Published online Apr 26, 2023. doi: 10.12998/wjcc.v11.i12.2825
Peer-review started: January 19, 2023
First decision: February 2, 2023
Revised: February 10, 2023
Accepted: March 24, 2023
Article in press: March 24, 2023
Published online: April 26, 2023
Processing time: 96 Days and 15.5 Hours
Pancreatic neuroendocrine tumors (NETs) account for about 1%–2% of pancreatic tumors and about 8% of all NETs. Computed tomography (CT), magnetic resonance imaging, and endoscopic ultrasound are common imaging modalities for the diagnosis of pancreatic NETs. Furthermore, somatostatin receptor imaging is of great value for diagnosing pancreatic NETs. Herein, we report the efficacy of technetium-99m methoxy-2-isobutylisonitrile (99mTc-MIBI) single photon emission CT (SPECT)/CT for detecting pancreatic NETs.
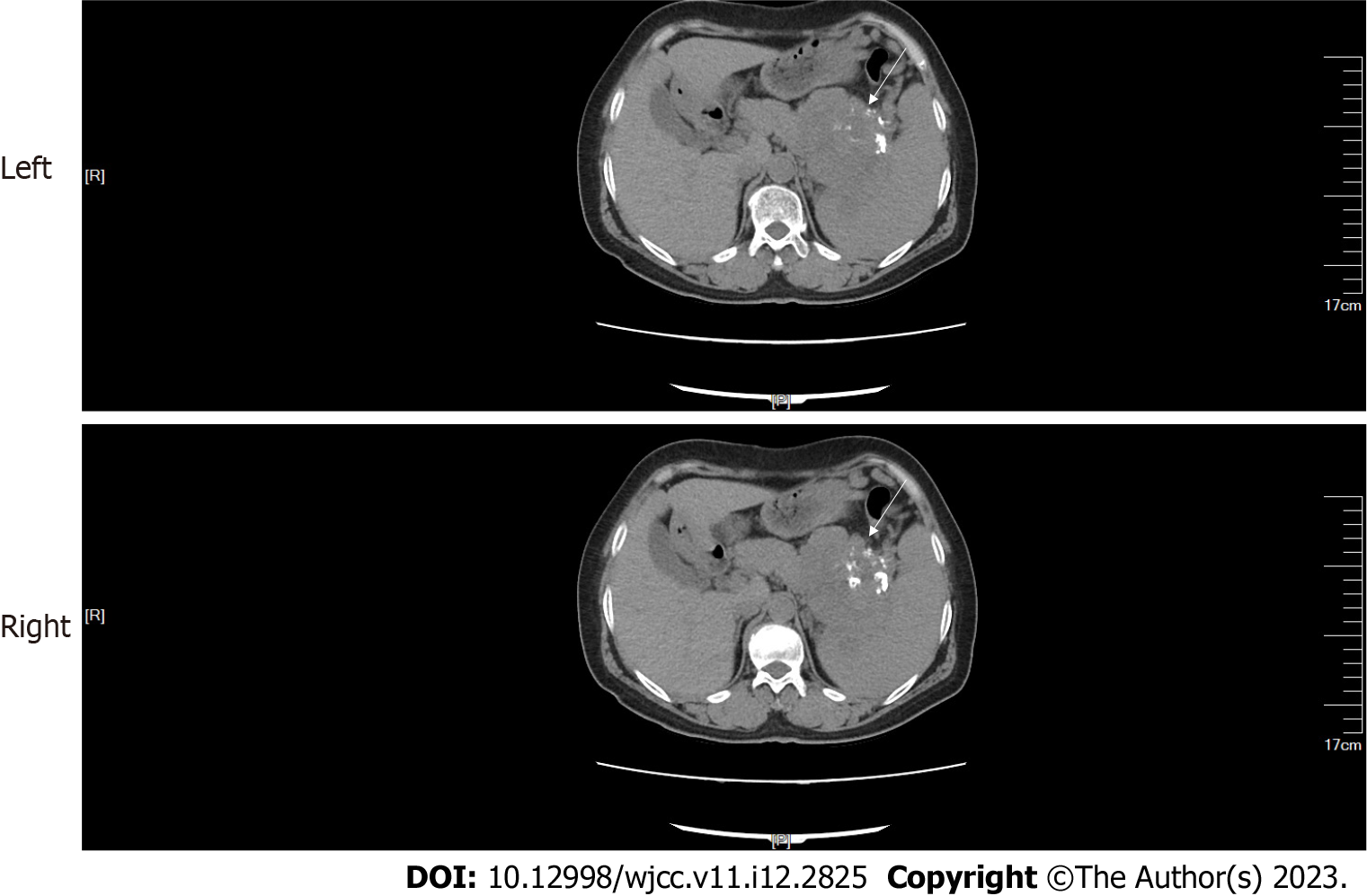
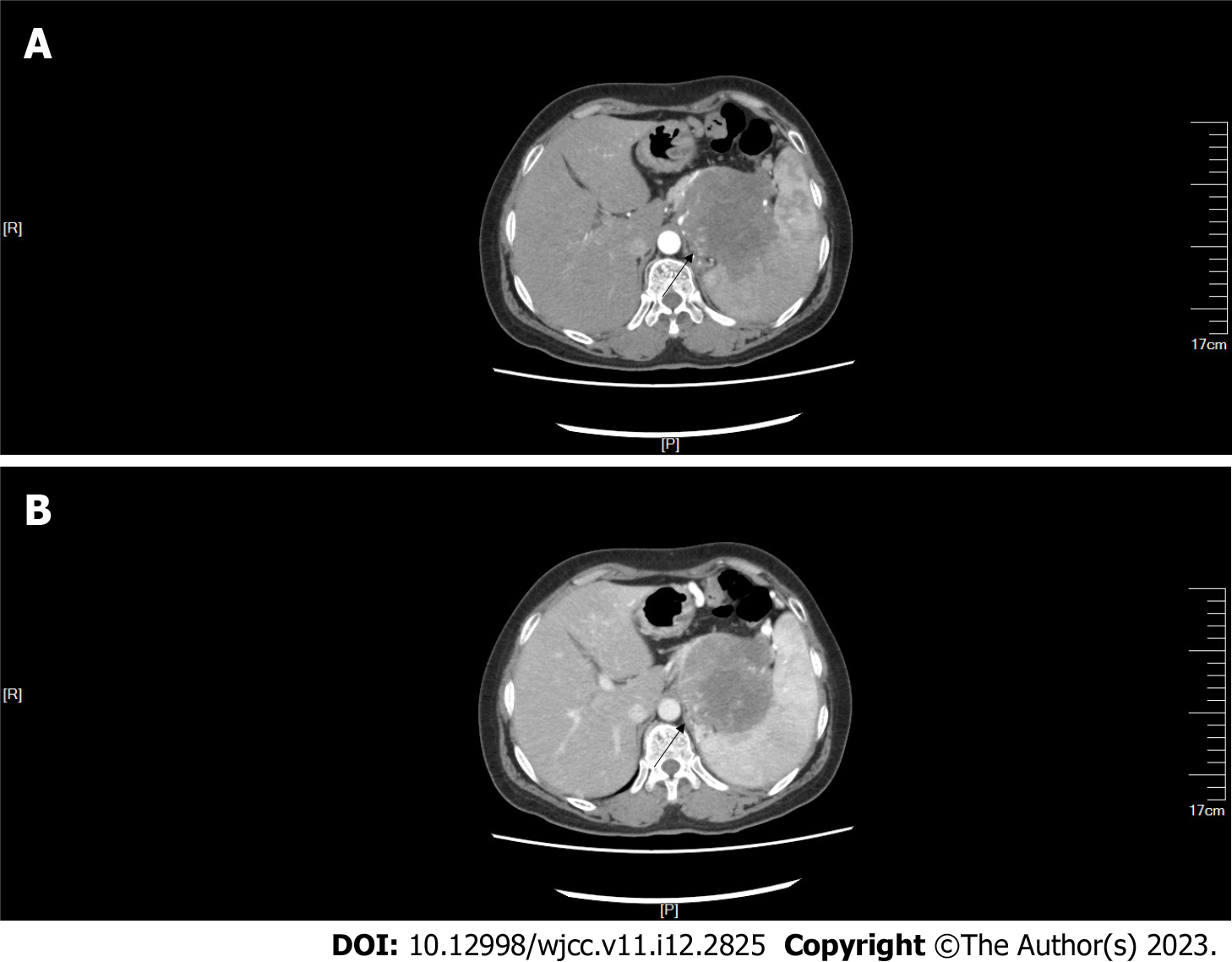
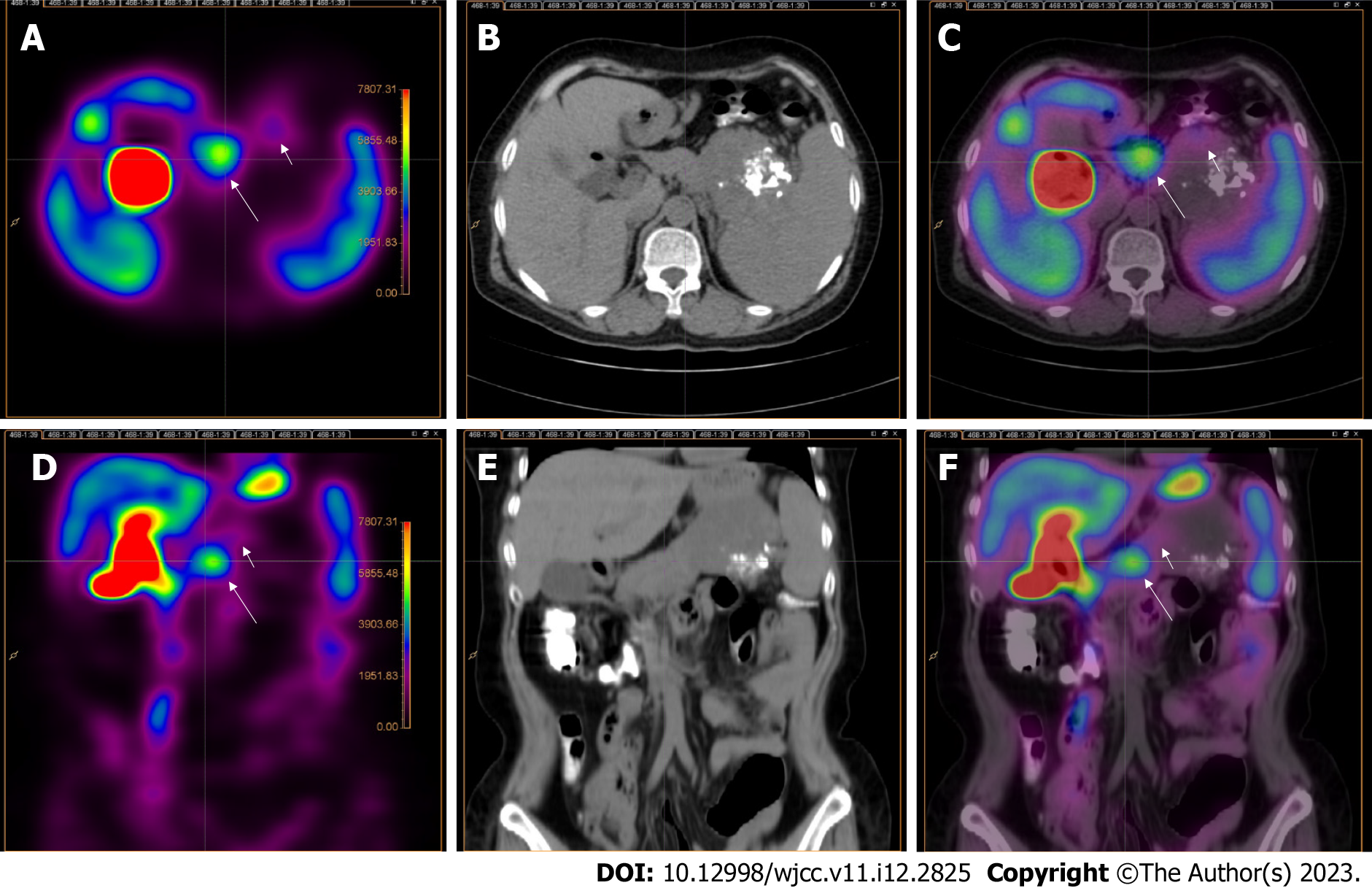
A 57-year-old woman presented to our hospital with a 1-d history of persistent upper abdominal distending pain. The distending pain in the upper abdomen was aggravated after eating, with nausea and retching. Routine blood test results showed a high neutrophil percentage, low leukomonocyte and monocyte percentages, and low leukomonocyte and eosinophil counts. Amylase, liver and kidney function, and tumor markers alpha-fetoprotein, carcinoembryonic antigen, and cancer antigen (CA) 125, CA72-4, CA19-9, and CA153 were normal. Abdominal CT showed a mass, with multiple calcifications between the pancreas and the spleen. The boundary between the mass and the pancreas and spleen was poorly defined. Contrast-enhanced CT revealed that the upper abdominal mass was unevenly and gradually enhanced. 99mTc-MIBI SPECT/CT revealed that a focal radioactive concentration, with mild radioactive concentration extending into the upper abdominal mass, was present at the pancreatic body and tail. The 99mTc-MIBI SPECT/CT manifestations were consistent with the final pathological diagnosis of pancreatic NET.
99mTc-MIBI SPECT/CT appears to be a valuable tool for detecting pancreatic NETs.
Core Tip: Neuroendocrine tumors (NETs) are rare. The gastroenteropancreatic tract is the most common site for NETs. Pancreatic NETs account for about 1%-2% of pancreatic tumors and about 8% of all NETs. Endoscopic ultrasound, computed tomography (CT), and magnetic resonance imaging are common imaging modalities for the diagnosis of pancreatic NETs. In addition, somatostatin receptor imaging is of great value for the diagnosis of pancreatic NETs. We experienced a case of pancreatic NET detected by technetium-99m methoxy-2-isobutylisonitrile (99mTc-MIBI) single-photon emission CT/CT, which was consistent with the final pathological diagnosis of pancreatic NET.
- Citation: Liu CJ, Yang HJ, Peng YC, Huang DY. Pancreatic neuroendocrine tumor detected by technetium-99m methoxy-2-isobutylisonitrile single photon emission computed tomography/computed tomography: A case report. World J Clin Cases 2023; 11(12): 2825-2831
- URL: https://www.wjgnet.com/2307-8960/full/v11/i12/2825.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i12.2825
Neuroendocrine tumors (NETs) are relatively rare tumors arising from cells in the diffuse neuroendocrine system, occurring mainly in the gastroenteropancreatic (GEP) tract and lungs[1]. The GEP tract is the most common site for NETs[2]. Pancreatic NETs account for about 1%-2% of all pancreatic tumors[3] and about 8% of all NETs[4]. The diagnostic imaging modalities for pancreatic NETs include computed tomography (CT), magnetic resonance imaging, endoscopic ultrasound, and somatostatin receptor imaging[5]. However, the application of technetium-99m methoxy-2-isobutylisonitrile (99mTc-MIBI) single photon emission CT (SPECT)/CT for detecting pancreatic NET has not been reported.
A 57-year-old woman presented with upper abdominal distending pain lasting 1 d.
The patient had persistent pain that was aggravated after eating, accompanied by nausea and retching. Her abdominal CT findings revealed an upper abdominal tumor originating from the spleen or pancreas. Therefore, she was admitted to the hospital for further examination and treatment.
The patient underwent traumatic abdominal exploratory surgery more than 30 years ago.
An old surgical scar with a longitudinal length of about 6 cm was observed on the upper abdomen. A mass was palpable in the left-upper abdomen, which was hard, poor in mobility, and slightly tender.
Routine blood test results showed a high neutrophil percentage, low lymphocyte and monocyte percentages, and a low eosinophil count. Routine urine and stool test results were normal. The levels of electrolytes (sodium, chlorine, calcium, and magnesium) were normal. Amylase, liver and kidney function, and tumor markers alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen (CA) 125, CA72-4, CA19-9, and CA153 were also normal.
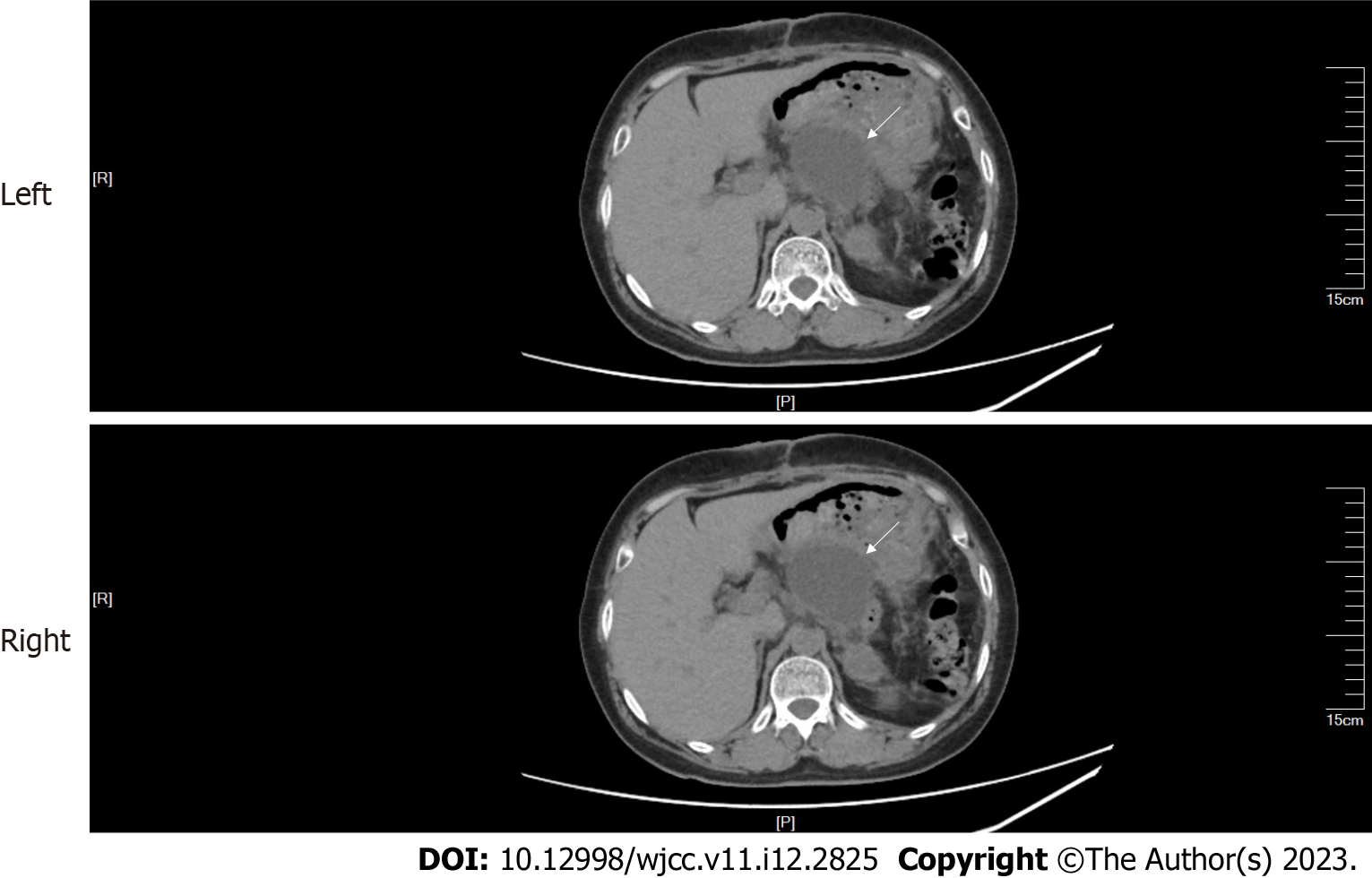
CT and contrast-enhanced CT of the abdomen: Abdominal CT showed a mass with multiple calcifications between the pancreas and the spleen. The boundary between the mass and the pancreas and spleen was poorly defined (Figure 1). The maximum cross section of the mass was about 9.0 cm × 8.6 cm. Contrast-enhanced CT revealed that the upper abdominal mass was unevenly and gradually enhanced (Figure 2).
Abdominal 99mTc-MIBI SPECT/CT: 99mTc-MIBI SPECT/CT was performed 5 d after contrast-enhanced CT imaging. Scanning began 30 min after the injection of 740 MBq of 99mTc-MIBI. Abdominal 99mTc-MIBI SPECT/CT was performed using a PRECEDENCE SPECT/CT (Philips Medical Systems, Eindhoven, the Netherlands) system. CT scanning was performed in a spiral mode over the entire abdomen at 250 mAs per slice, 120 Kv, and with a slice thickness of 3.0 mm. Immediately after CT scanning, SPECT acquisition of the abdomen was performed. The SPECT system was equipped with low-energy, high-resolution parallel-hole collimators. The SPECT acquisition followed an elliptical orbit, with a step-and-shoot acquisition of 64 angles over 360° (180° per detector) and an acquisition time of 20 s per frame. The SPECT data were reconstructed with attenuation correction from CT acquisition and iterative reconstruction via AutoSPECT Pro software with astonish, four iterations, and 16 subsets. The 99mTc-MIBI SPECT/CT fused images were processed using Fusion Viewer (version 2.1) procedures. The SPECT slice thickness was the same as that of CT.
The 99mTc-MIBI SPECT/CT images showed the presence of a focal radioactive concentration, with mild radioactive concentration extending into the upper abdominal mass at the pancreatic body and tail (Figure 3).
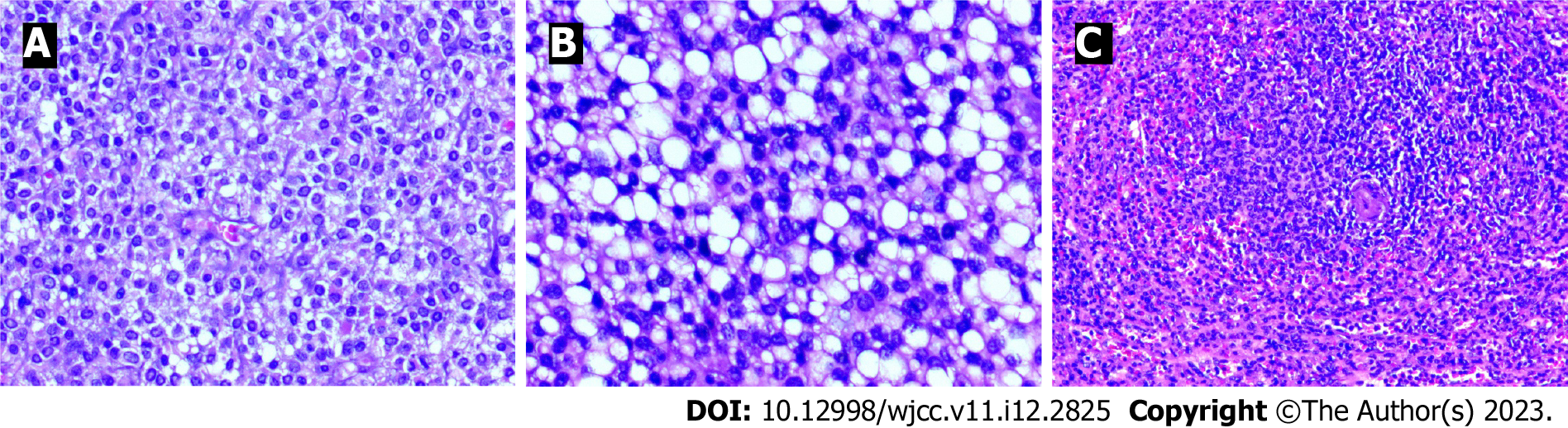
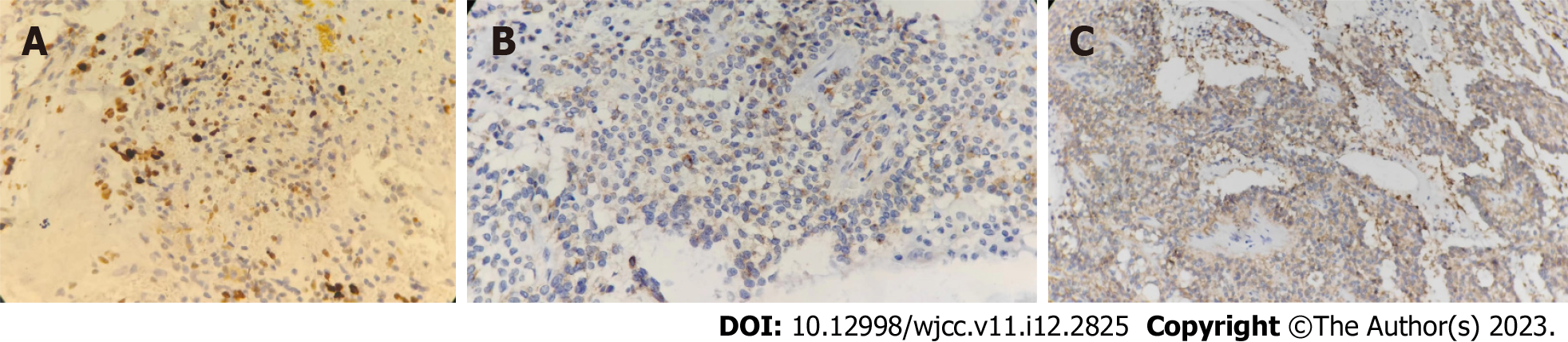
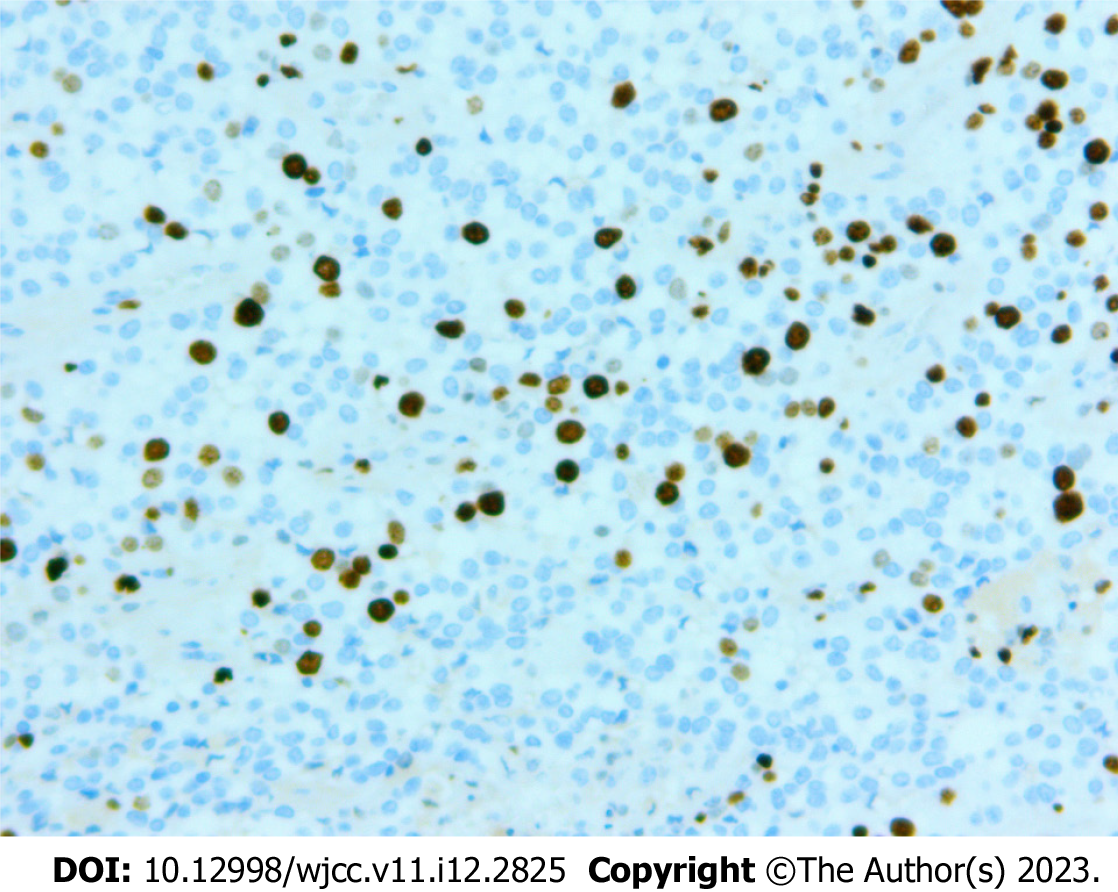
The pathology of the pancreatic lesion indicated a well-differentiated pancreatic NET by hematoxylin and eosin staining (Figure 4A and B). The pathology of the spleen showed normal spleen cells by hematoxylin and eosin staining (Figure 4C). Immunohistochemical analysis showed that the tumor cells were positive for insulinoma-associated protein 1, synaptophysin, and cluster of differentiation 56 (Figure 5). The Ki-67 (marker of proliferation Ki-67) proliferative index was assessed at 10% (Figure 6).
Two days after 99mTc-MIBI SPECT/CT, the pancreatic body and tail, the upper abdominal mass discovered by CT (the mass with multiple calcifications between the pancreas and the spleen), and the spleen were excised. During the operation, a pancreatic lesion was seen to expand outward and extend into the spleen. This was inconsistent with the abdominal CT findings.
The patient was followed up 35 d after surgery. She complained of dull pain in her upper abdomen. Abdominal CT results showed encapsulated effusion in the surgical area (Figure 7), with no obvious abnormality in the remaining area. The patient’s condition improved after ultrasound-guided closed abdominal drainage.
Pancreatic NETs are often divided into functional and nonfunctional pancreatic NETs. The majority of pancreatic NETs are nonfunctional[5]. The symptoms of a nonfunctional pancreatic NET include abdominal or back pain, nausea, vomiting, pancreatitis, and obstructive jaundice[5]. Patients with functional pancreatic NETs often present with symptoms caused by hormone production of the tumor, leading to an early diagnosis[6].
Endoscopic ultrasound, CT, and magnetic resonance imaging are common imaging modalities for the diagnosis of pancreatic NETs. In addition, somatostatin receptor imaging is of great value for the diagnosis of pancreatic NETs[7], but the method is not easily available in our hospital.
More than 90% of well-differentiated GEP NETs express somatostatin receptors[8]. Functional imaging technique of gallium-68 dota-octreotate (68Ga-DOTATATE) positron emission tomography (PET)/CT uses radiolabeled somatostatin analogs to localize NETs. Research showed that the sensitivity of 68Ga-DOTATATE PET/CT was about 95% for detecting pancreatic NETs[7]. Unlike 68Ga-DOTATATE, 99mTc-MIBI is a nonspecific tumor imaging agent. However, compared with 68Ga-DOTATATE PET/CT, 99mTc-MIBI SPECT/CT is a relatively cheap and easily available imaging modality. Herein, we present a case of pancreatic NETs detected by 99mTc-MIBI SPECT/CT.
99mTc-MIBI is a lipophilic univalent cationic agent. Driven by cytoplasmic and mitochondrial transmembrane potential gradients, 99mTc-MIBI penetrates reversibly into the cytoplasm and concentrates in mitochondria[9]. In one study, there were greater electrical gradients from outside the cell to the mitochondria in carcinoma cells than in normal epithelial cells, and the uptake of 99mTc-MIBI increased tenfold in carcinoma cells[9].
99mTc-MIBI SPECT/CT may be a useful tool for detecting lymph node and lung metastases in patients with differentiated thyroid carcinoma[10]. Additionally, 99mTc-MIBI SPECT can be used to differentiate benign from malignant solitary pulmonary nodules and thyroid nodes[11,12]. Lu et al[13] reported a case of mediastinal typical carcinoid tumor detected by 99mTc-MIBI SPECT/CT.
Our patient was a 57-year-old woman with symptoms that started 1 d previously. The age for the occurrence of pancreatic NETs is equivalent to the mean age (57-58 years) previously identified for this type of tumor[5]. The patient’s abdominal CT showed a mass with multiple calcifications between the pancreas and spleen. The boundary between the mass and pancreas and spleen was poorly defined. These findings indicated that the tumor might have originated from the spleen or pancreas. Contrast-enhanced CT revealed that the mass was unevenly and gradually enhanced. This indicated the possibility of a malignant tumor. However, 99mTc-MIBI SPECT/CT showed the presence of a focal radioactive concentration, with mild radioactive concentration extending into the upper abdominal mass at the pancreatic body and tail. This finding strongly suggested that the upper abdominal mass originated from the pancreas. The CT manifestations of the pancreatic tissue corresponding to the focal radioactive concentration were solid and homogenous. A previous study reported that pancreatic NETs tended to appear as solid and homogenous lesions on CT imaging[7]. In our case study, the 99mTc-MIBI SPECT/CT manifestations were consistent with the final pathological diagnosis of pancreatic NET.
99mTc-MIBI SPECT/CT appears to be valuable for diagnosing pancreatic NETs. However, subsequent large-sample studies are needed to confirm this finding.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Akbulut S, Turkey; Ampollini L, Italy; Saglam S, Turkey S-Editor: Zhao S L-Editor: Filipodia P-Editor: Zhao S
| 1. | Ohmoto A, Morizane C. Genomic Profiles and Current Therapeutic Agents in Neuroendocrine Neoplasms. Curr Drug Targets. 2020;21:389-405. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Iabichino G, Di Leo M, Arena M, Rubis Passoni GG, Morandi E, Turpini F, Viaggi P, Luigiano C, De Luca L. Diagnosis, treatment, and current concepts in the endoscopic management of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol. 2022;28:4943-4958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 3. | Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K, Doi R, Shimatsu A, Nishida T, Komoto I, Hirata Y, Nakamura K, Igarashi H, Jensen RT, Wiedenmann B, Imamura M. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2459] [Article Influence: 307.4] [Reference Citation Analysis (4)] |
| 5. | Scott AT, Howe JR. Evaluation and Management of Neuroendocrine Tumors of the Pancreas. Surg Clin North Am. 2019;99:793-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 618] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 7. | Hu HF, Li Z, Chen K, Liu MQ, Ye Z, Chen XM, Zhang Y, Yu XJ, Xu XW, Ji SR. Multimodality imaging differentiation of pancreatic neuroendocrine tumors and solid pseudopapillary tumors with a nomogram model: A large single-center study. Front Surg. 2022;9:970178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 8. | Grey N, Silosky M, Lieu CH, Chin BB. Current status and future of targeted peptide receptor radionuclide positron emission tomography imaging and therapy of gastroenteropancreatic-neuroendocrine tumors. World J Gastroenterol. 2022;28:1768-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 9. | Moretti JL, Hauet N, Caglar M, Rebillard O, Burak Z. To use MIBI or not to use MIBI? Eur J Nucl Med Mol Imaging. 2005;32:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Okudan B, Seven B, Gülaldı NCM, Çapraz M, Açıkgöz Y. The Value of (99m)Tc-MIBI SPECT/CT in the Postoperative Assessment of Patients with Differentiated Thyroid Carcinoma. Curr Med Imaging. 2022;18:404-408. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Xia G, An C, Ming Z, Guo H, Liu L, Li Y. 18F-FDG-PET/CT versus 99Tcm-MIBI-SPECT: which is better for detection of solitary pulmonary nodules ? J BUON. 2017;22:1246-1251. [PubMed] |
| 12. | Schenke SA, Campennì A, Tuncel M, Bottoni G, Sager S, Bogovic Crncic T, Rozic D, Görges R, Özcan PP, Groener D, Hautzel H, Klett R, Kreissl MC, Giovanella L. Diagnostic Performance of (99m)Tc-Methoxy-Isobuty-Isonitrile (MIBI) for Risk Stratification of Hypofunctioning Thyroid Nodules: A European Multicenter Study. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Lu C, Wang Z, Wang G, Wang X, Liu X. Superior mediastinal typical carcinoid detected by 99mTc-MIBI SPECT/CT imaging: A case report. Medicine (Baltimore). 2017;96:e9457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |