Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3491
Peer-review started: January 25, 2023
First decision: March 14, 2023
Revised: March 25, 2023
Accepted: April 13, 2023
Article in press: April 13, 2023
Published online: May 26, 2023
Processing time: 120 Days and 13 Hours
Through significant advances in the treatment of peripheral arterial occlusive disease, acute ischemia of the lower extremity is still associated with significant morbidity, limb threat and mortality. The two main causes of acute ischemia in lower extremities are arterial embolism and atherosclerotic arteries. Timely recognition and treatment of acute limb ischemia in emergency situations is essential in order to minimize the duration of ischemia.
To investigate the application effect of angiojet thrombolysis in the treatment of acute lower extremity arterial embolization.
Sixty-two patients with acute lower extremity arterial embolization admitted to our hospital from May 2018 to May 2020 were selected. Among them, the observation group (twenty-eight cases) had received angiojet thrombolysis, and the control group (thirty-four cases) had received femoral artery incision and thrombectomy. After thrombus clearance, significant residual stenosis of the lumen was combined with balloon dilation and/or stent implantation. When the thrombus removal was not satisfactory, catheter-directed thrombolysis was performed. The incidence of postoperative complications, recurrence rate and recovery of the two groups were compared.
There were no significant differences in postoperative recurrence (target vessel reconstruction rate), anklebrachial index and the incidence of postoperative complications between the two groups (P > 0.05); there were statistically significant differences in postoperative pain score and postoperative rehabilitation between the two groups (P < 0.05).
The application of angiojet in the treatment of acute lower limb artery thromboembolism disease is safe and effective, minimally invasive, quicker recovery after operation, less postoperative complications, which is more suitable for the treatment of femoral popliteal arterial thromboembolism lesions. If the thrombus removal is not satisfactory, the combination of coronary artery aspiration catheter and catheterized directed thrombolysis can be used. Balloon dilation and stent implantation can be considered for obvious lumen stenosis.
Core Tip: Acute lower extremity arterial embolism is a common clinical emergency. It has the characteristics of rapid onset and rapid development. If left untreated, irreversible damage to limb tissue can occur within hours. Therefore, the key to the treatment of acute lower extremity arterial embolism is to seize the time and implement effective treatment plan is of great significance. Angiojet thrombectomy is a minimally invasive technique to remove thrombectomy through percutaneous insertion of catheters. The objective of this study was to investigate the application effect of angiojet thrombectomy in the treatment of acute lower extremity arterial embolization.
- Citation: Meng XH, Xie XP, Liu YC, Huang CP, Wang LJ, Liu HY, Fang X, Zhang GH. Observation of the effect of angiojet to treat acute lower extremity arterial embolization. World J Clin Cases 2023; 11(15): 3491-3501
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3491.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3491
Acute lower extremity arterial embolism is a common clinical emergency. It has the characteristics of acute onset and rapid development; if treatment is not pursued in time, within hours, it can cause irreversible damage to limb tissue, even threatening life and reducing the quality of life[1,2]. Therefore, the key to the treatment of acute lower extremity arterial embolism is to pursue treatment in a timely manner and implement an effective treatment plan, which is of great significance to reduce the amputation rate and mortality. At present, the main clinical methods for acute lower extremity arterial embolization include femoral artery incision and thrombectomy and catheter-directed thrombolysis. Among them, femoral artery incision and thrombectomy can quickly and partially restore the lower limb arterial blood supply, which can effectively reduce the incidence of amputation in patients. However, it has disadvantages such as the high amount of thrombectomy trauma, large reperfusion injury reaction, high risk of general anaesthesia, and a long operation time[3,4]. Angiojet thrombectomy is a minimally invasive technique for thrombectomy by percutaneous insertion of a thrombectomy catheter. This study intends to investigate the application effect of angiojet thrombectomy in the treatment of acute lower extremity arterial embolization.
A total of sixty-two patients with acute lower extremity arterial embolization who were treated in our hospital from May 2018 to May 2020 were selected as the research subjects, including thirty-eight males and twenty-four females aged 55 to 83 years, with an average age of 69.52 ± 7.45 years, a disease duration from 2.67 to 64.17 h, an average disease duration (13.22 ± 10.85) h, a preoperative ankle brachial index (ABI) from 0.21 to 0.46, an average ABI of (0.33 ± 0.06), a body mass index (BMI) from 18.10 to 24.62 and average BMI of (21.87 ± 1.91) (Table 1). The inclusion criteria: (1) The diagnosis of acute lower extremity arterial embolism is clear, that is, the patient had sudden symptoms of pain, paraesthesia, paralysis, pulselessness and pallor in the affected limb, and the acute lower extremity artery embolism is confirmed by computed tomography examination and d-dimer that is significantly increased; (2) Clinical classification of lower extremity ischaemia: Rutherford grade 3 or lower; and (3) The patients and their families gave informed consent and signed the informed consent. The exclusion criteria: (1) Patients with immune system diseases such as thromboangiitis obliterans; (2) Patients with a recent history of active bleeding; (3) Irreversible necrosis that occurred in the affected limb of the patient, and amputation is inevitable; (4) Patients with contraindications to anticoagulation and antiplatelet medications; (5) Those with severe liver, kidney, and cardiac insufficiency who cannot tolerate surgery; and (6) Patients and their families who refuse to sign the informed consent. Sixty-two patients were divided into an observation group (twenty-eight cases) and a control group (thirty-four cases) according to the random number table method. The observation group included seventeen males and eleven females, aged 55 to 83 years, with an average age of 69.00 ± 7.29 years; the preoperative ABI ranged from 0.21 to 0.46, with an average ABI of 0.33 ± 0.05; and the BMI ranged from 18.19 to 24.62, with an average BMI of 21.97 ± 1.88. The disease duration ranged from 2.65 to 64.12 h, and the average disease duration was 13.21 ± 10.84 h (Table 2). There were fourteen patients with smoking, eleven patients with hypertension, ten patients with diabetes mellitus, nine patients with hyperlipidaemia, nine patients with coronary heart disease, and twenty-two patients with atrial fibrillation. The control group consisted of twenty-one males and thirteen females, aged 57 to 81 years, with an average age of 69.94 ± 7.66 years; the preoperative ABI ranged from 0.21 to 0.46, with an average ABI of 0.33 ± 0.06; and the BMI ranged from 18.10 to 24.59, with an average BMI of 21.79 ± 1.97. The disease duration ranged from 2.70 to 63.93 h, and the average disease duration was 13.25 ± 10.87 h (Table 2). There were eighteen patients with smoking, fourteen patients with hypertension, fifteen patients with diabetes, eleven patients with hyperlipidaemia, twelve patients with coronary heart disease, and twenty-six patients with atrial fibrillation. There was no significant difference in the general data between the two groups (P > 0.05) (Table 2 and 3), and the data was comparable. This study was approved by the hospital ethics committee, and all patients and their families signed informed consent forms.
| Groups | Gender (male) | Gender (female) | Age (yr) | BMI | Pre-operation ABI | Course of disease (h) |
| Enrolled patients (n = 62) | 38 (61%) | 24 (39%) | 55-83 (69.52 ± 7.45) | 18.10-24.62 (21.87 ± 1.91) | 0.21-0.46 (0.33 ± 0.06) | 2.67-64.17 (13.22 ± 10.85) |
| Groups | Age (yr) | Pre-operation ABI | BMI | Course of disease (h) |
| Control group (n = 34) | 57-81 (69.94 ± 7.66) | 0.21-0.46 (0.34 ± 0.06) | 18.10-24.59 (21.79 ± 1.97) | 2.70-63.93 (13.25 ± 10.87) |
| Observation group (n = 28) | 55-83 (69.00 ± 7.29) | 0.21-0.46 (0.33 ± 0.05) | 18.19-24.62 (21.97 ± 1.88) | 2.65-64.12 (13.21 ± 10.84) |
| t value | 0.059 | 0.538 | 0.381 | 0.015 |
| P value | 0.956 | 0.592 | 0.705 | 0.989 |
| Groups | Control group (n = 34) | Observation group (n = 28) | χ2 value | P value |
| Gender (male) | 21 (62) | 17 (61) | 0.007 | 0.932 |
| Gender (female) | 13 (38) | 11 (39) |
Surgical methods: All patients in the observation group had undergone angiojet thrombectomy. After satisfactory local anaesthesia, angiography confirmed that the puncture was located in the common femoral artery. High-pressure angiography was performed to confirm the location and length of the lesion, the patency of the deep femoral artery, the superficial femoral artery and the condition of the subknee outflow tract. After systemic heparinization, according to different conditions, an appropriate guide wire was selected, a single curved catheter was used to open the occluded segment, and an angiojet thrombus aspiration catheter was introduced for thrombus aspiration. The risk of distal embolism should be minimized during aspiration. If there was residual local stenosis, balloon dilation could be combined, and stent placement could be performed if necessary. If distal embolism occurred, a six-French guiding catheter could be introduced into the femoral popliteal segment for local aspiration; if embolism occurred in the anterior tibial artery, posterior tibial artery or peroneal artery, a four-French angiojet thrombus aspiration catheter or coronary thrombus aspiration catheter could be combined.
All patients in the control group underwent femoral artery incision and thrombectomy. After general anaesthesia was induced, a longitudinal incision was made in the ipsilateral groin, approximately 7 cm-10 cm long, the skin and subcutaneous tissue were incised layer by layer, and the femoral artery was exposed. The common femoral artery, superficial femoral artery, and deep femoral artery were dissociated and cuffed. After systemic heparinization, the common femoral artery was transversely incised. According to the different embolization sites, different diameters of fogarty catheters were used for thrombectomy. If thrombectomy of the inferior knee artery is performed, attention should be given to the catheter guide wire and true lumen opening. Similarly, if there was residual local stenosis, balloon dilation could be combined, and stenting could be performed if necessary.
Before the end of the operation, the blood flow velocity and thrombus load were judged by angiography in both groups.
The control group was given prophylactic anti-infective therapy (within 30 min before surgery), and both groups were given vasodilator and anticoagulant therapy after surgery. Among them, the antiplatelet drug was bayaspirin or clopidogrel, and the anticoagulant drug was warfarin or rivaroxaban. The specific medication plan, time and dosage were determined according to the patient's comprehensive condition.
Both groups were followed up for twenty months. The observation indicators included: (1) Amputation, death and other prognoses of the two groups after the operation; (2) The curative effect of the two groups. For the postoperative curative effect analysis, the Coley criterion was adopted: (I) Recovery: The distal limb pulse after treatment returned to normal, with no muscle and skin necrosis and no sensorimotor disturbance; (II) Good: The distal limb pulse was weaker than that of the contralateral side, and the symptoms disappeared; (III) Fair: After treatment, the blood supply of the distal limb was partially restored but compensatory; (IV) Poor: After the treatment, the artery of the distal limb was not patent, which was compensated by collateral vessels, and the symptoms of ischaemia were still present; and (V) Amputation and death (total effective rate = (number of cured cases + number of good cases + number of normal cases)/total number of cases × 100%); (3) The changes in ankle brachial index (ABI) values were evaluated in the two groups, and the changes in ABI values before and after surgery were taken as the judgement standard; (4) The postoperative recurrence of the two groups was evaluated, and the second operation was taken as the standard; (5) The postoperative rehabilitation conditions of the two groups, were compared including pain score (the pain score standard was evaluated by the Numerical Rating Scale (NRS) III), time to get out of bed, operation time, and postoperative hospital stay; and (6) The postoperative complications between the two groups, including ischaemia-reperfusion injury syndrome, puncture site/incision infection, lymphatic leakage, distal embolism, haemoglobinuria, haemorrhage and haematoma formation, were compared.
Data processing had used SPSS 22.0 software. Measurement data were expressed as mean ± standard deviation (mean ± SD), and t-test was used for pairwise comparison. The enumeration data were expressed in the form of percentage (%), and the χ2 test was used for analysis. P < 0.05 was considered statistically significant.
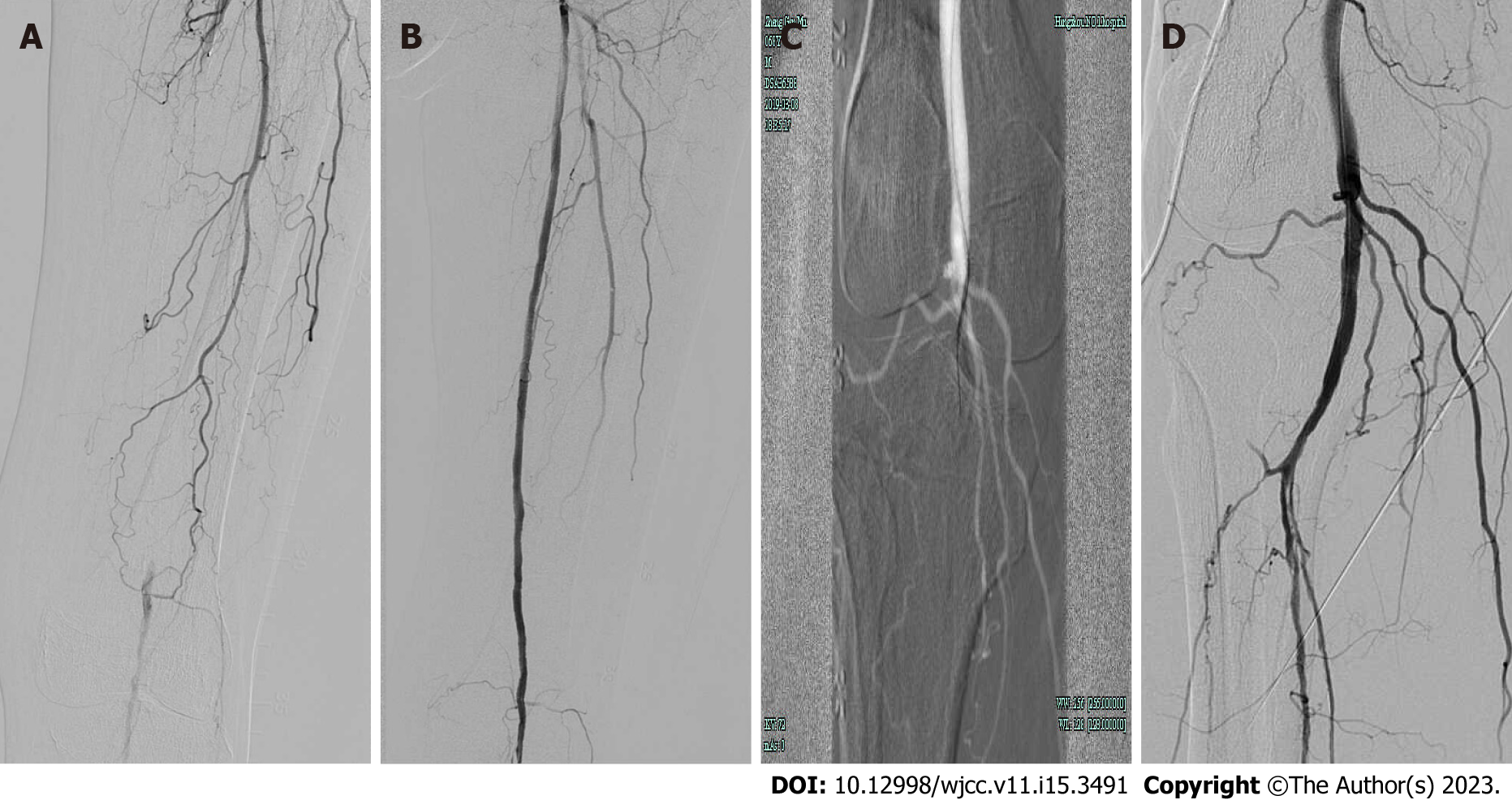
Compared with the control group (97.06%), the total effective rate of the observation group (100.00%) was not significantly different (P > 0.05). Two patients in both groups received catheterized directed thrombolysis (CDT) treatment, and postoperative thrombus removal and lumen opening were satisfactory. In the control group, two patients with distal arterial embolization were treated with a 3-french fogarty catheter. In the observation group, four patients had distal artery embolization during the operation, and a coronary artery suction catheter was used to aspirate the thrombus. Postoperative thrombus removal and lumen opening were satisfactory. Twenty-one patients in the control group were treated with balloon dilation (percutaneous endovascular angioplasty (PTA), and 18 patients in the observation group were treated with balloon dilation. The lumen opening was satisfactory, and there was no significant difference in the above results (P > 0.05) (Table 4). Twenty-eight patients underwent thrombus aspiration, the aspiration time ranged from 105 to 234 s, and the operation success rate was 100%. Postoperative angiography was used to evaluate the thrombus clearance rate (Figure 1).
| Groups | Recovery | Good | Fair | Poor | Amputa-tion/death | Total effective rate |
| Control group (n = 34) | 18 (52.94) | 10 (29.41) | 5 (14.71) | 1 (2.94) | 0 (0) | 33 (97.06) |
| Observation group (n = 28) | 16 (57.14) | 8 (28.57) | 4 (14.29) | 0 (0.00) | 0 (0) | 28 (100.00) |
| χ2 value | 0.837 | |||||
| P value | 0.360 |
The pain score, operation time, time to get out of bed, and postoperative hospital stay in the observation group were all lower than those in the control group, and the differences were statistically significant (P < 0.05) (Table 5).
| Group | Pain score (point) | ABI difference before and after operation | Operation time (h) | Time to get out of bed (h) | Hospital stay (d) |
| Control group (n = 34) | 3.97 ± 0.94 | 0.59 ± 0.13 | 3.51 ± 0.45 | 25.18 ± 1.77 | 8.04 ± 0.75 |
| Observation group (n = 28) | 1.71 ± 0.71 | 0.56 ± 0.11 | 2.53 ± 0.32 | 16.32 ± 2.48 | 5.80 ± 0.98 |
| t value | 10.480 | 0.739 | 9.579 | 16.390 | 10.150 |
| P value | 0.000 | 0.463 | 0.000 | 0.000 | 0.000 |
There was no significant difference in postoperative puncture site infection, haemorrhage, haematoma, lymphatic leakage, distal embolism, haemoglobinuria, or recurrence rate between the two groups (P > 0.05). However, the incidence of puncture site infection, bleeding, haematoma and lymphatic leakage in the observation group was 0, which was significantly lower than that in the control group. The statistically insignificant difference between the two groups may be related to the insufficient sample size. The number of patients with ischaemia-reperfusion injury syndrome and postoperative complications in the observation group was significantly lower than that in the control group (P < 0.05), and the postope
| Groups | Ischemia reperfusion injury syndrome (case) | Infection of puncture site/incise-on (case) | Bleeding at puncture site/incise-on (case) | Puncture site/incise-on hematoma (case) | Lymphatic leakage (case) | Recurrence (case) | Distal embolism (case) | Hemoglob-inuria (cases) | Patients with complications (cases) |
| Control group (n = 34) | 23 (0.68) | 2 (0.06) | 4 (0.12) | 3 (0.09) | 3 (0.09) | 4 (0.12) | 2 (0.06) | 0 (0) | 24 (0.71) |
| Observation group (n = 28) | 10 (0.36) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0.11) | 4 (0.14) | 3 (0.11) | 10 (0.36) |
| χ2 value | 6.289 | 1.702 | 3.521 | 2.596 | 2.596 | 0.017 | 1.240 | 3.828 | 7.540 |
| P value | 0.012 | 0.192 | 0.061 | 0.107 | 0.107 | 0.897 | 0.265 | 0.050 | 0.006 |
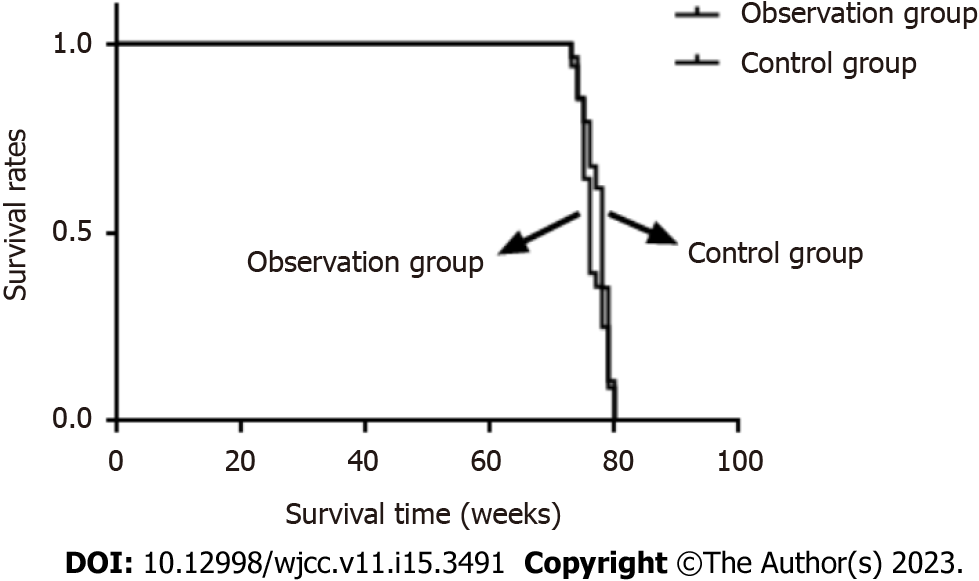
In the observation group, one patient (3.60%) died after the operation due to multiple organ failure; one patient (2.90%) in the control group died due to natural death, and there was no significant difference in mortality between the two groups (P > 0.05). The survival time of the observation group was 76.18 ± 2.51 wk and that of the control group was 77.32 ± 2.06 wk, and there was no significant difference in the survival time between the two groups (P > 0.05) (Tables 7 and 8). The survival curves of the two groups are shown in Figure 2.
| Group | Death |
| Control group (n = 34) | 1 (2.90) |
| Observation group (n = 28) | 1 (3.60) |
| χ2 value | 0.020 |
| P value | 0.889 |
| Group | Survival time (wk) |
| Control group (n = 34) | 77.32 ± 2.06 |
| Observation group (n = 28) | 76.57 ± 2.06 |
| t value | 1.432 |
| P value | 0.158 |
The risk of amputation with acute lower limb ischaemia is between 15% and 30%, and the perioperative morbidity and mortality are high[5]. Acute lower limb ischaemia mainly includes acute arterial thrombosis based on lower limb arteriosclerotic occlusive disease, lower limb artery embolism, and acute lower limb artery thrombosis of an unknown cause. It is important to distinguish these conditions because the treatment and prognosis are different[6]. Embolic disease is characterized by the presence of a foreign body (embolus) that partially or completely occludes the vascular lumen. Previously thought to be the cause of rheumatic heart disease, the majority of acute lower extremity arterial embolisms (60%) are now thought to be associated with atrial fibrillation or mural thrombosis after myocardial infarction and, rarely, an embolus from valvular growth or atrial myxoma[7]. Aneurysms may also produce emboli, so the abdomen and popliteal fossa should be palpated carefully, and atherosclerosis may also cause distal emboli upon plaque rupture[8]. Hypertension, diabetes, and hyperlipidaemia can all induce the formation of cardiovascular plaques and increase the risk of thrombosis[3,9]. Coronary heart disease is either an incomplete or a complete blockage of the cardiovascular system caused by the formation of plaques in the coronary arteries. If the plaques rupture, thrombosis may occur[10]. Atrial fibrillation can lead to blood pooling in the atrium, thereby forming a thrombus, and atrial fibrillation can easily lead to thrombus shedding[11]. Currently, the majority of acute lower extremity ischaemia (approximately 70%) is caused by arterial thrombosis, which usually occurs in the presence of preexisting vascular disease and is common in patients with diabetes[12].
Traditional arterial incision and fogarty catheter thrombectomy are very effective in the treatment of acute arterial embolization of the lower extremities, especially in cases involving a single large blood vessel. However, in some cases, such as the presence of sclerotic occlusive lesions in lower extremity arterioles or peripheral artery lesions, the early clinical results are still unsatisfactory, which may be related to the existence of potential stenosis occlusive lesions. Even secondary vascular injuries by fogarty balloon catheters may limit clinical success[13]. Diabetic patients are generally at higher risk for severe peripheral arterial disease in the lower infra-knee artery and are at higher risk for poor outcomes after thrombectomy using the fogarty balloon catheter. The arterial walls of diabetic patients are fragile, prone to spasms, and severely calcified, so fogarty catheters are difficult to pass through and reach the target vessel. In these cases, bypass surgery may be used as the primary option or as a second-line treatment when thromboembolic resection fails[14]. However, conventional arteriotomy and fogarty catheter thrombectomy or bypass surgery usually have a longer anaesthesia duration, higher surgical risk, more severe trauma, higher rates of thrombus retention, and higher rates of thrombus recurrence[15]. In addition, the disadvantages of open surgery include scarring of the incision, difficulty in reoperation with femoral artery dissection, increased likelihood of lymphatic leakage, incision infection, and inability of the catheter to pass through the sclerosis to occlude the diseased artery. CDT is becoming accepted for acute limb ischaemia. Based on the available clinical evidence, CDT achieves the same outcomes as open surgery in terms of the limb salvage rate and target lesion opening rate. Compared with open surgery, CDT has the advantages of less trauma and a faster recovery time. However, patients with acute limb ischaemia tend to be older, have comorbidities and have a high risk of bleeding. Therefore, due to the strict requirements of CDT, this method is only suitable for a small number of patients. In addition, thrombolysis can cause bleeding, especially fatal cerebral haemorrhage[16]. In recent years, the application of percutaneous mechanical thrombectomy in the treatment of acute limb ischaemia has shown many advantages; it is a minimally invasive endovascular treatment, can be used for rapid thrombectomy, and requires lower doses or no thrombolytic drugs; all of these factors indicate that it has better application prospects[17].
AngioJet is an effective mechanical thrombectomy device for removing thrombi, restoring and maintaining arterial patency and alleviating symptoms and can shorten the time of special bed care for patients with severe conditions[18]. Some domestic scholars believe that it is not necessary to use the spraying mode first but directly use the suction mode[19]; however, foreign researchers believe that spraying combined with the suction mode can achieve a better thrombolysis effect[20]. Angiojet is a hemolytic thrombectomy device that may cause hemoglobinemia and haemoglobinuria when patients are repeatedly operated on, and this occurs mainly in patients with renal insufficiency[21]. It is recommended to rehydrate patients with isotonic saline during and after surgery and alkalize urine with sodium bicarbonate. AngioJet thrombus aspiration has been applied in different diseases, including acute thrombosis and stent thrombosis, and the success rate of effective intraoperative blood flow recovery is between 60% and 90%[22]. The PEARL clinical study reported the results of mechanical thrombolysis with rheological drugs in the treatment of acute lower extremity ischaemia. The operation was successful in 83% of the 283 patients, and half of the operations were completed without the need for assisted CDT. At twenty months of follow-up, the amputation-free survival and mortality were 81% and 91%, respectively, 91% of the patients were free from bleeding requiring blood transfusion, and 95% of the patients were free from renal failure. A subgroup analysis showed that patients without subknee artery disease and patients without CDT who underwent haemolytic thrombectomy had a better prognosis[23]. In addition to the abovementioned intravascular haemolysis and acute renal insufficiency, a series of complications related to the mechanism of action of this system may occur, including vascular injury and acute embolization, stent collapse during thrombectomy, and distal embolization[24]. There may be embolism in the distal arteries after angiojet thrombectomy and thrombolysis. If the distal main artery is embolized, the 4-french angiojet catheter can be exchanged, and PTA can be used to address the distal arterial embolism. If the residual stenosis is mainly from an old thrombus due to arteriosclerosis or embolization, PTA and stent implantation should be performed; if the residual stenosis is mainly residual fresh thrombus, thrombus aspiration can be performed again. If it is invalid, CDT therapy can be performed.
In this study, angiojet thrombectomy and arterial incision thrombectomy were compared in terms of surgical efficacy, operation-related indicators, postoperative rehabilitation, postoperative complications, embolism recurrence and other aspects to explore the clinical application value of angiojet thrombectomy and provide a reference for follow-up treatment and recurrence prevention. The results of this study showed that there was no significant difference between angiojet thrombectomy and arteriotomy thrombectomy in terms of overall efficacy and postoperative embolism recurrence. In addition, compared with arterial incision and thrombectomy, angiojet thrombectomy significantly reduced the operation time, reduced postoperative pain, shortened patients' time to get out of bed and stay in hospital, and reduced the incidence of postoperative complications, which was conducive to postoperative recovery, early discharge and improvement of postoperative quality of life. In this study, 8 patients in the observation group were treated with PTA and/or stent implantation during the operation, and all patients achieved good therapeutic effects.
In conclusion, angiojet thrombectomy is a safe and effective means for the treatment of acute lower extremity arterial embolism, and it effectively reduces the operation time, postoperative pain and postoperative complication rate and speeds up the postoperative recovery.
In conclusion, angiojet thrombectomy is a safe and effective means for the treatment of acute lower extremity arterial embolism, which effectively reduces operation time, postoperative pain and postoperative complication rate and speeds up postoperative recovery. Stenosis or residual thrombus (which cannot be aspirated), can be combined with PTA and/or stenting. This study is a retrospective study with a small number of cases and a short follow-up time. The clinical application and utility of angiojet thrombectomy is limited. Therefore, a larger sample size is needed to evaluate the relevant risk factors and more clinical experience. In addition, prospective clinical studies are needed to accumulate experience and improve techniques to better grasp indications.
Acute lower extremity arterial embolism is a clinical emergency, if not treated in time, easy to lead to limb ischemia necrosis, eventually amputation, serious damage to the physical and mental health of the patient.
In the past, the effect of conventional drug thrombolytic therapy was slow and limited, and the optimal treatment time was easily delayed. Timely treatment of acute limb ischemia is essential to minimize the duration of ischemia.
To investigate the effect of angiojet in acute lower extremity arterial embolization.
Through the collection and analysis of patients with acute lower limb artery embolization admitted to our hospital from May 2018 to May 2020, the postoperative complication rate, recurrence rate and recovery of the two groups of patients who underwent vascular injection thrombolysis and femoral artery incision thrombolysis were compared.
There were no significant differences in postoperative recurrence, anklebrachial index and the incidence of postoperative complications between the two groups; there were statistically significant differences in postoperative pain score and postoperative rehabilitation between the two groups.
It is safe and effective that the application of angiojet in the treatment of acute lower extremity arterial thromboembolism.
Prospective studies should be conducted to expand the sample size and extend follow-up time to assess related risk factors and summarize more clinical experience, so as to accumulate experience, improve techniques and better grasp indications.
We thank all the doctors in the radiology department of Hangzhou First People's Hospital for their technical support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia; Schoenhagen P, United States S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Yang M, Chen L, Zhang M, Huang X, Zhao W, Wang H. Evidence-Based Nursing Model in Interventional Thrombolysis for Acute Lower Extremity Arterial Embolism. Contrast Media Mol Imaging. 2022;2022:4488797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Zhao L, Cai H, Song Q. Clinical Study on Treatment of Acute Lower Extremity Arterial Embolism With Straub Thrombus Removal System. Front Surg. 2022;9:891649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | de Donato G, Pasqui E, Setacci F, Palasciano G, Nigi L, Fondelli C, Sterpetti A, Dotta F, Weber G, Setacci C. Acute on chronic limb ischemia: From surgical embolectomy and thrombolysis to endovascular options. Semin Vasc Surg. 2018;31:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Araujo ST, Moreno DH, Cacione DG. Percutaneous thrombectomy or ultrasound-accelerated thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev. 2022;1:CD013486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Khan S, Hawkins BM. Acute Limb Ischemia Interventions. Interv Cardiol Clin. 2020;9:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | McNally MM, Univers J. Acute Limb Ischemia. Surg Clin North Am. 2018;98:1081-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Meng XH, Xie LS, Xie XP, Liu YC, Huang CP, Wang LJ, Zhang GH, Xu D, Cai XC, Fang X. Cardiac myxoma shedding leads to lower extremity arterial embolism: A case report. World J Clin Cases. 2022;10:10606-10613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 8. | Shao C, Wang J, Tian J, Tang YD. Coronary Artery Disease: From Mechanism to Clinical Practice. Adv Exp Med Biol. 2020;1177:1-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Suo Y, Zhang Z, Fu H, Zhang Y, Yuan M, Wang Y, Goudis CA, Tse G, Liu T, Li G. Inhibition of renin-angiotensin axis reduces the risk of thrombus formation in the left atrial appendage in patients with hypertension complicated by atrial fibrillation. J Renin Angiotensin Aldosterone Syst. 2018;19:1470320318782623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Dattoli-García CA, Jackson-Pedroza CN, Gallardo-Grajeda AL, Gopar-Nieto R, Araiza-Garygordobil D, Arias-Mendoza A. Infarto agudo de miocardio: revisión sobre factores de riesgo, etiología, hallazgos angiográficos y desenlaces en pacientes jóvenes. Arch Cardiol Mex. 2021;91:485-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Liang S, Zhou L, Ye K, Lu X. Limb Salvage After Percutaneous Mechanical Thrombectomy in Patients with Acute Lower Limb Ischemia: A Retrospective Analysis from Two Institutions. Ann Vasc Surg. 2019;58:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Chang Z, Yan H, Zhen Y, Zheng J, Liu Z. Lower Limb Arterial Calcification and Acute Thrombosis Risk in Patients with Peripheral Artery Disease. Ann Vasc Surg. 2020;63:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Davis FM, Albright J, Gallagher KA, Gurm HS, Koenig GC, Schreiber T, Grossman PM, Henke PK. Early Outcomes following Endovascular, Open Surgical, and Hybrid Revascularization for Lower Extremity Acute Limb Ischemia. Ann Vasc Surg. 2018;51:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Stoberock K, Kaschwich M, Nicolay SS, Mahmoud N, Heidemann F, Rieß HC, Debus ES, Behrendt CA. The interrelationship between diabetes mellitus and peripheral arterial disease. Vasa. 2021;50:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | de Athayde Soares R, Matielo MF, Brochado Neto FC, Cury MVM, Duque de Almeida R, de Jesus Martins M, Pereira de Carvalho BV, Sacilotto R. Analysis of the results of endovascular and open surgical treatment of acute limb ischemia. J Vasc Surg. 2019;69:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Huang H, Gu J, Su H, Chen L, He X, Kong J, Shi Y, Lu Z, Yuan Y. Solitaire™ Stent Thrombectomy System in the Treatment of Acute Lower-Limb Ischemia: Comparisons in Safety and Effectiveness with Conventional Catheter-Directed Thrombolysis Therapy. Biomed Res Int. 2022;2022:6997221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Yang X, Li X, Yin M, Wang R, Ye K, Lu X, Li W, Cheng Y, Qin J. Percutaneous Mechanical Thrombectomy for Acute Limb Ischemia With Aorto-iliac Occlusion. Front Surg. 2022;9:831922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Ascher E, Kibrik P, Rizvi SA, Alsheekh A, Marks N, Hingorani A. Fast-track thrombolysis protocol for acute limb ischemia. J Vasc Surg. 2021;73:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Gong M, Zhou Y, He X, Chen L, Su H. Mechanical Thrombectomy Using Kissing Y-Solitaire as a Rescue Treatment for Refractory Acute Popliteal and Infrapopliteal Occlusion in Elderly Patients. J Vasc Interv Radiol. 2021;32:1601-1605. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Garcia MJ, Lookstein R, Malhotra R, Amin A, Blitz LR, Leung DA, Simoni EJ, Soukas PA. Endovascular Management of Deep Vein Thrombosis with Rheolytic Thrombectomy: Final Report of the Prospective Multicenter PEARL (Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths) Registry. J Vasc Interv Radiol. 2015;26:777-85; quiz 786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Esteras R, Cannata-Ortiz P, Del Palacio-Tamarit M, Guerrero-Hue M, García-Caballero C, Egido J, Gimeno J, Ortiz A, Gracia-Iguacel C, Moreno JA. Podocyte and tubular involvement in AngioJet-induced kidney injury. Clin Kidney J. 2021;14:424-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Gandhi SS, Ewing JA, Cooper E, Chaves JM, Gray BH. Comparison of Low-Dose Catheter-Directed Thrombolysis with and without Pharmacomechanical Thrombectomy for Acute Lower Extremity Ischemia. Ann Vasc Surg. 2018;46:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Leung DA, Blitz LR, Nelson T, Amin A, Soukas PA, Nanjundappa A, Garcia MJ, Lookstein R, Simoni EJ. Rheolytic Pharmacomechanical Thrombectomy for the Management of Acute Limb Ischemia: Results From the PEARL Registry. J Endovasc Ther. 2015;22:546-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Chan PG, Goh GS. Safety and efficacy of the AngioJet device in the treatment of thrombosed arteriovenous fistula and grafts: A systematic review. J Vasc Access. 2018;19:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |