Published online Apr 26, 2023. doi: 10.12998/wjcc.v11.i12.2839
Peer-review started: December 30, 2022
First decision: January 17, 2023
Revised: February 27, 2023
Accepted: March 22, 2023
Article in press: March 22, 2023
Published online: April 26, 2023
Processing time: 116 Days and 17.1 Hours
Papillary thyroid cancer (PTC) is one of the well-differentiated thyroid tumors. Cutaneous metastasis from differentiated thyroid cancers occurs in < 1% of primary thyroid carcinomas but produces the worst survival prognosis. The multi-targeting tyrosine kinase inhibitor anlotinib has been approved to treat refractory advanced non-small-cell lung cancer as well as advanced soft-tissue and clear cell sarcomas in China.
In a patient with scalp metastasis caused by PTC, thyroid and skull metastasis tumor sizes were significantly reduced after a trial of neoadjuvant anlotinib therapy for 3 cycles. Anlotinib maintenance medication after thyroidectomy further reduced the metastatic skull tumor size thereby preventing the require
The outcome of the present trial confirmed the potential of anlotinib therapy to treat scalp metastasis induced by PTC and point the way for the treatment of similar diseases in the future.
Core Tip: Cutaneous metastasis to the scalp is a site in patients which occurs after papillary thyroid cancers (PTC). Tyrosine kinase inhibitors represent a new approach to treat certain types of thyroid cancer. Here we report a case of scalp metastasis caused by PTC, in which neoadjuvant therapy of anlotinib before thyroidectomy was beneficial in reducing the thyroid and skull metastasis tumor sizes. Anlotinib maintenance regimen after thyroidectomy reduced the size of the metastatic skull tumor further, which successfully avoided the need for craniotomy.
- Citation: Zhang LY, Cai SJ, Liang BY, Yan SY, Wang B, Li MY, Zhao WX. Efficacy of anlotinib combined with radioiodine to treat scalp metastasis of papillary thyroid cancer: A case report and review of literature. World J Clin Cases 2023; 11(12): 2839-2847
- URL: https://www.wjgnet.com/2307-8960/full/v11/i12/2839.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i12.2839
Papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC) are the two most common differentiated thyroid tumors (DTC)[1,2], with lymph nodes metastasis occurring frequently, but cutaneous metastasis to the scalp being a rare site. The average survival time for patients with thyroid carcinoma skin metastasis was about 19 mo from the end of the last century[1]. Most patients with thyroid cancer undergo surgery combined with subsequent radioactive iodine (RAI) therapy to clear any remaining tumor tissue and to prevent thyroid cancer recurrence[3]. RAI therapy is also given for more advanced cancers and/or cancers that have spread to lymph nodes or distant areas[4]. Apart from these regular treatment regimens, novel target therapies including a tyrosine kinase inhibitor (TKI) has become a new approach to treat certain types of thyroid cancer.
For example, sorafenib was approved by the United States Food and Drug Administration (FDA) in 2014 for the treatment of radioiodine-resistant DTC with metastasis[5]. Anlotinib is a new TKI that targets the vascular endothelial growth factor, fibroblast growth factor and platelet-derived growth factor receptors as well as c-kit[6]. Anlotinib was approved as third-line therapy for refractory advanced non-small-cell lung cancer in 2018, and as second-line treatment for advanced soft-tissue and clear cell sarcomas in 2019 in China[7]. In a preclinical study, anlotinib inhibited the cell viability of PTC, suppressed migration of thyroid cancer cells in vitro and also xenograft thyroid tumor growth in mice[8]. In a phase 2b study of medullary thyroid cancer patients treated with anlotinib, the median progression free survival (PFS) was found to be significantly prolonged (20.7 vs 11.1 mo)[9]. Another recent case of a patient with recurrent and metastatic radioactive iodine-refractory DTC (RAIR-DTC) treated with anlotinib showed a partial response after 2 cycles of anlotinib treatment and a PFS of 37 mo[10].
A 72-year-old woman presented to our hospital with a scalp mass on November 3, 2020.
A scalp mass biopsy puncture indicated that thyroid cancer metastasis.
The patient had no previous history of any illnesses.
The patient had no previous personal and family history.
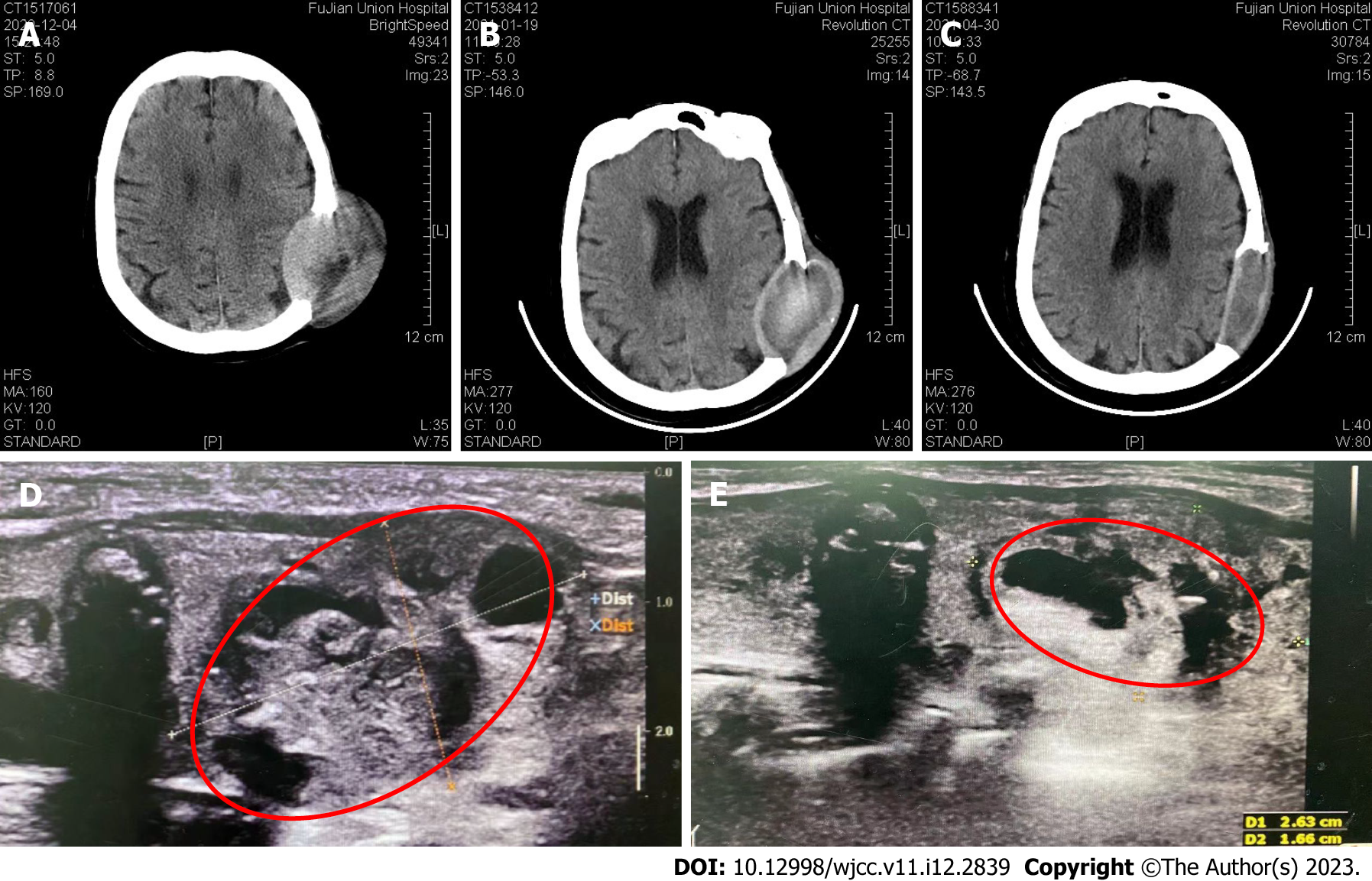
On December 4, 2020, a 3.0 cm × 3.0 cm nodule was palpable on the left thyroid and a 4.0 cm × 3.0 cm nodule was palpable on the right thyroid. In addition, a 6.5 cm × 5.0 cm mass was palpable in the left temporoparietal junction region.
Immunohistochemical results: TTF-1 (+), CK7 (+), NapsinA (+), CDX-2 (-).
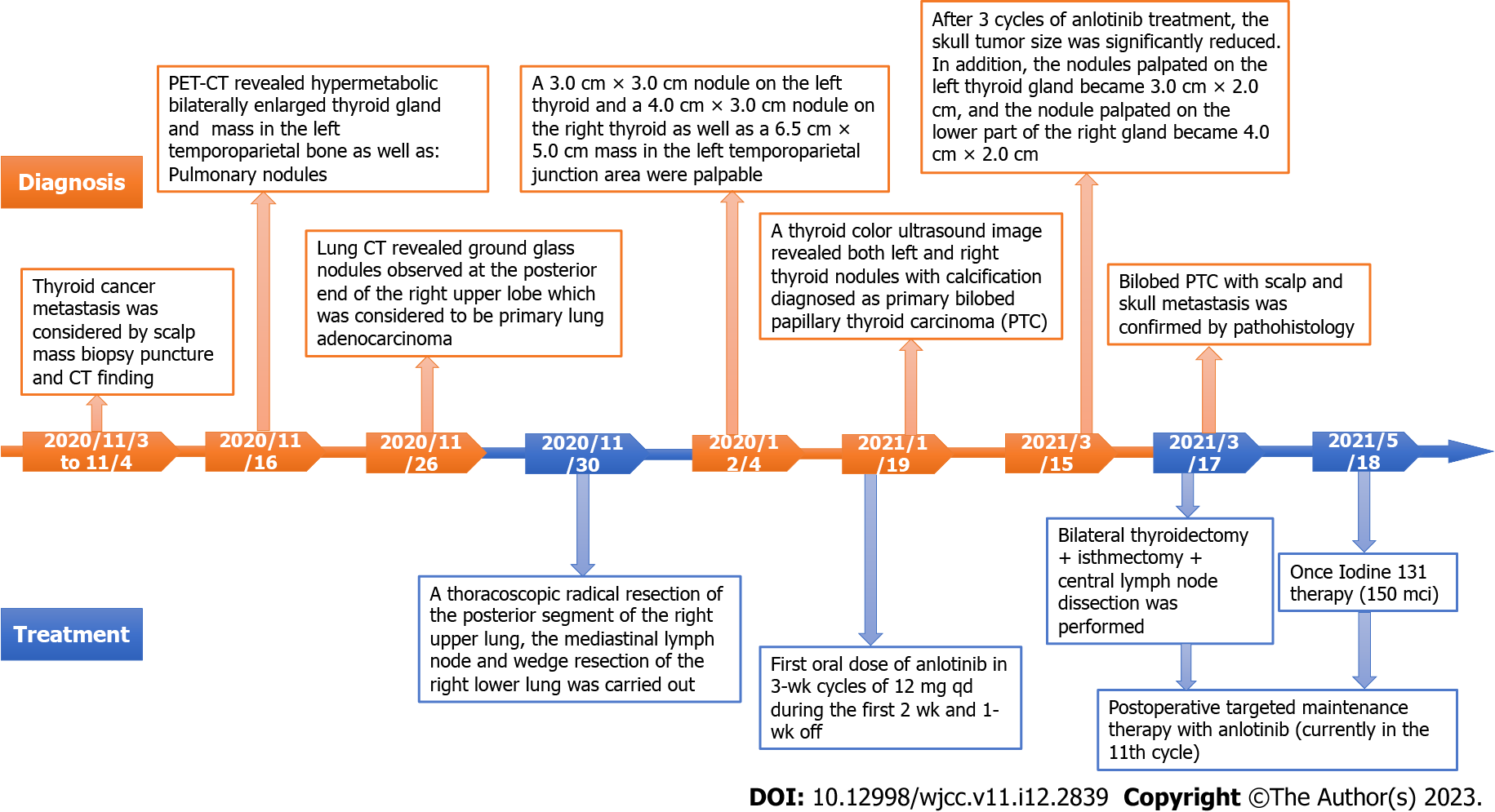
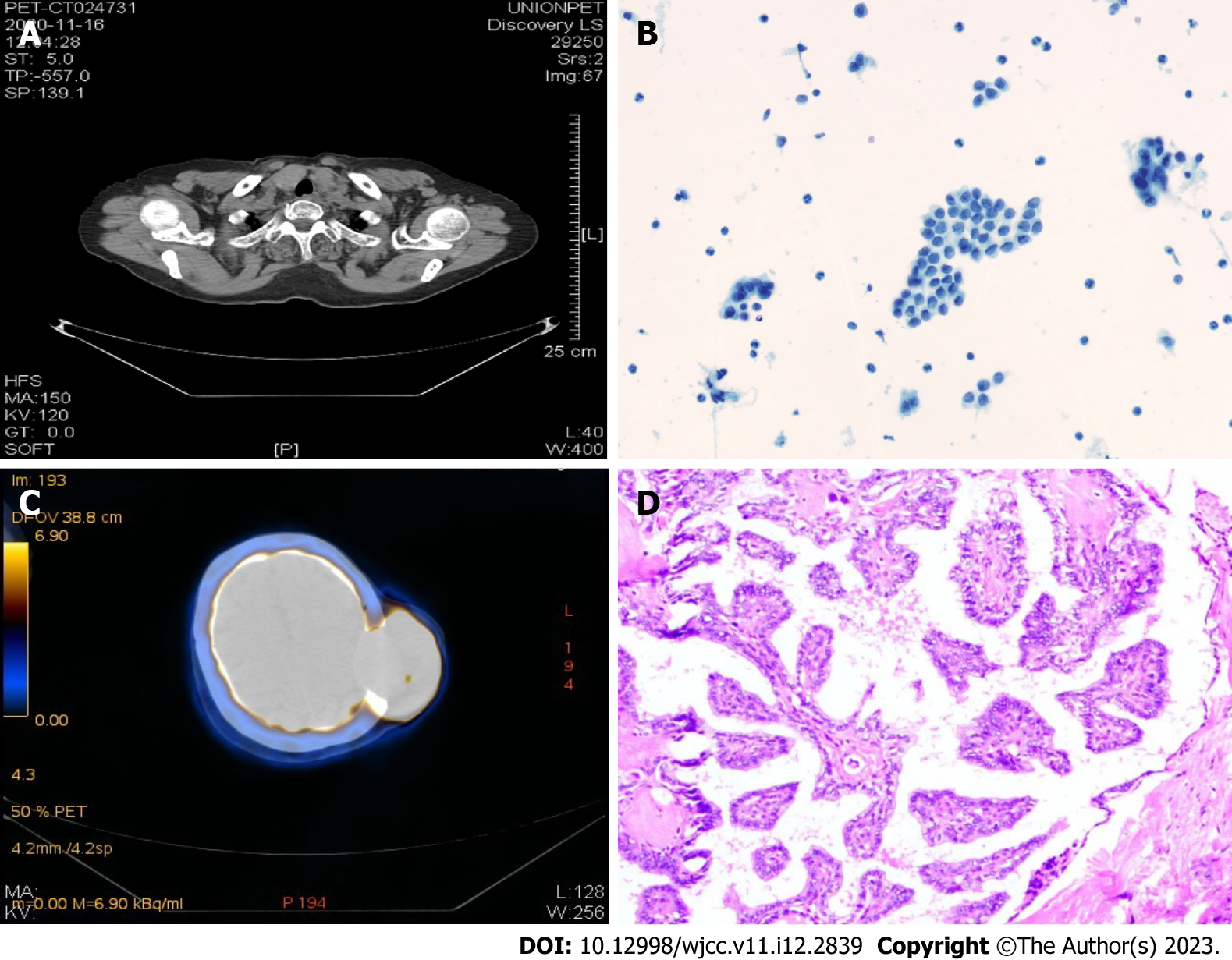
A scheme of the diagnoses and treatments in chronological order is provided in Figure 1. On November 16, 2020, a positron emission tomography-computed tomography (PET-CT) imaging diagnosis was conducted in which a hypermetabolic mass was revealed in the left parietal skull bone, indicating skin metastasis (Figure 2A), which was confirmed by a fine-needle aspiration biopsy diagnosis (Figure 2B). In addition, a bilaterally enlarged thyroid gland with increased metabolism was found (Figure 2C).
Lung CT was performed on November 26, 2020, which revealed ground glass nodules at the posterior end of the right upper lung lobe and in the dorsal portion of the right lower lung lobe. The ground glass nodules observed at the posterior end of the right upper lobe may correlate with the possibility of primary lung adenocarcinoma and oblique fissure. Both lungs exhibited scattered ground glass patches, likely due to chronic inflammation.
On January 19, 2021, A thyroid color ultrasound image revealed a left thyroid nodule with calcification (Chinese TI-RADS category 4) and a right thyroid nodule with calcification (Chinese TI-RADS category 3c)[11] (Figure 3).
According to the findings, bilobed PTC with scalp and skull metastasis was diagnosed based on histopathology data and PET-CT imaging. Next-generation sequencing (NGS) by an Illumina Novaseq 6000 system (Illumina, San Diego, CA, United States) revealed mutations in the BRAF, KRAS and IGF1R genes of the PTC. In addition, neck metastasis of the PTC and primary lung cancer were provisionally diagnosed.
On November 30, 2020, a thoracoscopic radical resection of the right upper lung cancer (resection of the posterior segment of the right upper lung + mediastinal lymph node sampling) plus wedge resection of the right lower lung were carried out. The operation went without complications and the postoperative recovery of the patient was good.
On January 19, 2021, the patient was given the first oral dose of anlotinib in 3-wk cycles of 12 mg qd during the first 2 wk and 1-wk off (Figure 3B). After 3 cycles of anlotinib treatment, the skull tumor size was significantly reduced. The nodule palpated on the left thyroid gland became 3.0 cm × 2.0 cm, and the nodule palpated on the lower part of the right gland became 4.0 cm × 2.0 cm. In addition, preoperative color ultrasonography also showed that the thyroid tumor size was significantly reduced after neoadjuvant anlotinib therapy (Figure 3E).
A thyroidectomy was performed on March 17, 2021 with the left thyroid lobule and isthmus and the right thyroid lobule and lymph nodes in the central region successfully removed. The histopathology confirmed PTC (Figure 2D). Targeted maintenance therapy of anlotinib was administered after the operation in order to avoid craniotomy (Figure 3C).
By postoperative day 2 (March 18 2021), the neck incision had healed and the patient recovered well. The intact parathyroid hormone concentration was 1.10 pmol/L and the electrolytic calcium concentration was 2.21 mmol/L.
Once iodine-131 therapy (dosage: 150 mci) was performed after the operation on May 18, 2021 and continued in combination with anlotinib (currently in the 11th cycle), a regimen which successfully avoided the need for craniotomy.
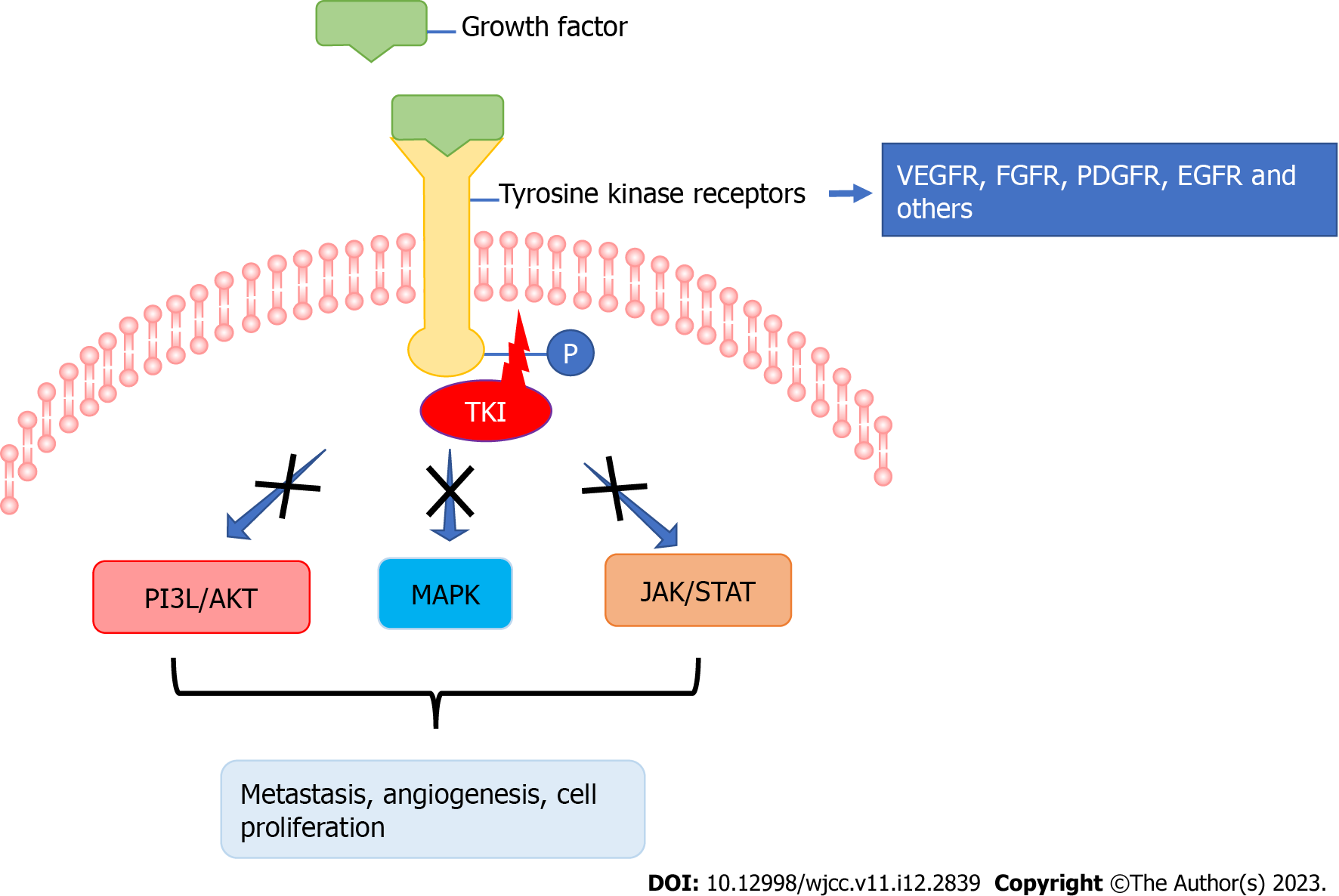
The 4 main subtypes of thyroid carcinoma are papillary, follicular, medullary and anaplastic forms[12], with PTC being the most frequently occurring followed by FTC[13,14]. In this case report, the potential of anlotinib therapy to treat scalp metastasis induced by PTC was demonstrated. Scalp metastasis of thyroid cancer has been also been described in other case reports[15-22] (Table 1). Most of the patients were females of advanced age with FTC and in the majority of the cases the scull metastasis had been diagnosed before thyroid cancer, as in the present case. Only 2 patients received post-operative TKI medication and the lung was the major organ of cancer lesions, which were supposed to be metastatic (Table 1). TKIs are small molecules which inhibit the activity of tyrosine kinases (Figure 4).
| Gender | Age | Type | Time of diagnosis | Metastasis | Treatment | Ref. |
| Female | 51 | Follicular | Before thyroid cancer | Lung, bone | Surgery, I-131 | [15] |
| Female | 64 | Papillary | After medical history of total thyroidectomy | Lung, liver | Excisional biopsy I-131 | [16] |
| Female | 37 | Follicular | Before thyroid cancer | Lung, bone, cranium | Resection, I-131, post-operative TKI | [17] |
| Female | 60 | Follicular | Before thyroid cancer | No | Surgery | [18] |
| Female | 70 | Follicular | Before thyroid cancer | Lung | Surgery, I-131 | [19] |
| Female | 75 | Follicular | 1 year after thyroidectomy | Lung | Surgery, I-131 | [20] |
| Male | 65 | Papillary | Before thyroid cancer | Vertebral and iliac lesions | Shave biopsy, I-131, post-operative sorafenib | [21] |
| Female | 49 | Follicular | 4 years after thyroidectomy | No | Surgery, I-131 | [22] |
TKI therapy is usually the final option for metastatic lesions, which are normally incurable by surgery and/or RAI therapy combined, but encouragingly a study involving 147 patients demonstrated that TKI treatment improved the prognosis of patients with distant metastasis and progressive disease[23]. A number of TKIs have been approved by the FDA to treat thyroid cancer, including vandetanib, cabozantinib, sorafenib and lenvatinib (Table 2). Vandetanib and cabozantinib were the first and second approved drugs for the therapy of advanced and progressive and/or symptomatic medullary thyroid cancer[24,25]. Sorafenib was approved for the treatment of 131I-refractory DTC after the findings of the phase 3 DECISION clinical trial[26]. Lenvatinib is an anti-angiogenic TKI that has been approved for the treatment of advanced and progressive 131I- refractory DTC[27]. A real-world study conducted in the United States demonstrated that treatment with first-line lenvatinib followed by subsequent second-line therapy delivered a clear clinical benefit[28]. A study carried out in Korea mainly focused on the adverse events of TKIs (lenvatinib and sorafenib) elicited in patients with DTC. Seventy-one cases (lenvatinib, n = 23; sorafenib, n = 48) were involved without new safety concerns being identified for either drug. Most AEs were managed with dose modification and medical therapy[29]. In former in vitro experiments, anlotinib reduced the viability of PTC and anaplastic thyroid cancer cell lines, most likely due to aberrant spindle assembly, G2/M arrest and TP53 activation, while in a murine model anlotinib suppressed migration and growth of thyroid tumor xenografts[8]. Recently, a phase 2 trial of anlotinib in Chinese patients with radioiodine-refractory DTC showed promising results with a median PFS of 40.54 mo in the treatment group compared to 8.38 mo in the placebo group at a data cutoff date of January 2020[30].
| Medication | Target | Indication | Ref. |
| Vandetanib | RET-tyrosine kinase, VEGFR and EGFR | Unresectable locally advanced or metastatic thyroid cancer | [24] |
| Cabozantinib | c-Met- tyrosine kinase, VEGFR 2, RET-tyrosine kinase, AXL | RAIR locally advanced or metastatic thyroid cancer after VEGFR-targeted therapy | [25] |
| Sorafenib | VEGFR 1-3, PDGFR and RAF and RET-kinases | Metastatic RAIR thyroid cancer | [26] |
| Lenvatinib | VEGFRs 1-3, FGFRs 1- 4, PDGFR-α, RET and KIT signaling | RAIR thyroid cancer | [27] |
Nevertheless, the present case report can be considered as a kind of pilot study. Besides conservative treatments, anlotinib has been used as neoadjuvant therapy, which significantly reduced the sizes of a metastatic skull tumor and of the primary PTC, while in combination with anlotinib maintenance medication a craniotomy was avoided.
NGS analysis of the PTC found mutations in the BRAF, KRAS and IGF1R genes, which did not match with the previously described undeferential thyroid cancer specific mutations of the TERT promoter, RET fusion and TP53[31]. On the other hand, BRAF mutations have been found to be key drivers of DTC, leading to the proposal of BRAF-directed therapies[32]. In line with the findings of the present study, a recent case report found that anlotinib was effective for the treatment of a RAIR-DTC patient with TERT promoter and BRAF mutations[10]. Another case report of a RAIR-DTC PTC patient with a BRAFV600E mutation noted that the patient developed tumor progression, with clinical symptoms that worsened after dabrafenib-trametinib withdrawal[33]. In our case we will continue to monitor the outcome of anlotinib maintenance therapy.
A limitation of the present study was the lack of NGS and histopathology data about the lung lesions and the neck lymph nodes, which had been provisionally diagnosed as primary lung cancer and PTC metastasis into the neck.
Neoadjuvant therapy of anlotinib before surgery was beneficial in reducing the thyroid tumor volume, but also that of the metastatic skull tumor. A craniotomy was thus avoided by using anlotinib maintenance therapy. Our findings point the way for the treatment of similar diseases in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia; Watanabe T, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Dahl PR, Brodland DG, Goellner JR, Hay ID. Thyroid carcinoma metastatic to the skin: a cutaneous manifestation of a widely disseminated malignancy. J Am Acad Dermatol. 1997;36:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Koller EA, Tourtelot JB, Pak HS, Cobb MW, Moad JC, Flynn EA. Papillary and follicular thyroid carcinoma metastatic to the skin: a case report and review of the literature. Thyroid. 1998;8:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Carballo M, Quiros RM. To treat or not to treat: the role of adjuvant radioiodine therapy in thyroid cancer patients. J Oncol. 2012;2012:707156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Lee SL. Radioactive iodine therapy. Curr Opin Endocrinol Diabetes Obes. 2012;19:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Thomas L, Lai SY, Dong W, Feng L, Dadu R, Regone RM, Cabanillas ME. Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist. 2014;19:251-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 433] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 7. | Li S. Anlotinib: A Novel Targeted Drug for Bone and Soft Tissue Sarcoma. Front Oncol. 2021;11:664853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Ruan X, Shi X, Dong Q, Yu Y, Hou X, Song X, Wei X, Chen L, Gao M. Antitumor effects of anlotinib in thyroid cancer. Endocr Relat Cancer. 2019;26:153-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Li D, Chi Y, Chen X, Ge M, Zhang Y, Guo Z, Wang J, Chen J, Zhang J, Cheng Y, Li Z, Liu H, Qin J, Zhu J, Cheng R, Xu Z, Zheng X, Tang P, Gao M. Anlotinib in Locally Advanced or Metastatic Medullary Thyroid Carcinoma: A Randomized, Double-Blind Phase IIB Trial. Clin Cancer Res. 2021;27:3567-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Su Y, Cheng S, Qian J, Zhang M, Li T, Zhang Y, Diao C, Zhang L, Cheng R. Case Report: Anlotinib Therapy in a Patient With Recurrent and Metastatic RAIR-DTC Harboring Coexistent TERT Promoter and BRAF(V600E) Mutations. Front Oncol. 2021;11:626076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Xue E, Zheng M, Zhang S, Huang L, Qian Q, Huang Y. Ultrasonography-Based Classification and Reporting System for the Malignant Risk of Thyroid Nodules. J Nippon Med Sch. 2017;84:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Barbaro D, Desogus N, Boni G. Pituitary metastasis of thyroid cancer. Endocrine. 2013;43:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Shen J, Wang S, Zhao X, Shao X, Jiang X, Dai Y, Xu S, Pan X. Skull metastasis from follicular thyroid carcinoma: report of three cases and review of literature. Int J Clin Exp Pathol. 2015;8:15285-15293. [PubMed] |
| 14. | Cabanillas ME, Ryder M, Jimenez C. Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocr Rev. 2019;40:1573-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 15. | Cihan BY, Koc A, Tokmak TT. The Role of Radiotherapy in Skull Metastasis of Thyroid Follicular Carcinoma. Klin Onkol. 2019;32:300-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Aghasi MR, Valizadeh N, Soltani S. A 64 year-old female with scalp metastasis of papillary thyroid cancer. Indian J Endocrinol Metab. 2011;15:S136-S137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Huang TC, Cheng YK, Chen TW, Hsu YC, Liu EW, Chen HH. A 'silent' skull metastatic follicular thyroid carcinoma mimicking as a benign scalp tumor in a pregnant woman. Endocrinol Diabetes Metab Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Singh V, Singh A, Bhadada SK, Nada R. Isolated dural metastasis of follicular carcinoma of the thyroid presenting as scalp swelling. J Cancer Res Ther. 2020;16:S248-S249. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Monti E, Dono M, Gonella E, Spina B, Pitto F, Petrogalli F, Conte L, Ambrosetti E, Minuto MN, Ansaldo GL, Morbelli S, Zupo S, Giusti M. An H-TERT Mutated Skin Metastasis as First Occurrence in a Case of Follicular Thyroid Carcinoma. Front Endocrinol (Lausanne). 2019;10:513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Nwaeze O, Obidike S, Mullen D, Aftab F. Follicular variant papillary thyroid carcinoma with a twist. Int J Surg Case Rep. 2015;8C:107-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Phelan PS, Mull JL, Rajput MZ, Musiek AC. Concurrent metastases of papillary thyroid carcinoma to the scalp and Meckel's cave. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Sager S, Yilmaz S, Doner RK, Niyazoglu M, Halac M, Kanmaz B. A rare case of solitary subcutaneous scalp metastasis from follicular thyroid carcinoma revealed with positron emission tomography/computed tomography: a case report and review. J Cancer Res Ther. 2014;10:431-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Iwasaki H, Yamazaki H, Takasaki H, Suganuma N, Sakai R, Nakayama H, Hatori S, Toda S, Masudo K. Treatment outcomes of differentiated thyroid cancer with distant metastasis improve by tyrosine kinase inhibitors. Oncol Lett. 2019;17:5292-5300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1067] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 25. | Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, Hoff AO, Hitre E, Bowles DW, Hernando J, Faoro L, Banerjee K, Oliver JW, Keam B, Capdevila J. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1126-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 26. | Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1122] [Article Influence: 102.0] [Reference Citation Analysis (1)] |
| 27. | Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1346] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 28. | Kish JK, Chatterjee D, Wan Y, Yu HT, Liassou D, Feinberg BA. Lenvatinib and Subsequent Therapy for Radioactive Iodine-Refractory Differentiated Thyroid Cancer: A Real-World Study of Clinical Effectiveness in the United States. Adv Ther. 2020;37:2841-2852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Kim SY, Kim SM, Chang H, Kim BW, Lee YS, Chang HS, Park CS. Safety of Tyrosine Kinase Inhibitors in Patients With Differentiated Thyroid Cancer: Real-World Use of Lenvatinib and Sorafenib in Korea. Front Endocrinol (Lausanne). 2019;10:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Chi Y, Gao M, Zhang Y, Shi F, Cheng Y, Guo Z, Ge M, Qin J, Zhang J, Li Z, Zhou X, Huang R, Chen X, Liu H, Cheng R, Xu Z, Zheng X, Li D, Tang P. Anlotinib in locally advanced or metastatic radioiodine-refractory differentiated thyroid carcinoma: A randomized, double-blind, multicenter phase II trial. Ann Oncol. 2020;31 Suppl 6:S1347. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Li Q, Zhang X, Feng J, Cheng D, Cai L, Dai Z, Zhao S, Li J, Huang J, Fang Y, Zhu H, Wang D, Wang S, Ma T, Lu X. Case Report: Next-Generation Sequencing Reveals Tumor Origin in a Female Patient With Brain Metastases. Front Oncol. 2021;11:569429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Pozdeyev N, Rose MM, Bowles DW, Schweppe RE. Molecular therapeutics for anaplastic thyroid cancer. Semin Cancer Biol. 2020;61:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Martín-Soberón MC, Ruiz S, De Velasco G, Yarza R, Carretero A, Castellano D, Sepúlveda-Sánchez JM. Pneumatosis intestinalis in a radioactive iodine-refractory metastasic thyroid papillary carcinoma with BRAF(V600E) mutation treated with dabrafenib-trametinib: a case report. J Med Case Rep. 2021;15:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |