL-specific lipopigment[14-16] may accumulate around the nucleus of retinal neurons.
Age of onset and disease duration in CLN6 type ANCL are associated with genetic variation across a broad phenotypic spectrum. More than 70 mutations in the CLN6 have been linked with late infantile NCL, early juvenile NCL and ANCL (Kufs type A)[17,18]. The clinical manifestations of this reported case are consistent with the manifestations of Kufs type A. However, the patient had paresthesia of deep sensation, superficial sensation and combined sensation accompanied by depressive symptoms. These symptoms are particularities of this case.
The electroencephalogram of patients with NCL typically show paroxysmal diffuse spikes, polyspikes and multifocal spikes[19]. MRI typically shows varying degrees of atrophy of the cerebrum, brainstem and cerebellum, with cerebellar atrophy being the most obvious. In the late stage of the disease, long T2 signal in the periventricular white matter, decreased T2 signal in the basal ganglia and cerebral cortex are observed[20,21]. Although the epileptiform waves of our patient were not captured, the head MRI showed that the patient had obvious brain atrophy.
NCL histopathological findings suggest neuronal degeneration in the cerebral and cerebellar cortex and accumulation of autofluorescent ceroidlipochromes in nerves and peripheral tissues[22,23]. Transmission electron microscopy ultrastructural examination reveals sparse storage deposits in lymphocytes, storage material coating, membrane-bound storage material as dense lipid pigments with fingerprints and amorphous materials. The ultrastructural analysis of skin biopsies reveals distinct storage inclusion in sweat gland epithelium, endothelial cells and smooth muscle cells. The inclusion body is a mixed type with curvilinear and fingerprint bodies. Different types of NCL have different sediment shapes, and mixed deposits may occur in atypical cases[24,25]. Nevertheless, the pathological examination is still not completely clear.
There are currently three types of ANCL: CLN1; CLN2; and CLN10. The mechanism of action of these three enzymes and the relationship between the functions and clinical phenotype are unclear. At present, there are still 10%-20% of cases that cannot be correctly typed. The diagnosis can be assisted by methods such as serum enzyme detection or genomics detection[26,27].
The diagnosis of NCL is primarily based on the age of onset, clinical manifestations, pathological examination results and genetic testing. Our patient was a 37-year-old female whose onset occurred 3 years before diagnosis. The initial symptoms were unsteady walking and seizures, with cognitive decline, paresthesia, depression and pyramidal tract sign (ankle clonus +). Before diagnosis, the patient also experienced cerebellar ataxia symptoms such as walking instability and shaking limbs. The patient’s brain MRI showed brain atrophy and leukoaraiosis. Combined with the patient’s clinical history and evidence of similar symptoms in the patient’s sister, genetic testing confirmed CLN6 type NCL. Unfortunately, symptomatic treatment was the only available therapy. At the 1-year follow-up, the patient’s symptoms had progressed; she was bedridden, unable to walk, and experiencing poorly controlled seizures, recurrent seizures, and stiffness in extremities.
There is currently no effective treatment for NCL. However, enzyme replacement therapy, immunosuppressive therapy, gene carrier therapy, stem cell therapy and drug therapy[28] have achieved promising results in animal models, clinical trials and various literature reports. Despite the lack of a cure for the disease, symptomatic treatment can slow the progression of the disease, stabilize organ function and increase lifespan.
Attending Physician Xue-Qiang Wang sincerely thanks Prof. Yu Zhai, Director Chuanbi Chen and Wen-Jie Zhao for their insightful comments on the report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pitton Rissardo J, Brazil; Sotelo J, Mexico S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Nita DA, Mole SE, Minassian BA. Neuronal ceroid lipofuscinoses. Epileptic Disord. 2016;18:73-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Nicolaou P, Tanteles GA, Votsi C, Zamba-Papanicolaou E, Papacostas SS, Christodoulou K, Christou YP. A Novel CLN6 Variant Associated With Juvenile Neuronal Ceroid Lipofuscinosis in Patients With Absence of Visual Loss as a Presenting Feature. Front Genet. 2021;12:746101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 3. | Mink JW, Augustine EF, Adams HR, Marshall FJ, Kwon JM. Classification and natural history of the neuronal ceroid lipofuscinoses. J Child Neurol. 2013;28:1101-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Huber RJ. Molecular networking in the neuronal ceroid lipofuscinoses: insights from mammalian models and the social amoeba Dictyostelium discoideum. J Biomed Sci. 2020;27:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Butz ES, Chandrachud U, Mole SE, Cotman SL. Moving towards a new era of genomics in the neuronal ceroid lipofuscinoses. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 6. | Cárcel-Trullols J, Kovács AD, Pearce DA. Cell biology of the NCL proteins: What they do and don't do. Biochim Biophys Acta. 2015;1852:2242-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | Shiro Y, Yamashita A, Watanabe K, Yamazaki T. CLN6's luminal tail-mediated functional interference between CLN6 mutants as a novel pathomechanism for the neuronal ceroid lipofuscinoses. Biomed Res. 2021;42:129-138. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Warrier V, Vieira M, Mole SE. Genetic basis and phenotypic correlations of the neuronal ceroid lipofusinoses. Biochim Biophys Acta. 2013;1832:1827-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Nosková L, Stránecký V, Hartmannová H, Přistoupilová A, Barešová V, Ivánek R, Hůlková H, Jahnová H, van der Zee J, Staropoli JF, Sims KB, Tyynelä J, Van Broeckhoven C, Nijssen PC, Mole SE, Elleder M, Kmoch S. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am J Hum Genet. 2011;89:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Badilla-Porras R, Echeverri-McCandless A, Weimer JM, Ulate-Campos A, Soto-Rodríguez A, Gutiérrez-Mata A, Hernández-Con L, Bogantes-Ledezma S, Balmaceda-Meza A, Brudvig J, Sanabria-Castro A. Neuronal Ceroid Lipofuscinosis Type 6 (CLN6) clinical findings and molecular diagnosis: Costa Rica's experience. Orphanet J Rare Dis. 2022;17:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Heine C, Koch B, Storch S, Kohlschütter A, Palmer DN, Braulke T. Defective endoplasmic reticulum-resident membrane protein CLN6 affects lysosomal degradation of endocytosed arylsulfatase A. J Biol Chem. 2004;279:22347-22352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Mole SE, Michaux G, Codlin S, Wheeler RB, Sharp JD, Cutler DF. CLN6, which is associated with a lysosomal storage disease, is an endoplasmic reticulum protein. Exp Cell Res. 2004;298:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Wheeler RB, Sharp JD, Schultz RA, Joslin JM, Williams RE, Mole SE. The gene mutated in variant late-infantile neuronal ceroid lipofuscinosis (CLN6) and in nclf mutant mice encodes a novel predicted transmembrane protein. Am J Hum Genet. 2002;70:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Martin JJ, Libert J, Ceuterick C. Ultrastructure of brain and retina in Kufs' disease (adult type-ceroid-lipofuscinosis). Clin Neuropathol. 1987;6:231-235. [PubMed] |
| 15. | Berkovic SF, Carpenter S, Andermann F, Andermann E, Wolfe LS. Kufs' disease: a critical reappraisal. Brain. 1988;111 (Pt 1):27-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 138] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Constantinidis J, Wisniewski KE, Wisniewski TM. The adult and a new late adult forms of neuronal ceroid lipofuscinosis. Acta Neuropathol. 1992;83:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Arsov T, Smith KR, Damiano J, Franceschetti S, Canafoglia L, Bromhead CJ, Andermann E, Vears DF, Cossette P, Rajagopalan S, McDougall A, Sofia V, Farrell M, Aguglia U, Zini A, Meletti S, Morbin M, Mullen S, Andermann F, Mole SE, Bahlo M, Berkovic SF. Kufs disease, the major adult form of neuronal ceroid lipofuscinosis, caused by mutations in CLN6. Am J Hum Genet. 2011;88:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat. 2012;33:42-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Veneselli E, Biancheri R, Buoni S, Fois A. Clinical and EEG findings in 18 cases of late infantile neuronal ceroid lipofuscinosis. Brain Dev. 2001;23:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Biswas A, Krishnan P, Amirabadi A, Blaser S, Mercimek-Andrews S, Shroff M. Expanding the Neuroimaging Phenotype of Neuronal Ceroid Lipofuscinoses. AJNR Am J Neuroradiol. 2020;41:1930-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Verma R, Raut TP, Tiwari N, Malhotra KP, Hussain N, Malhotra HS. Late infantile neuronal ceroid lipofuscinosis: A case report with review of literature. Ann Indian Acad Neurol. 2013;16:282-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Mole SE. Neuronal ceroid lipofuscinoses (NCL). Eur J Paediatr Neurol. 2006;10:255-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Guerreiro R, Bras JT, Vieira M, Warrier V, Agrawal S, Stewart H, Anderson G, Mole SE. CLN6 disease caused by the same mutation originating in Pakistan has varying pathology. Eur J Paediatr Neurol. 2013;17:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Satodate R, Monma N, Sakuma T, Ito N, Itoh M. Ultrastructure of peripheral lymphocytes in generalized ceroid-lipofuscinosis. Report of a case. Pathol Res Pract. 1982;173:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Cotman SL, Karaa A, Staropoli JF, Sims KB. Neuronal ceroid lipofuscinosis: impact of recent genetic advances and expansion of the clinicopathologic spectrum. Curr Neurol Neurosci Rep. 2013;13:366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Goebel HH, Wisniewski KE. Current state of clinical and morphological features in human NCL. Brain Pathol. 2004;14:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Rosenberg JB, Chen A, Kaminsky SM, Crystal RG, Sondhi D. Advances in the Treatment of Neuronal Ceroid Lipofuscinosis. Expert Opin Orphan Drugs. 2019;7:473-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |