Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3481
Peer-review started: March 8, 2023
First decision: March 24, 2023
Revised: March 27, 2023
Accepted: April 21, 2023
Article in press: April 21, 2023
Published online: May 26, 2023
Processing time: 77 Days and 20.3 Hours
Chronic otitis media (COM) is an inflammatory disease that lasts for a long time. It is common in developing countries. Hearing loss can result from COM. The relationship between variations in middle ear anatomy and COM was investigated in our study.
To compare the prevalence of middle ear anatomic variations between the cases with COM and healthy individuals.
This retrospective study included 500 patients with COM and 500 healthy controls. The presence of those variants was determined: Koerner’s septum, facial canal dehiscence, high jugular bulb, jugular bulb dehiscence, jugular bulb diverticulum, sigmoid sinus anterior location and deep tympanic recesses.
A total of 1000 temporal bones were examined. The incidences of these variants were respectively (15.4%-18.6%), (38.6%-41.2%), (18.2%-4.6%), (2.6%-1.2%), (1.2%-0%), (8.6%-0%), (0%-0%). It was observed that only high jugular bulb (P < 0.001) and anteriorly located sigmoid sinus frequencies (P = 0.002) in the case group were statistically significantly higher than the control groups.
COM is a multifactorial disease and variants of middle ear have always been important in terms of potential risk for complication during surgery but rarely associated with COM as an etiology or as a consequence of the disease. We didn't find a positive correlation between COM and Koerner’s septum and facial canal defect. We ended up with a significant conclusion with the variants of dural venous sinuses -high jugular bulb, dehiscence of jugular bulb, diverticulum of jugular bulb and anteriorly located sigmoid sinus- that have been studied less and frequently associated with inner ear illnesses.
Core Tip: Chronic otitis media (COM) is a chronic inflammatory disease. It is frequently seen in developing countries. COM can cause hearing loss. In our study, the relationship between the variations of middle ear anatomy and COM was investigated. Not many studies have been done on this subject in the literature. Our study was conducted by comparing patients and control groups. The relationship between different anatomical variations in the population and COM was investigated.
- Citation: Gökharman FD, Şenbil DC, Aydin S, Karavaş E, Özdemir Ö, Yalçın AG, Koşar PN. Chronic otitis media and middle ear variants: Is there relation? World J Clin Cases 2023; 11(15): 3481-3490
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3481.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3481
Chronic otitis media (COM) is a chronic inflammatory disease of the tympanic cavity and mastoid cavity mucosa that is characterized by a non-intact tympanic membrane and is a worldwide public health problem that primarily affects developing countries[1].
COM is classified clinically into two groups: Active and inactive, with multiple subgroups within each. This disease is one of the most common causes of conductive hearing loss; additionally, it can be progressive, with middle ear bones eroding and inner ear destruction leading to sensorineural hearing loss. Even though they are extremely rare, intracranial complications can occur[2].
Because of its high prevalence and the morbidities it causes, it plays an important role in the daily work routines of ear nose throat doctors and radiologists.
Chronic middle ear inflammation is caused by both genetic factors that cause functional and anatomic variations in the middle ear that predispose to COM and multiple environmental factors. Depending on the stage and complication of the disease, it is treated medically or surgically[3].
Middle ear anatomy is unique for every ear and person with multiple variations. High resolution computed tomography (HRCT) of the temporal bone provides plenty of information about the complications and the factors that predisposes to disease or to decide the kind of procedure to operate before surgery and to warn the surgeon against the risk of possible complications that might happen due to the variants with its high resolution and perfect bone detail.
The surgery procedure of choice for eliminating the infected mastoid cells is tympanomastoidectomy. Knowing the anatomic variations of that middle ear is essential before the operation in order to perform it properly; otherwise, recurrence and complications are unavoidable[4].
There hasn't been enough research done on the anatomic variations of the middle ear in the literature. The purpose of this study is to compare the prevalence of anatomic variations between case and control groups, as well as to define and compare the potential erosive effect of COM on the middle ear anatomic structures.
This retrospective study included 500 patients with COM and 500 healthy controls. In both groups, the examinations were performed if a clinician deemed it necessary. Both groups were examined between March 2020 and August 2022.
Patients with maxillofacial anomalies, temporal bone fracture, prior ear surgery or mastoidectomy and younger than 18 years old were excluded from the study. Moreover, images which have low diagnostic quality due to motion artifacts were not included in the study.
We selected patients for our control group if COM was written as a final diagnosis in their electronic records and if there was mucosal thickening and soft tissue densities in middle ear space and mastoid cavity on imaging.
All images were examined by a radiologist who is 25 years experienced in head and neck radiology.
Seven variants were identified for review: Koerner’s septum (KS), facial canal dehiscence, high jugular bulb, jugular bulb dehiscence, jugular bulb diverticulum, anteriorly located sigmoid sinus and deep tympanic recesses.
All studies were performed using a standardized temporal bone protocol with a revolution EVO scanner (General Electric Company, United States) with 100 kV (peak), 190 mA, 0.53:1 pitch, Display field of view (DFOV) 18.0 cm acquired at 0.625 mm section thickness.
To identify variants, the following criteria were used.
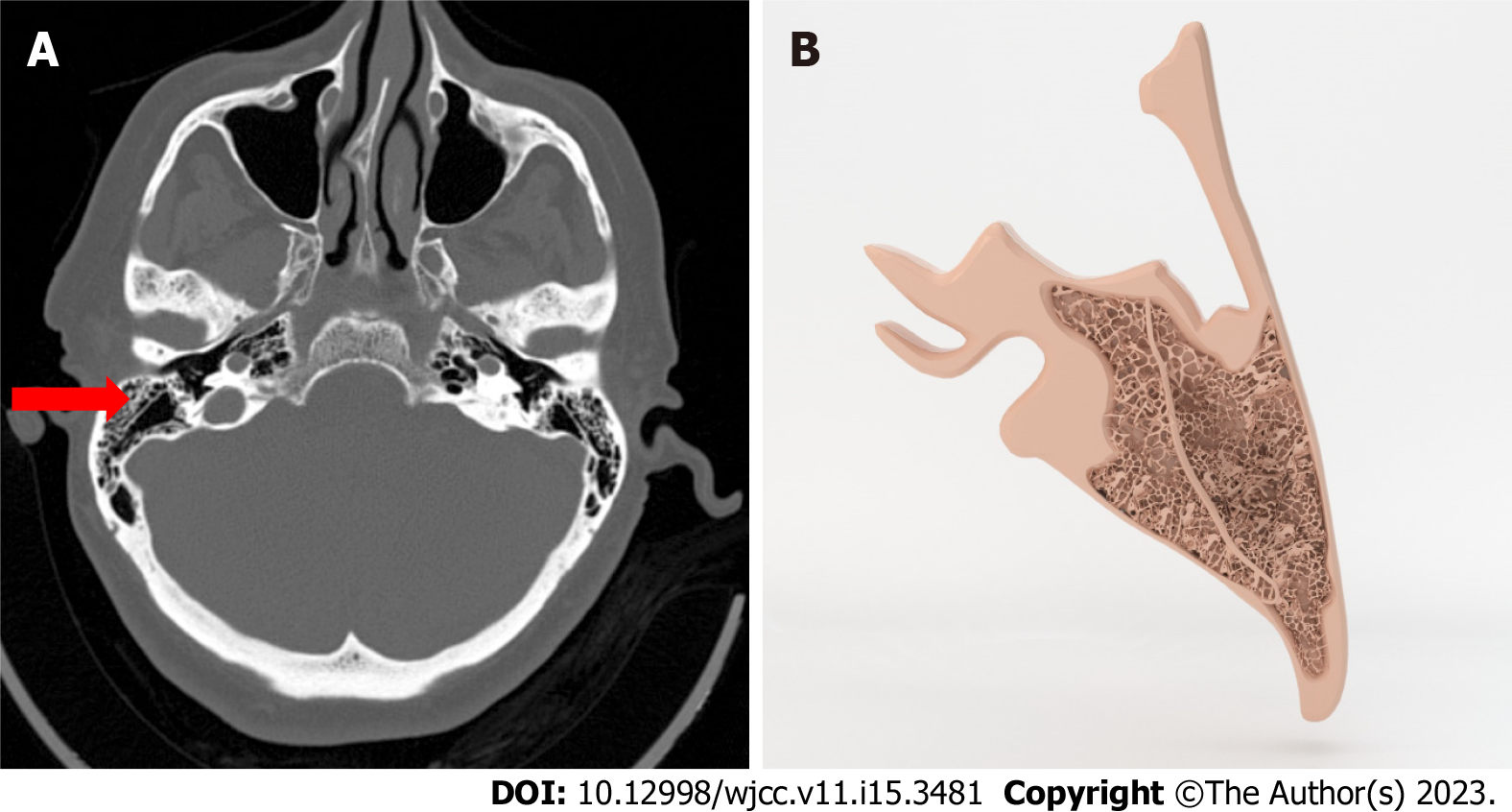
KS: KS was diagnosed on computerized tomography (CT) at the level of the antrum in the mastoid bone, with the appearance of a bone segment that is thicker and longer than the walls of air cells (Figure 1)[1].
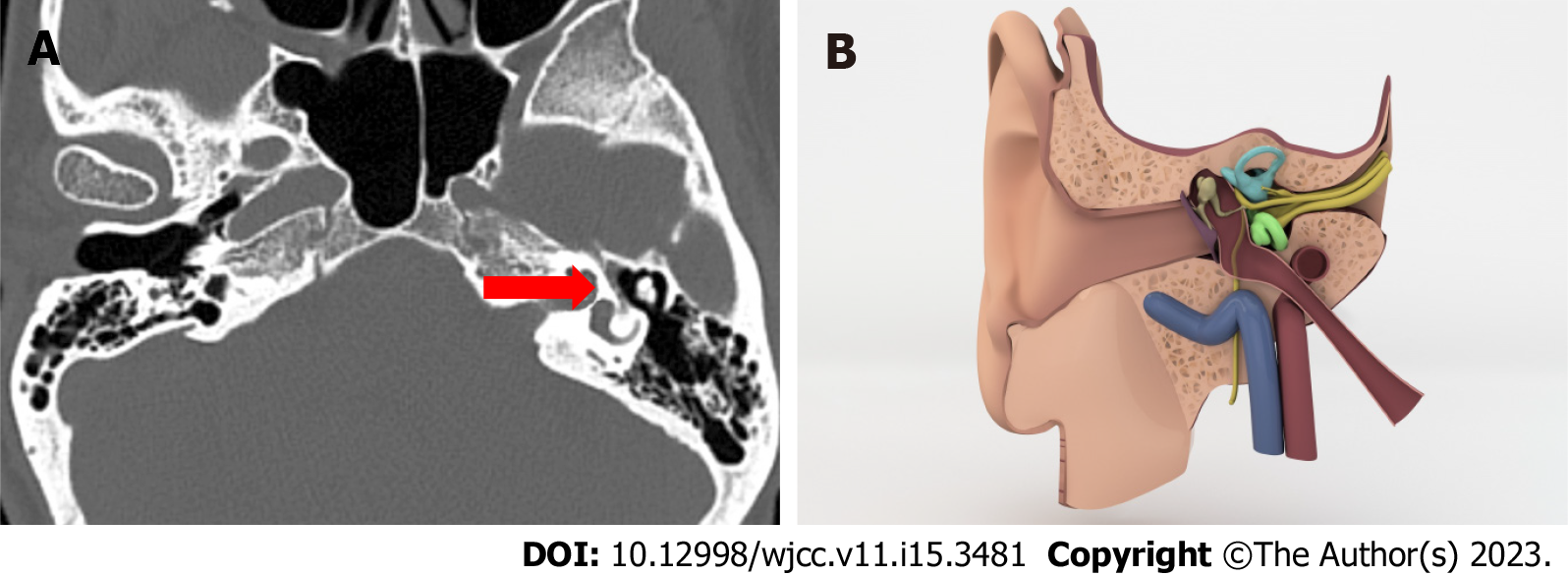
Facial canal dehiscence: Facial nerve dehiscence is diagnosed when there is a loss of integrity in the bone channel (Figure 2)[5].
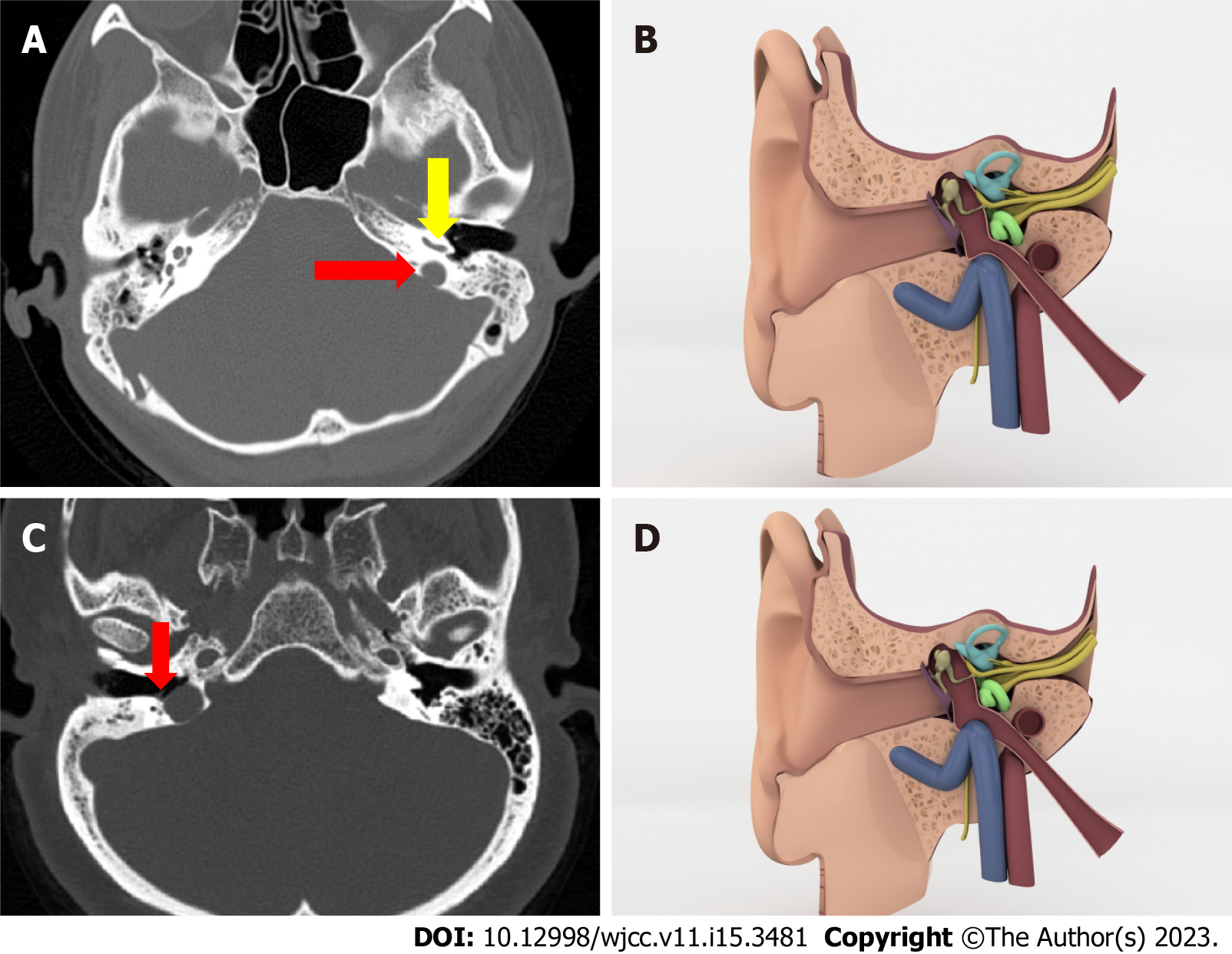
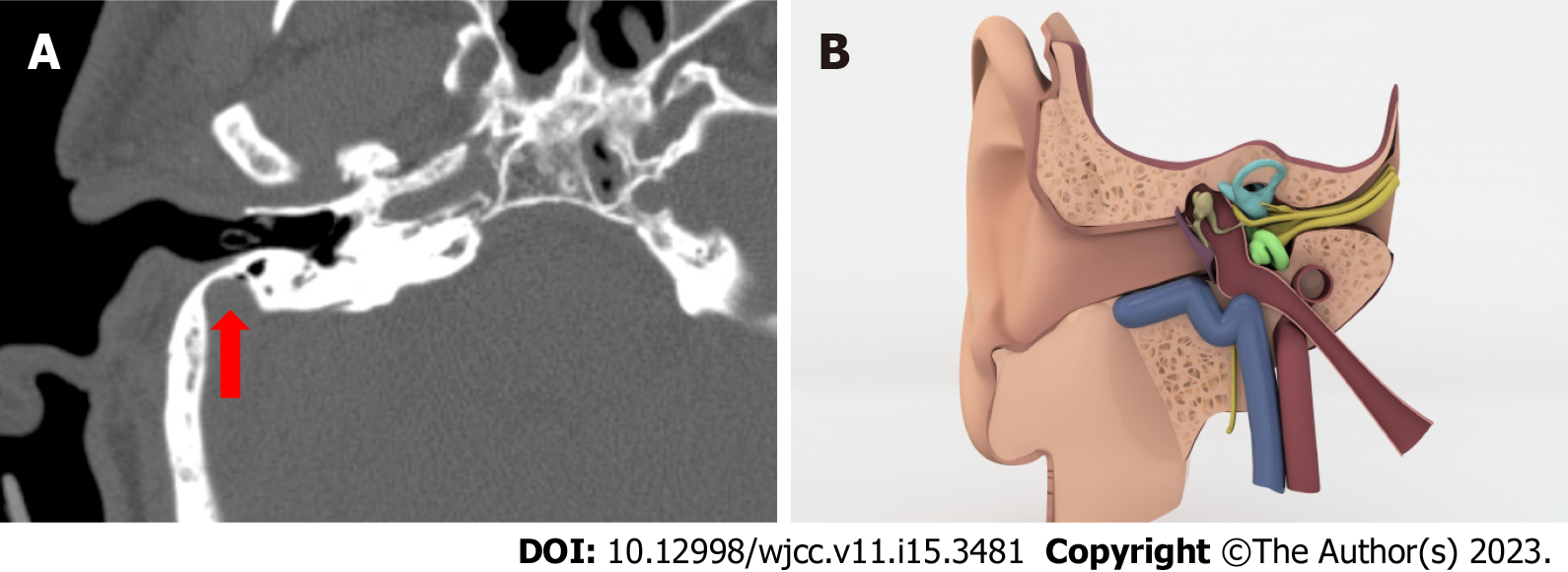
High riding jugular bulb: There are several criteria in the literature that indicate a high jugular bulb. We used the criterion that the roof of bulb reaches the cochlea’s basal return level (Figure 3A and B)[6].
Jugular bulb dehiscence: When there is no lamina bone that separates the JB from the middle ear cavity and the bulb is in direct contact with the middle ear cavity, we diagnosed jugular bulb dehiscence (Figure 3C and D)[7].
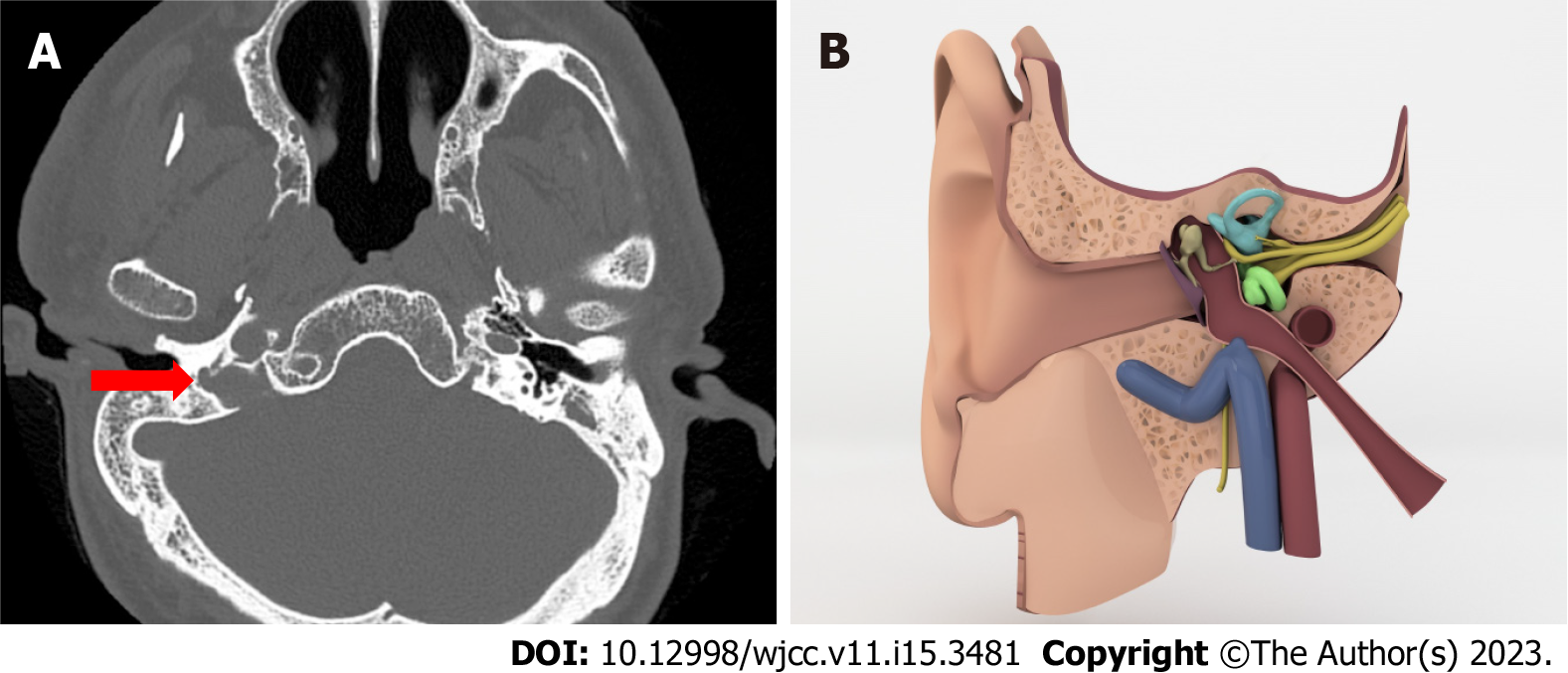
Jugular bulb diverticulum: The jugular bulb diverticulum is a polypoid protrusion that extends superior to the bulb, as well as less medially and posteriorly (Figure 4)[7].
Anteriorly located sigmoid sinus: There must be no anteroposterior distance between the posterior part of the bonny external auditory canal and the sigmoid sinus to diagnose the anteriorly located sigmoid sinus (Figure 5)[8].
Deep tympanic recess: If the depth of the recess is 6 mm or deeper on axial plane[9].
The Chi squared test and Fisher exact test (if necessary) were used to analyze categorical variables. The descriptive statistics were mean ± SD, median [min max] for numerical variables and numbers and percentages (%) for the categorical variables. The SPSS Windows version 23.0 package program was used for the statistical analyses and P values less than 0.05 were considered statistically significant.
There are 500 patients in both control and case series. Case series has 260 male, 240 female patients and median age is 48.32 (± 15.03). Control series has 225 male, 275 female patients, median age is 45.86 (± 15.48)
In terms of clinical features, both groups were compared in Table 1.
| Variantsa | Case (n = 500) | Control (n = 500) | P value |
| Koerner’s septum + | 77 (15.4) | 93 (18.6) | 0.178 |
| Koerner’s septum - | 423 (84.6) | 407 (81.4) | |
| Facial canal dehiscence + | 193 (38.6) | 206 (41.2) | 0.401 |
| Facial canal dehiscence - | 307 (61.4) | 294 (58.8) | |
| High jugular bulb + | 91 (18.2) | 23 (4.6) | < 0.001 |
| High jugular bulb - | 409 (81.8) | 477 (95.4) | |
| Jugular dehiscence + | 13 (2.6) | 6 (1.2) | 0.105 |
| Jugular dehiscence - | 487 (97.4) | 494 (98.8) | |
| Jugular diverticulum + | 4 (0.8) | 0 (0) | - |
| Jugular diverticulum - | 496 (99.2) | 0 (0) | |
| Anterior located sigmoid sinus + | 43 (8.6) | 19 (3.8) | 0.002 |
| Anterior located sigmoid sinus - | 457 (91.4) | 481 (96.2) | |
| Deep tympanic recess + | 0 (0) | 0 (0) | - |
| Deep tympanic recess - | 500 (100) | 500 (100) |
The high jugular bulb frequency was found to be significantly different between the case (91 individuals; 18.2%) and control (23 individuals; 4.6%) groups (P < 0.001).
The incidence of anteriorly located sigmoid sinus was significantly higher in case groups (43 individuals; 8.6%) than in control subjects (19 individuals; 3.8%) (P = 0.002).
However, we didn’t find a statistically significant difference in frequency with other variants.
In terms of the involvement sites of 500 cases diagnosed with COM and 500 healthy individuals are compared in Table 2.
| Case (n = 500) | Control (n = 500) | P value | |
| Koerner’s septum | |||
| Right | 36 (46.8) | 31 (33.3) | 0.057 |
| Left | 29 (37.7) | 34 (36.6) | |
| Bilateral | 12 (15.6) | 28 (30.1) | |
| Facial canal dehiscence | |||
| Right | 68 (35.4) | 49 (23.8) | 0.003 |
| Left | 84 (43.8) | 85 (41.3) | |
| Bilateral | 40 (20.8) | 72 (35) | |
| High jugular bulb | |||
| Right | 39 (42.9) | 10 (50) | 0.763 |
| Left | 40 (44) | 7 (35) | |
| Bilateral | 12 (13.2) | 3 (15) | |
| Jugular dehiscence | |||
| Right | 8 (61.5) | 3 (50) | 0.812 |
| Left | 4 (30.8) | 2 (33.3) | |
| Bilateral | 1 (7.7) | 1 (16.7) | |
| Jugular diverticulum | |||
| Right | 3 (75) | 0 (0) | --- |
| Left | 1 (25) | 0 (0) | |
| Bilateral | 0 (0) | 0 (0) | |
| Anterior located sigmoid sinus | |||
| Right | 26 (60.5) | 10 (62.5) | 0.547 |
| Left | 14 (32.6) | 6 (37.5) | |
| Bilateral | 3 (7) | 0 (0) | |
| Deep tympanic recess | |||
| Right | 0 (0) | 0 (0) | --- |
| Left | 0 (0) | 0 (0) | |
| Bilateral | 0 (0) | 0 (0) |
There was a significant difference in facial canal dehiscence sides between case and control groups. (P = 0.003) While facial canal dehiscence is more common on the right side in case series (68, 35.4%), it has been observed that it is more common in the bilateral region in control series (P = 0.03).
Aside from clinic and audiologic tests, imaging modalities are used as a supplement to diagnose middle ear pathologies and plan surgery protocols. HRCT of the temporal bone is the modality of choice for its superior high resolution of bone anatomy.
It is critical for radiologists and otologists to understand temporal bone variants in order to understand the etiology and treatment of COM, which is a multifactorial disease.
It is clinically significant that the air cells of the mastoid bone, which is formed by the union of squamous and petrous bones, are separated by a thin bone bridge named petrosquamous suture, as known as KS which is a common variant.
Previous researches in the literature have suggested that the KS inhibits pneumatization and drainage of the middle ear space by blocking aditus at the antrum and epitympanum. So it is thought as a reason of chronic middle ear diseases.
It’s also a reason of complication in mastoid surgery[10,11].
Antrum, the largest air cell, is located deeper than the KS that’s why it makes it more difficult to reach the antrum during surgery. Furthermore, during mastoid surgery, a well-developed, thick KS can be confused with the lateral wall of the sinus, and as a result, deep mastoid air cells cannot be cleaned out[10,11].
In our study, we found 77 patients (15.4%) with KS, and there are 93 cases (18.6%) with KS in the control series and statistically, it is not concluded to be significant with COM. Moreover we had more patients with this variant in our control group.
It is still important to report KS thus it is potentially a reason of complication in surgery.
The chorda tympani runs through a bone labyrinth known as the fascial canal. It enters the temporal bone via the internal acoustic canal and exits through the stylomastoid foramen. It has three distinct parts along the course in the temporal bone. Respectively, labyrinth, tympanic and mastoid. Even though canal dehiscence can occur anywhere along the bonny canal, the tympanic segment is the longest and most common location[12].
Facial canal dehiscence can be linked to ossification issues in inutero life. Despite the fact that it can be congenital, there are numerous studies that show a link between facial canal dehiscence and COM. It is probably a result of the erosive effect. That is why some consider it a pathology rather than a variant[13].
In imaging, a canal dehiscence should have precise edges so it can be differentiated from low mineralization. However micro dehiscence cannot be visualized on the temporal HRCT because of the thin structure and tortuous course of the canal. Which means, surgeons shouldn’t depend on the CT report completely and should make a decent assessment of the canal intraoperatively in terms of preventing chorda tympani injury which may require revision operations[14].
In our study we have 193 patients in case series (38.5%) and 205 patients in control series (41%) with facial canal dehiscence. These numbers make it the most common variant in all of six variants we look for. We ended up with similar numbers so it is not statistically significant. So our results are more compatible with congenital theory.
Yet, it is essential to report facial canal dehiscence as inflammation of the middle ear can spread through the facial nerve and cause facial palsy[15] and its vulnerability in terms of getting harmed during surgery.
Jugular bulb is the venous part between the horizontal part of the sigmoid sinus and the upper end of the internal jugular vena. The morphology of the dural venous sinuses and the jugular bulb can vary greatly and is usually asymmetric for individuals[16].
Jugular bulb is absent at birth and develops around the age of two as a result of changing venous sinus dynamics after adapting to the erect position[17].
Although there is no agreement on the changing positions of sigmoid sinus and jugular bulb, general opinion is some pathologies that shorten the distance between the outer ear and the sigmoid sinus and rises the jugular bulb by reducing the pneumatization of the mastoid air cells[18].
The roof of the jugular bulb is lower anatomically than the floor of the hypotympanum, and a bone lamina should separate the jugular bulb from the middle ear space[18].
Authors suggested more than one criteria to decide if the jugular bulb is high. Any of them can be used. In this study we used the one which says if the jugular bulb is higher than the basal turn of the cochlea and projects into the middle ear space.
In the literature, the prevalence of the high riding jugular bulb, the most common variant of jugular bulb, is 3.5%-7.5%. It is mostly on the right side where the venous system is dominant and it is accepted as a physiologic variant.
In our study, 90 cases (18%) in the case series and 23 cases (4.6%) in the control series have a high riding jugular bulb. This time we found it statistically significant. We ended up with a prevalence in the control series that is comparable to the literature, but it is significantly higher in the case series. In the case series, 38 patients have a high riding jugular bulb on the right side, 40 patients have it on the left side and 12 patients have it bilateral. There is not a right side dominancy unlike literature.
Jugular bulb dehiscence is when jugular bulb doesn’t have a bone lamina and is in contact with middle ear cavity. This condition creates a negative pressure effect in the cavity. This aggravates chronic middle ear inflammation and retracts tympanic membrane[19].
In our study, 13 patients (2.6%) have it, while 6 patients (1.2%) have it in the control series, indicating that there is no statistically significant relationship. However, it is worth noting that case series has twice as many people as case series.
Jugular bulb diverticulum is considered as a true venous anomaly and is a reason of retrotympanic vascular mass. It can occur by two different entities. Either there is a dehiscence on the floor of middle ear wall or there is not a dehiscence but jugular fossa is large without a dehiscent floor[20].
We only have four patients in the case series and none in the control series, and there is no statistically significant relationship.
Anterior located sigmoid sinus is a rare variant like jugular diverticulum.
We have 43 patients (8.6%) in our case group and 17 patients (3.4%) in our control group. There are statistically significantly more patients in the case group than in the control group.
Jugular bulb and other venous variants are frequently associated with tinnitus, but they should not be considered as a cause of tinnitus before ruling out other possible etiologies because they could be often found in the general population. As far as we know, COM and jugular bulb and sigmoid sinus variants have never been associated in the literature before and our study is the first one.
Deep tympanic recess was not found in the total 1000 patients.
We aimed to find out a cause and effect relation between some of temporal bone variants and COM that’s a multifactorial disease, characterized by the long term and destructive inflammation of the middle ear space and mastoid cellular. We didn't find a positive correlation between COM and KS and facial canal defect. There are more studies about both variants than others in the literature. On the other hand we ended up with a significant conclusion with the variants of dural venous sinuses -high jugular bulb, dehiscence of jugular bulb, diverticulum of jugular bulb and anteriorly located sigmoid sinus- that have been studied less and frequently associated with inner ear illnesses. Even though there is no agreement on why and how these variants occur, our study adds a new perspective to the literature by associating the variants with COM. However more studies should be done in the future to find out if these variants occur due to COM or COM occurs due to variants.
Our article examines the anatomical and variational differences in chronic otitis media (COM) patients.
COM is a multifactorial disease and variants of middle ear have always been important in terms of potential risk for complication during surgery but rarely associated with COM as an etiology or as a consequence of the disease.
The objectives of our research are to determine its relationship with anatomical variations in COM.
Our study is a retrospective study. The following conditions were investigated in our study: Koerner's septum (KS), facial canal dehiscence, high jugular bulb, jugular bulb dehiscence, jugular bulb diver
Only the case group's high jugular bulb and anteriorly located Sigmoid Sinus frequencies were statistically significantly higher than the control groups.
We discovered no link between COM and KS or facial canal defect. We came to a significant conclusion with the dural venous sinus variants -high jugular bulb, jugular bulb dehiscence, jugular bulb diverticulum, and anteriorly located sigmoid sinus- that have been studied less and frequently associated with inner ear illnesses.
The relationship between COM and anatomical variations is investigated in our study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Sangani V, United States; Zhang Y, China S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Wojciechowski T, Skadorwa T, Drożdż A, Ciszek B, Szopiński K. The radioanatomical assessment of the Körner's septum. Surg Radiol Anat. 2019;41:669-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, Grasso D, Barbiero C, Tamburlini G. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7:e36226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 682] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 3. | Schilder AG, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, Venekamp RP. Otitis media. Nat Rev Dis Primers. 2016;2:16063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 336] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 4. | Kusak A, Rosiak O, Durko M, Grzelak P, Pietruszewska W. Diagnostic imaging in chronic otitis media: does CT and MRI fusion aid therapeutic decision making? - a pilot study. Otolaryngol Pol. 2018;73:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Nager GT, Proctor B. The facial canal: normal anatomy, variations and anomalies. II. Anatomical variations and anomalies involving the facial canal. Ann Otol Rhinol Laryngol Suppl. 1982;97:45-61. [PubMed] |
| 6. | Sterkers O, Bozorg Grayeli A, Julien N, Bouccara D, Rihane S, Chaigne P. [Jugular bulb diverticulum mimicking Menière's disease. Surgical treatment]. Ann Otolaryngol Chir Cervicofac. 1993;110:363-371. [PubMed] |
| 7. | Vattoth S, Shah R, Curé JK. A compartment-based approach for the imaging evaluation of tinnitus. AJNR Am J Neuroradiol. 2010;31:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Potter GD. The ear, the surgeon and the radiologist. Hickey lecture, 1973. Am J Roentgenol Radium Ther Nucl Med. 1973;118:501-510. [PubMed] |
| 9. | Saito R, Igarashi M, Alford BR, Guilford FR. Anatomical measurement of the sinus tympani. A study of horizontal serial sections of the human temporal bone. Arch Otolaryngol. 1971;94:418-425. [PubMed] |
| 10. | Toros SZ, Karaca CT, Habeşoğlu TE, Noşeri H, Ertugay CK, Naiboğlu B, Egeli E. Is there a relation between mastoid aeration and Körner's septum? Eur Arch Otorhinolaryngol. 2010;267:1523-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Ozer E, Bayazit YA, Kara C, Mumbuç S, Kanlikama M, Gümüşburun E. Körner's septum (petrosquamosal lamina) and chronic ear disease. Surg Radiol Anat. 2004;26:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Măru N, Cheiţă AC, Mogoantă CA, Prejoianu B. Intratemporal course of the facial nerve: morphological, topographic and morphometric features. Rom J Morphol Embryol. 2010;51:243-248. [PubMed] |
| 13. | Spector JG, Ge X. Ossification patterns of the tympanic facial canal in the human fetus and neonate. Laryngoscope. 1993;103:1052-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Kozerska M, Skrzat J, Spulber A, Walocha J, Wroński S, Tarasiuk J. Micro-CT study of the dehiscences of the tympanic segment of the facial canal. Surg Radiol Anat. 2017;39:375-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Chan KC, Wang PC, Chen YA, Wu CM. Facial nerve dehiscence at mastoidectomy for cholesteatoma. J Int Adv Otol. 2011;7:311-316. |
| 16. | Manjila S, Bazil T, Kay M, Udayasankar UK, Semaan M. Jugular bulb and skull base pathologies: proposal for a novel classification system for jugular bulb positions and microsurgical implications. Neurosurg Focus. 2018;45:E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Friedmann DR, Eubig J, McGill M, Babb JS, Pramanik BK, Lalwani AK. Development of the jugular bulb: a radiologic study. Otol Neurotol. 2011;32:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Prasad KC, Basava CH, Gopinathan PN, Induvarsha G, Harshita RT, Ashok BK. A Revisit to High Jugular Bulb: A Newer Clinical Grading. Indian J Otolaryngol Head Neck Surg. 2018;70:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Friedmann DR, Eubig J, Winata LS, Pramanik BK, Merchant SN, Lalwani AK. Prevalence of jugular bulb abnormalities and resultant inner ear dehiscence: a histopathologic and radiologic study. Otolaryngol Head Neck Surg. 2012;147:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Wadin K, Thomander L, Wilbrand H. Effects of a high jugular fossa and jugular bulb diverticulum on the inner ear. A clinical and radiologic investigation. Acta Radiol Diagn (Stockh). 1986;27:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |