Published online Apr 26, 2023. doi: 10.12998/wjcc.v11.i12.2766
Peer-review started: March 2, 2023
First decision: March 14, 2023
Revised: March 18, 2023
Accepted: March 24, 2023
Article in press: March 24, 2023
Published online: April 26, 2023
Processing time: 54 Days and 15.4 Hours
Obesity is a state in which excess heat is converted into excess fat, which accumulates in the body and may cause damage to multiple organs of the circulatory, endocrine, and digestive systems. Studies have shown that the accumulation of abdominal fat and mesenteric fat hypertrophy in patients with obesity makes laparoscopic surgery highly difficult, which is not conducive to operation and affects patient prognosis. However, there is still controversy regarding these conclusions.
To explore the relationship between body mass index (BMI) and short-term prognosis after surgery for colorectal cancer.
PubMed, Embase, Ovid, Web of Science, CNKI, and China Biology Medicine Disc databases were searched to obtain relevant articles on this topic. After the articles were screened according to the inclusion and exclusion criteria and the risk of literature bias was assessed using the Newcastle-Ottawa Scale, the prognostic indicators were combined and analyzed.
A total of 16 articles were included for quantitative analysis, and 15588 patients undergoing colorectal cancer surgery were included in the study, including 3775 patients with obesity and 11813 patients without obesity. Among them, 12 articles used BMI ≥ 30 kg/m2 and 4 articles used BMI ≥ 25 kg/m2 for the definition of obesity. Four patients underwent robotic colorectal surgery, whereas 12 underwent conventional laparoscopic colorectal resection. The quality of the literature was good. Meta-combined analysis showed that the overall complica
Obesity increases the overall complication and SSI rates of patients undergoing colorectal cancer surgery but has no influence on the incidence of anastomotic leak, reoperation rate, and short-term mortality rate.
Core Tip: Colorectal rectal cancer (CRC) is a common malignant tumor in gastroenterology, ranking the third in the global incidence rate of malignant tumors, second only to lung cancer and breast cancer, with a mortality rate of about 8% of all malignant tumors. Like other malignant tumors, the etiology of CRC is still unclear. It may occur in the colon or anywhere in the rectum, but it is most common in the rectum and sigmoid colon, and the rest are in the cecum, ascending colon, descending colon and transverse colon in turn. So far, the radical treatment of CRC is still surgical treatment. The definition of radical cancer resection is to remove tumors visible to the naked eye, including primary and drainage lymph nodes. Although lesions can be removed during surgery, complete removal is still not easy for patients with extensive local diseases. For patients with advanced CRC, the tumor size is relatively large, there are many vascular variations, the field of vision of laparotomy is poor, and the operation is difficult.
- Citation: Li Y, Deng JJ, Jiang J. Relationship between body mass index and short-term postoperative prognosis in patients undergoing colorectal cancer surgery. World J Clin Cases 2023; 11(12): 2766-2779
- URL: https://www.wjgnet.com/2307-8960/full/v11/i12/2766.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i12.2766
Colorectal rectal cancer (CRC) is a common malignant tumor and ranks third in the incidence of malignant tumors worldwide; it is second only to lung and breast cancers, with a mortality rate of approximately 8%[1] of all malignant tumors. Similar to other malignancies, the cause of CRC remains unclear and can occur anywhere in the colon or rectum; however, it is most common in the rectum and sigmoid colon, whereas the remainder is found sequentially in the cecum, ascending, descending, and transverse colon[2]. Surgical treatment still remains the radical treatment of CRC, and radical resection of intestinal cancer is defined as the removal of macroscopic tumors, including primary and draining lymph nodes. Although the lesion can be removed during surgery, complete removal is still difficult in patients with extensive local disease[3]. For patients with advanced CRC, the tumor size is relatively large, with high vascular variation, and the visual field of laparotomy is poor, making the surgery difficult. In recent years, laparoscopy has emerged as an auxiliary operation with the advantages of a small surgical wound, an open operation field, and rapid postoperative recovery. It has been gradually applied to radical resection of CRC and has achieved an ideal clinical effect[4]. Obesity is a state in which excess heat in the human body is converted into excess fat, which accumulates in the body and may cause damage to the circulatory, endocrine, and digestive systems[5]. Studies have suggested that abdominal fat accumulation and mesenteric fat hypertrophy in patients with obesity make laparoscopic surgery very difficult, which is not conducive to the operation and affects patient prognosis[6]. However, other studies have also shown that obesity is not associated with a short-term prognosis after surgery[7]. The controversial findings may be related to the sample size in the study, individual differences between patients and tumors, or time to evaluate body mass index (BMI). We conducted a meta-analysis to explore the relationship between BMI and short-term prognosis after surgery for CRC.
In January 2023, we retrieved the PubMed, Embase, Ovid, Web of Science, CNKI, and China Biology Medicine disc databases in the keyword-free search mode. Key words included: "obesity" or "obese" or "BMI" or "laparoscopic colorectal resection.”
(1) Study type: All studies were observational cohort studies (either retrospective or prospective); (2) Research participants: The primary disease of all the research participants was CRC, including all malignant tumors occurring in the colon or rectum. It was clarified in the study that the patients were treated by surgical resection, and the resection operations included but was not limited to the following common laparoscopic operations: Right hemicolectomy, expanded right hemicolectomy, ileocolic resection, and left hemicolectomy; (3) Grouping or cohort division: There must be two or more definite cohorts in the study. The cohorts were grouped such that patients were divided into obese and non-obese groups according to BMI ≥ 30 kg/m2 or BMI ≥ 25 kg/m2[8]; and (4) Outcome indicators: Short-term prognosis indicators of the two groups, such as complication rate, mortality after surgery, reoperation rate, and hospital stay, must be provided in the study.
(1) Patients with non-primary CRC; (2) Patients who did not provide the grouping criteria for obesity or for whom cohort data of obese and non-obese were not available; the patient type was visceral obesity; (3) The study type was a non-observational cohort study, such as a case study; and (4) Studies with missing outcome indicators and for which data were not available, such as studies with only long-term prognostic survival analysis and no short-term prognostic survival indicators.
After literature retrieval, repetitive literature was excluded using software. The titles and abstracts were read by two researchers, and screening was conducted according to the inclusion and exclusion criteria. After the final literature was determined, the full text of the literature was obtained individually from the Internet. If the original text was not obtained, the author of the original text was contacted by phone or email to obtain the full text. The full texts of the articles were read for data extraction and further screening to remove articles with no data or incomplete indicators.
Literature quality and bias risk assessment were performed using the Newcastle-Ottawa Scale[9] for quality analysis of the included literature. The scale was used to evaluate the object selection, comparability, and outcome indicators in the literature. The maximum score was 9 points, and the quality was good, with a score of > 5 points. Higher scores indicated better literature quality and less bias, with a score of 5–7 indicating medium quality and 8–9 indicating high quality. Articles with scores < 5 were excluded.
Two researchers independently extracted literature data: Study type, date of publication, study location, age of the patient, tumor type, tumor stage, BMI, intraoperative indicators, postoperative indicators, and prognosis indicators. After the two researchers completed data extraction, they cross-examined each other's results and discussed and finalized the differences generated.
(1) The discrete variables were reported as effect quantity with OR and 95%CI; (2) Statistical comparisons were described using forest plot; (3) Literature heterogeneity was analyzed using I2 method and Q-test, and I2 > 50% or P < 0.1 indicated the heterogeneity of the results; (4) In case of no heterogeneity between the articles, the fixed-effects model (Mantel–Haenszel) was used; in case of heterogeneity between the articles, the Dersimonian–Laird method was used; (5) Survey of heterogeneity: Subgroup analysis was used to investigate heterogeneity; (6) Meta-regression analysis was used to investigate factors that were significant for effect size; (7) Sensitivity analysis: Diagnostic tests were used to analyze studies that might have influenced the pooled results; literature was culled one by one and the combined effects of the remaining articles were calculated to identify the articles that most affected the results; and (8) Publication bias was detected using the Egger's test and results of publication bias were presented using a trim-filled plot.
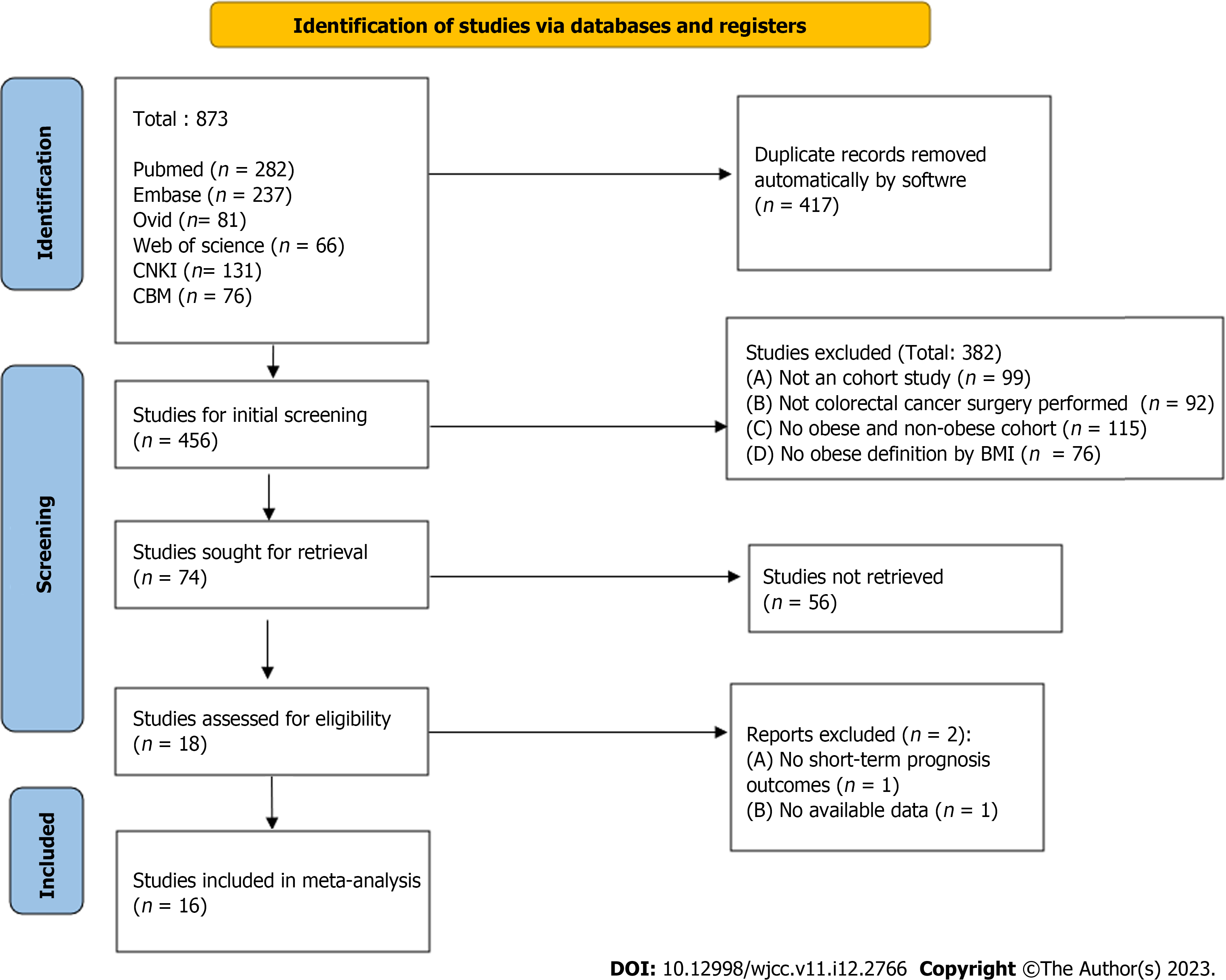
As shown in Figure 1, which is a flow chart of the literature selection process, 873 articles were initially retrieved. After initial deduplication and screening, 18 articles[6,10-26] were included in the final screening. However, articles[16] lacking a short-term prognosis indicator and articles[19] whose data could not be obtained were excluded; finally, 16 articles were included. A total of 15588 patients (3775 with obesity and 11813 without obesity) were included in the study. Among them, 12 articles used BMI ≥ 30 kg/m2 and 4 articles used BMI ≥ 25 kg/m2 for the definition of obesity. Four patients underwent robotic colorectal surgery and 12 underwent conventional laparoscopic resection. The basic characteristics, grouping information, and outcome indicator lists of all articles and patients are listed in Table 1.
| Ref. | Country | Patient's age (years) | Obesity definition | Overall number of people | Grouping number (O/N) | Surgical measures | Outcome indicators |
| Hannan et al[10], 2022 | Ireland | 67 | BMI: 30 kg/m2 | 107 | 34/73 | Robotic colorectal surgery | a, b, c, d, e |
| Lagares-Garcia et al[11], 2016 | United States | 59.9 ± 13.8 | BMI: 30 kg/m2 | 67 | 34/33 | Robotic colorectal surgery | a, b, c, d |
| Akiyoshi et al[12], 2011 | Japan | 63.9 (23–91) | BMI: 25 kg/m2 | 1,169 | 243/926 | Laparoscopic colorectal resection | a, b, e |
| Merkow et al[13], 2009 | United States | 68.02 ± 6.18 | BMI: 30 kg/m2 | 1,679 | 607/1072 | Laparoscopic colorectal resection | a, c, d, e |
| Choi et al[14], 2017 | Korea | 66.7 ± 11.2 | BMI: 25 kg/m2 | 313 | 80/233 | Laparoscopic colorectal resection | a, b, c, d, e |
| Zhang et al[15], 2021 | China | 55 (36–78) | BMI: 30 kg/m2 | 356 | 48/308 | Laparoscopic colorectal resection | a, b, c, d, e |
| Yamashita et al[16], 2021 | Japan | 70 (24–96) | BMI: 25 kg/m2 | 1,648 | 313/1335 | Laparoscopic colorectal resection | a, b, e |
| Bège et al[18], 2009 | France | 62 ± 10.7 | BMI: 30 kg/m2 | 210 | 24/186 | Laparoscopic colorectal resection | a, b, c, d, e |
| Hede et al[19], 2015 | Sweden | 72.7 ± 13.1 | BMI: 30 kg/m2 | 6,675 | 1528/5147 | Laparoscopic colorectal resection | a, d |
| Makino et al[21], 2014 | United States | 67.5 ± 11.7 | BMI: 30 kg/m2 | 152 | 76/76 | Laparoscopic colorectal resection | a, b, d, e |
| Bamboat et al[22], 2012 | United States | 65 | BMI: 30 kg/m2 | 245 | 68/177 | Laparoscopic colorectal resection | a, b, e |
| Miyamoto et al[23], 2014 | Japan | 65.9 ± 9.6 | BMI: 25 kg/m2 | 561 | 140/421 | Laparoscopic colorectal resection | a, b, d, e |
| Bayraktar et al[24], 2018 | Turkey | 60 ± 11 | BMI: 30 kg/m2 | 101 | 30/71 | Robotic colorectal surgery | a, b, e |
| Poulsen et al[25], 2012 | Denmark | 69 (37–97) | BMI: 30 kg/m2 | 425 | 93/332 | Laparoscopic colorectal resection | a, c, d |
| Bell et al[26], 2018 | Australia | 71.6 | BMI: 30 kg/m2 | 1464 | 299/1165 | Laparoscopic colorectal resection | a, c, d |
| Peacock et al[27], 2020 | United States | 54.1 ± 12.5 | BMI: 30 kg/m2 | 533 | 161/372 | Robotic colorectal surgery | a, b, c, e |
Among the 16 included articles, 10[6,10-15,17,18,26] had a quality score of 8–9, with little bias and high quality. A total of six articles[20-25] were scored 6–7, with a small amount of bias and medium quality. The overall quality was good (Table 2).
| Ref. | Case selection (/4) | Comparability (/2) | Outcome indicators (/3) | Total score (/9) |
| Hannan et al[10], 2022 | 4 | 2 | 3 | 9 |
| Lagares-Garcia et al[11], 2016 | 4 | 2 | 2 | 8 |
| Akiyoshi et al[12], 2011 | 4 | 2 | 2 | 8 |
| Merkow et al[13], 2009 | 4 | 2 | 3 | 9 |
| Choi et al[14], 2017 | 4 | 2 | 2 | 8 |
| Zhang et al[15], 2021 | 4 | 2 | 2 | 8 |
| Yamashita et al[16], 2021 | 4 | 2 | 3 | 9 |
| Bège et al[18], 2009 | 4 | 2 | 2 | 8 |
| Hede et al[19], 2015 | 4 | 2 | 3 | 9 |
| Makino et al[21], 2014 | 3 | 2 | 2 | 7 |
| Bamboat et al[22], 2012 | 3 | 2 | 2 | 7 |
| Miyamoto et al[23], 2014 | 2 | 2 | 2 | 6 |
| Bayraktar et al[24], 2018 | 2 | 2 | 2 | 6 |
| Poulsen et al[25], 2012 | 3 | 2 | 2 | 7 |
| Bell et al[26], 2018 | 2 | 2 | 2 | 6 |
| Peacock et al[27], 2020 | 4 | 2 | 2 | 8 |
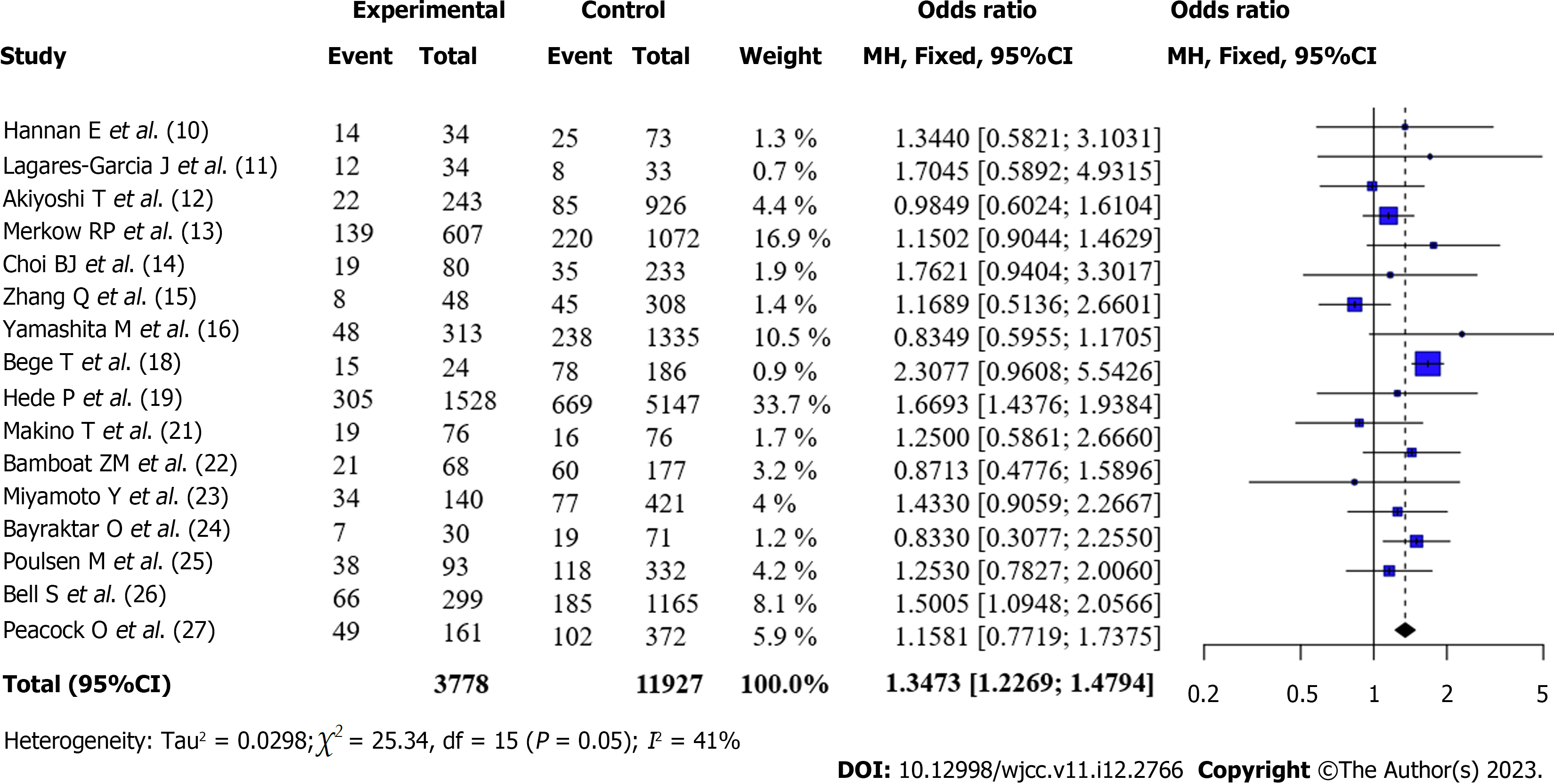
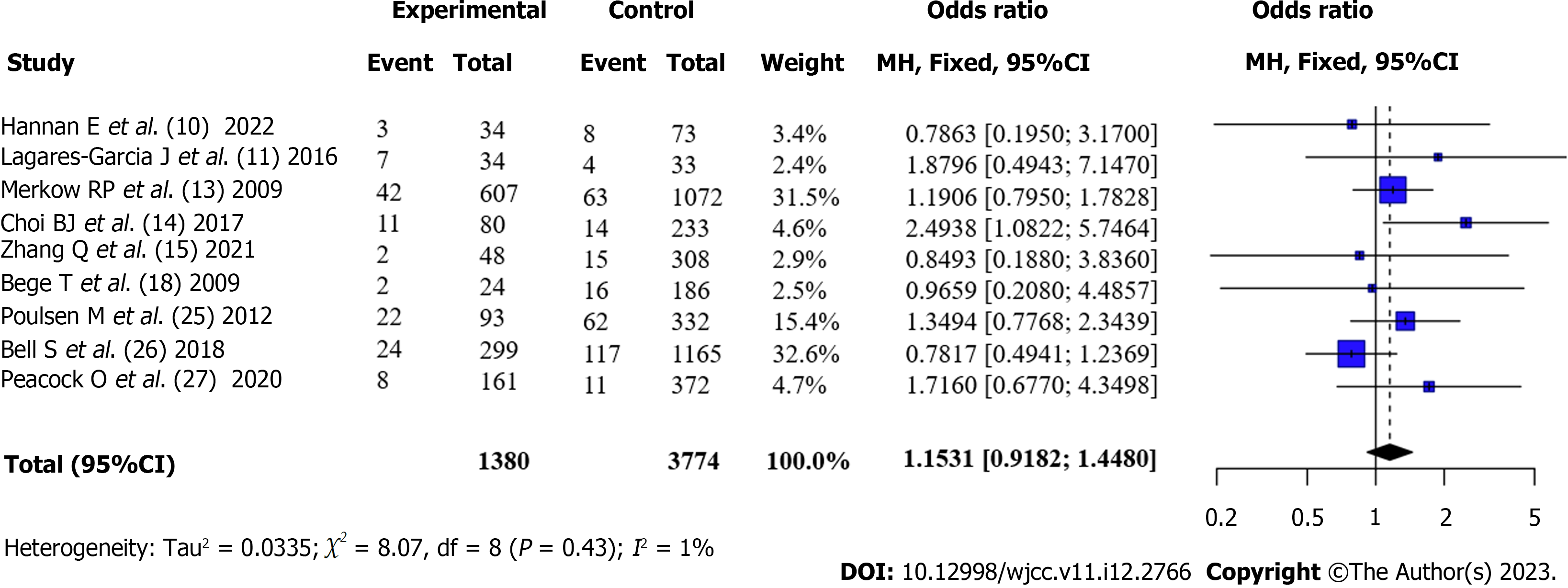
Total complication rate after surgery for CRC (Obese vs Non-obese): All articles[6,10-15,17,18,20-26] reported the total complication rates of patients with obesity and those without obesity after surgery. There was no statistical heterogeneity between the articles (I2 = 41%, P = 0.05). According to the fixed-effects model and meta-analysis, the complication rate of patients with obesity after surgery was higher than that of patients without obesity [OR = 1.35, 95%CI: 1.23-1.48, Z = 6.25, P < 0.0001] (Figure 2).
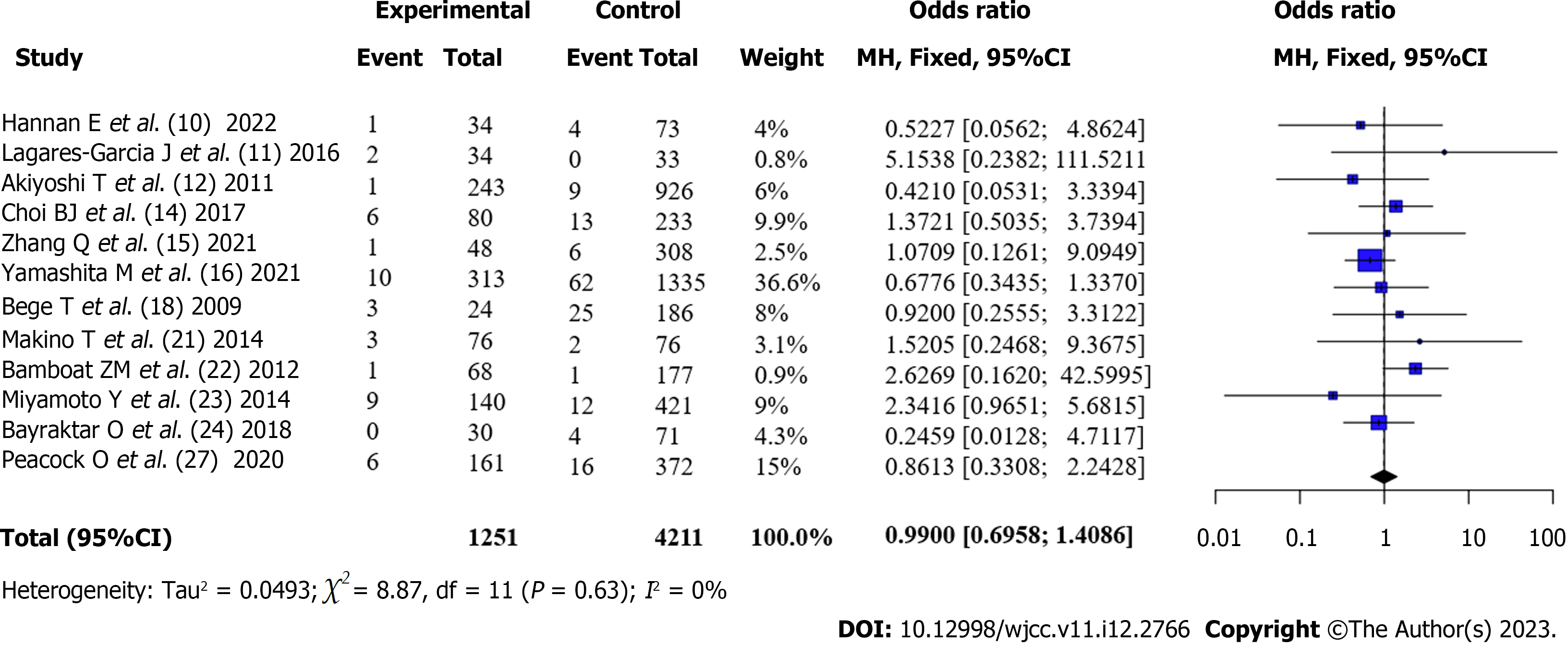
Incidence of postoperative anastomotic leak in patients with CRC (Obese vs Non-obese): Twelve articles[10-15,17,20-23,26] reported the incidence of anastomotic leak in patients with obesity and those without obesity after surgery. There was no statistical heterogeneity between the articles (I2 = 0%, P = 0.63). A fixed-effects model was used. The incidence of anastomotic leak in patients with obesity after surgery was not statistically different from that in patients without obesity according to the meta-analysis [OR = 0.99, 95%CI: 0.70-1.41, Z = -0.06, P = 0.956] (Figure 3).
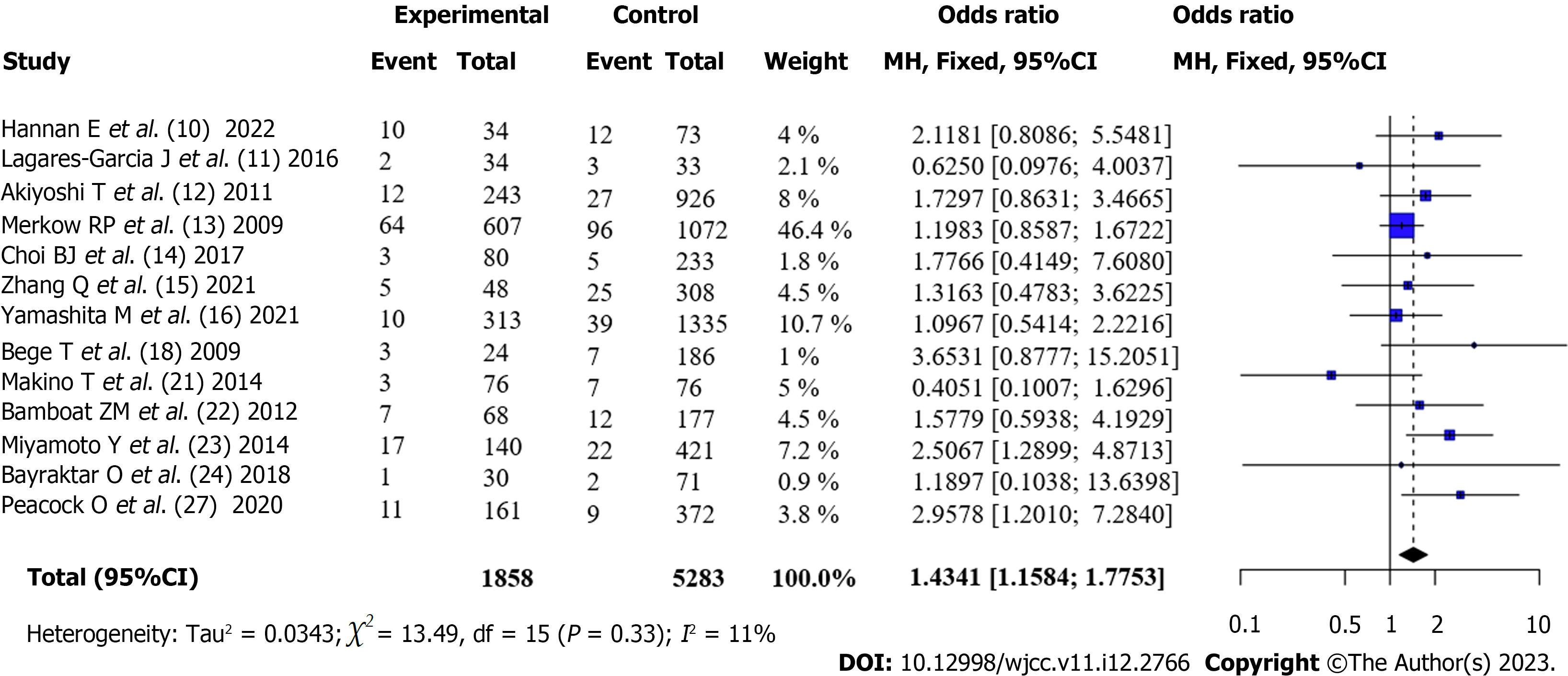
Incidence of SSI in patients with CRC after surgery (Obese vs Non-obese): The incidence of surgical site infection (SSI) between patients with obesity and those with obesity after surgery was reported in 13 articles[6,10-15,17,20-23,26]. There was no statistical heterogeneity between the articles (I2 = 11%, P = 0.33). According to the fixed-effects model and meta-analysis, the incidence of SSI in patients with obesity after surgery was higher than that in patients without obesity [OR = 1.43, 95%CI: 1.16-1.78, Z = 3.31, P < 0.001] (Figure 4).
Reoperation rate after surgery for CRC (Obese vs Non-obese): Nine articles[6,10,11,13,14,17,24-26] reported the postoperative reoperation rates of patients with obesity and those without obesity. There was no statistical heterogeneity between the articles (I2 = 1%, P = 0.43). According to the fixed-effects model and meta-analysis, the reoperation rate of patients with obesity after surgery was higher than that of patients without obesity, but the difference was not statistically significant [OR = 1.15, 95%CI: 0.92-1.45, Z = 1.23, P = 0.23] (Figure 5).
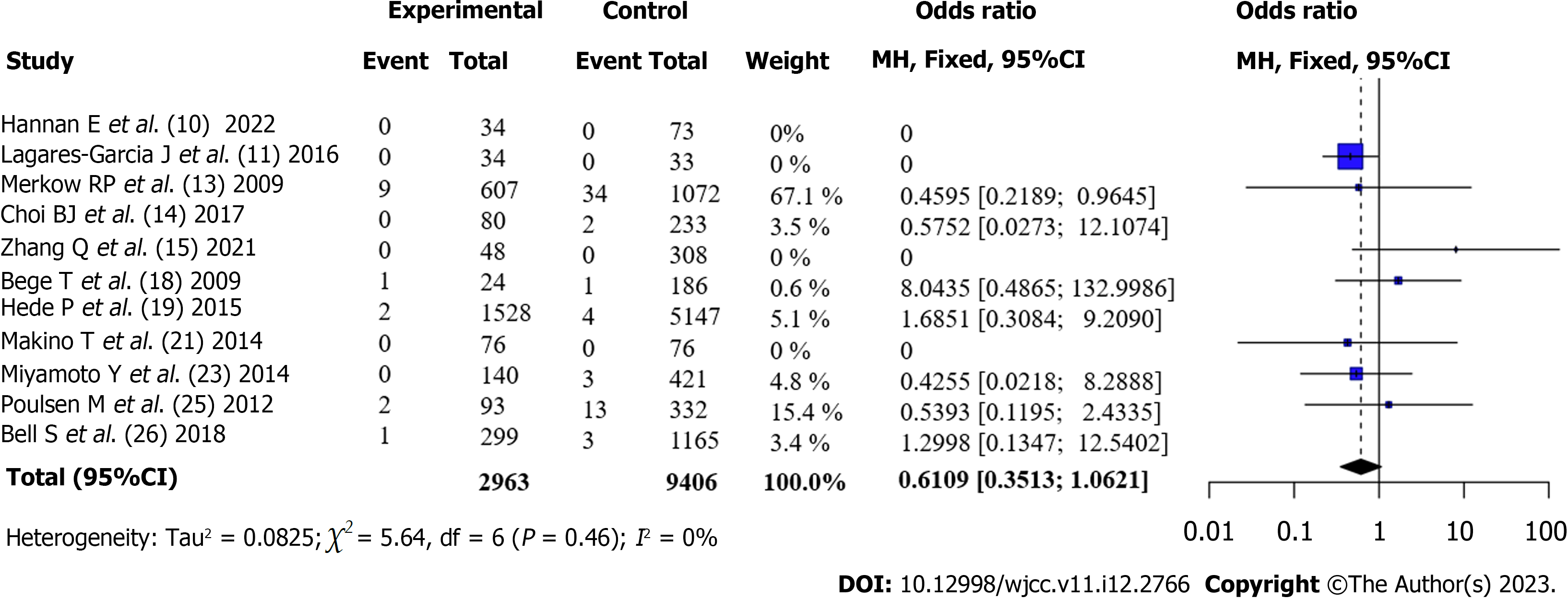
Postoperative mortality of patients undergoing CRC surgery (Obese vs Non-obese): Eleven articles[6,10,11,13,14,17,18,20,22,24,25] reported the short-term mortality rates of patients with obesity and those without obesity after surgery. There was no statistical heterogeneity between the articles (I2 = 0%, P = 0.46). Using a fixed-effects model and meta-analysis, it was found that the mortality rate of patients with obesity after surgery was lower than that of patients without obesity. However, the differences were not statistically significant [OR = 0.61, 95%CI: 0.35-1.06, Z = -1.75, P = 0.08], as shown in Figure 6.
Although there was no statistical heterogeneity between the articles in the analysis of the overall complication incidence index after surgery (I2 = 41%, P = 0.05), there was still some heterogeneity. We conducted subgroup analysis according to the “geographical location”, “surgery approach,” and “obese BMI” and found that after the articles were grouped according to the study site and “geographical location,” the inter-group heterogeneity test between subgroups was P < 0.05, indicating that the “geographical location” of the study site was one of the sources of heterogeneity in this study, as shown in Table 3.
| Grouping number | Subgroup grouping method | Subgroup | Number of articles | Effect size | Heterogeneity | |
| I2 (%) | tau2 | |||||
| 1 | Geographical location | Europe | 6 | OR = 1.59, (1.40, 1.80) | 0 | 0 |
| North America | 5 | OR = 1.14, (0.94, 1.37) | 0 | 0 | ||
| Asia | 5 | OR = 1.13, (0.84, 1.52) | 35.4 | 0.04 | ||
| 2 | Surgery approach | Robotic colorectal surgery | 4 | OR = 1.18, (0.85, 1.64) | 0 | 0 |
| Laparoscopic colorectal resection | 12 | OR = 1.28, (1.08, 1.51) | 53.3 | 0 | ||
| 3 | Obese body mass index | 30 kg/m2 | 12 | OR = 1.34, (1.14, 1.57) | 21.8 | 0.02 |
| 25 kg/m2 | 4 | OR = 1.14, (0.81, 1.58) | 51.3 | 0.06 | ||
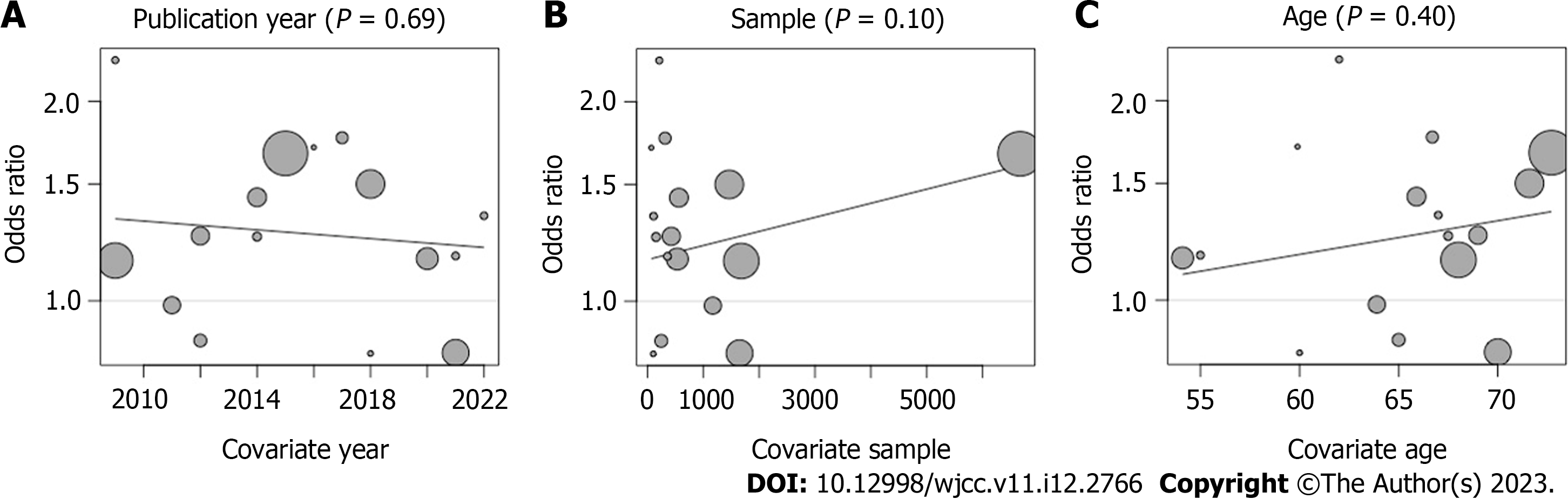
In the analysis of the overall complication incidence index after surgery, to understand the factors that may affect the results of Pooled ES, we used the "year of publication,” "sample size," and "patient's age" to regression pooled ES, and found that none of the three factors had a statistically significant effect on the results (P > 0.05), which indicates that none of the three factors was able to affect the results Figure 7.
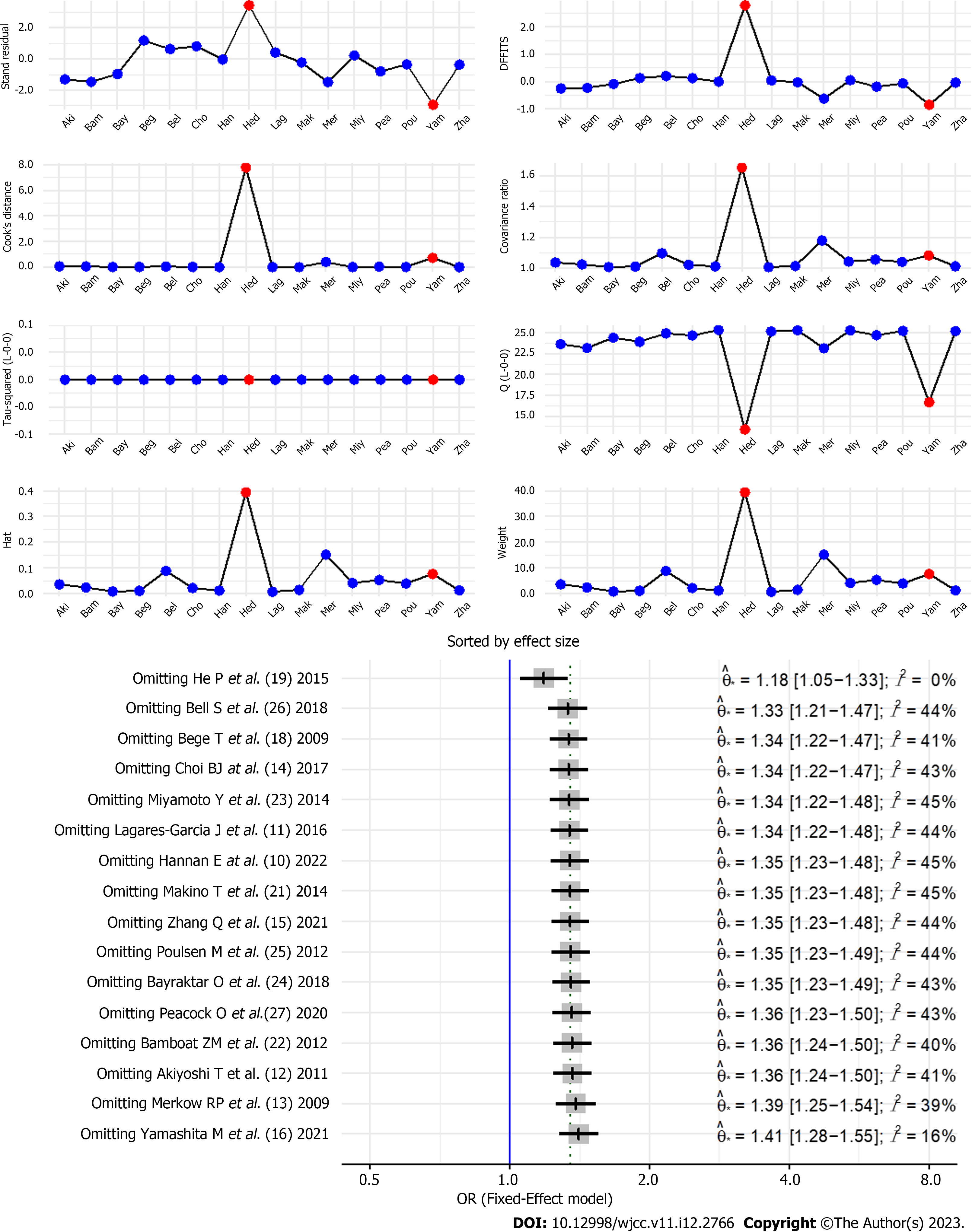
The results of the analysis of the postoperative complication rate indicators were verified using sensitivity diagnosis, and it was found that the literature significantly affected the results, which might be related to the fact that the sample size of the literature was much larger than that of other studies[18]. After literature[18] was excluded, the remaining 15 studies were excluded individually, and no significant deviations were found, indicating that the final pool result was stable, as shown in Figure 8.
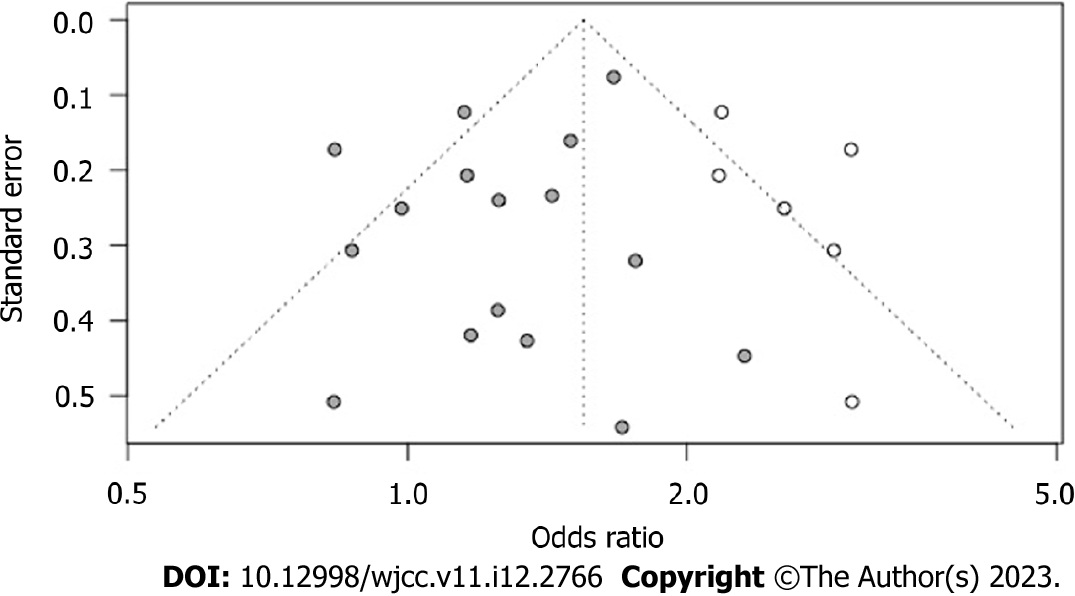
In the analysis of the incidence indicators of postoperative complications, publication bias was detected using Egger’s t-test, with t = 0.43 and P = 0.20, indicating no significant left-right asymmetry in the funnel plot, as shown in Figure 9.
Over the past few years, overweight and obesity have gradually become widespread epidemics. Obesity is related to the occurrence of several diseases. In addition to cardiovascular and cerebrovascular diseases, obesity is closely related to the occurrence of many cancers, including colorectal, endometrial, and breast cancers[27,28]. However, studies on obesity and surgical prognosis of these tumors are rare and controversial.
To explore the impact of being overweight on the short-term prognosis of CRC surgery, 16 observational cohort studies with 15588 participants were included in this meta-analysis. The results showed that being overweight can increase the overall incidence of complications after CRC surgery and the incidence of SSI. However, the impact on the incidence of anastomotic leak, reoperation rate, and mortality was not obvious. In recent years, laparoscopic colorectal cancer resection has been widely used in clinical colorectal cancer radical surgery because of its small wound, open operation field, few complications, rapid postoperative recovery, and other advantages, and its short-term and long-term efficacy has been widely recognized worldwide[29]. However, for some patients with CRC, the tumor size is relatively large and the visual field during laparotomy is poor. As the operation involves the anatomy and root treatment of multiple important blood vessels, such as ileocolic and colonic vessels, the difficulty of completing the operation under laparoscopy is greatly increased. In addition, with changes in the dietary habits of people, an increasing number of people are becoming obese. Obesity is not only an induction factor for multiple internal medicine diseases but also has many negative effects on surgery[30]. Patients with obesity and CRC are very likely to suffer from severe vascular injury during surgery due to abdominal fat accumulation, mesenteric fat hypertrophy, and narrow space in the abdominal cavity, which makes laparoscopic radical resection difficult. Obesity may also result in reduced oxygen circulation in wounds, insufficient collagen synthesis, insufficient antibiotic concentration, and impaired immune function, which make patients vulnerable to infection, causing complications, such as incision infection, intestinal obstruction, and anastomotic leak, which prolong the hospitalization time of patients and increase reoperation and mortality rates[4].
In this study, we conducted a regression analysis of the three factors that may affect the results of the meta-analysis, including "year of publication,” "sample size,” and "patient's age" and found that none of the three factors could affect the results of the meta-analysis. In the stability analysis, we removed the large sample literature that may have affected the results and found that the results were not fundamentally reversed. Publication bias analysis showed that there was no statistically significant asymmetry on either side of the funnel, which indicated that the results of this study were stable and reliable, and the evidence was sufficient.
In this study, we adopted a subgroup analysis approach to explore the sources of heterogeneity in the meta-analysis. As some included studies adopted BMI ≥ 30 kg/m2 as the definition of obesity, while others adopted BMI ≥ 25 kg/m2 as the definition of obesity, it is very likely that the different BMI definitions of obesity increased the heterogeneity between the articles. The World Health Organization defines grade I obesity as BMI of 30 kg/m2 and above, grade II obesity as BMI 35.00–39.99 kg/m2, and grade III obesity as BMI ≥ 40.00 kg/m2. However, due to the prevalence of obesity among different populations and different understandings of obesity, it is also common to adopt BMI ≥ 25 kg/m2 as the definition of obesity in regions (such as some countries in East Asia)[31]. However, in this subgroup analysis, it was found that the heterogeneity between the two standard groups was not statistically significant, which indicates that regardless of which definition standard was adopted, it had little impact on the results.
We further divided the 16 articles into three regions, namely Europe, America, and Asia, according to the regions where the research was conducted. The subgroup analysis found that the heterogeneity among the groups in the three regions was statistically significant and that the ethnic groups in the three regions had significant effects on the results of the meta-analysis. Studies have suggested that East Asian populations are highly vulnerable to obesity and cardiovascular diseases, which may be related to the surgical prognosis of CRC. However, the specific mechanisms require further investigation.
In recent years, great progress has been made in the robot-assisted radical resection of CRC. Panteleimonitis et al[32] in their research compared it with conventional laparoscopic surgery and found that for patients with obesity, robot-assisted colorectal cancer surgery compared with laparoscopic surgery had a shorter hospital stay and a lower readmission rate of 30 d; however, the operation time was longer. This suggests that robot-assisted colorectal cancer surgery may have a better prognosis than laparoscopic surgery for patients with obesity; however, the heterogeneity between the two surgeries was not statistically significant in this subgroup analysis, and there was no significant difference between the total complication rates of the two surgeries. Therefore, the advantages of robot-assisted colorectal surgery require further studies and validation using different research indicators.
Although the sample size included in this study was sufficient and the results of the sensitivity analysis were stable, there were still some limitations. First, the prognostic indicators were not complete enough, and there were no informative indicators, such as the patient's hospital stay, postoperative urinary tract infection, and acute renal failure; Second, the included studies were observational cohort studies, and process control for the studies was insufficient. Some patients who switched to laparotomy when laparoscopic surgery was ineffective were considered to have undergone laparoscopic surgery; and Third, some patients with benign tumors in some studies were included in this meta-analysis, and there may be some bias. Thus, further research on this topic is warranted.
Obesity (excessive BMI) can increase the overall complication and SSI rates of patients with CRC after radical surgery but has no significant effect on the incidence of anastomotic leak, reoperation rate of patients, and short-term mortality rate.
Obesity is a state in which excess heat is converted into excess fat, which accumulates in the body and may cause damage to multiple organs of the circulatory, endocrine, and digestive systems. Studies have shown that the accumulation of abdominal fat and mesenteric fat hypertrophy in patients with obesity makes laparoscopic surgery highly difficult, which is not conducive to operation and affects patient prognosis. However, there is still controversy regarding these conclusions.
Research on the state of obese patients converting excess heat into excess fat, which can accumulate in the body and cause damage to multiple organs of the circulatory, endocrine, and digestive systems. Abdominal fat accumulation and mesenteric fat in obese patients make laparoscopic surgery difficult, detrimental to surgery, and affecting patient prognosis.
To explore the relationship between body mass index (BMI) and short-term prognosis after surgery for colorectal cancer.
PubMed, Embase, Ovid, Web of Science, CNKI, and China Biology Medicine Disc databases were searched to obtain relevant articles on this topic. After the articles were screened according to the inclusion and exclusion criteria and the risk of literature bias was assessed using the Newcastle-Ottawa Scale, the prognostic indicators were combined and analyzed.
A total of 16 articles were included for quantitative analysis, and 15588 patients undergoing colorectal cancer surgery were included in the study, including 3775 patients with obesity and 11813 patients without obesity. Among them, 12 articles used BMI ≥ 30 kg/m2 and 4 articles used BMI ≥ 25 kg/m2 for the definition of obesity. Four patients underwent robotic colorectal surgery, whereas 12 underwent conventional laparoscopic colorectal resection. The quality of the literature was good. Meta-combined analysis showed that the overall complication rate of patients with obesity after surgery was higher than that of patients without obesity [OR = 1.35, 95%CI: 1.23-1.48, Z = 6.25, P < 0.0001]. The incidence of anastomotic leak after surgery in patients with obesity was not significantly different from that in patients without obesity [OR = 0.99, 95%CI: 0.70-1.41), Z = -0.06, P = 0.956]. The incidence of surgical site infection (SSI) after surgery in patients with obesity was higher than that in patients without obesity [OR = 1.43, 95%CI: 1.16-1.78, Z = 3.31, P < 0.001]. The incidence of reoperation in patients with obesity after surgery was higher than that in patients without obesity; however, the difference was not statistically significant [OR = 1.15, 95%CI: 0.92-1.45, Z = 1.23, P = 0.23]; Patients with obesity had lower mortality after surgery than patients without obesity; however, the difference was not statistically significant [OR = 0.61, 95%CI: 0.35-1.06, Z = -1.75, P = 0.08]. Subgroup analysis revealed that the geographical location of the institute was one of the sources of heterogeneity. Robot-assisted surgery was not significantly different from traditional laparoscopic resection in terms of the incidence of complications.
Obesity increases the overall complication and SSI rates of patients undergoing colorectal cancer surgery but has no influence on the incidence of anastomotic leak, reoperation rate, and short-term mortality rate.
Colorectal rectal cancer (CRC) is a common malignant tumor and ranks third in the incidence of malignant tumors worldwide; it is second only to lung and breast cancers, with a mortality rate of approximately 8% of all malignant tumors. Similar to other malignancies, the cause of CRC remains unclear and can occur anywhere in the colon or rectum; however, it is most common in the rectum and sigmoid colon, whereas the remainder is found sequentially in the cecum, ascending, descending, and transverse colon. Surgical treatment still remains the radical treatment of CRC, and radical resection of intestinal cancer is defined as the removal of macroscopic tumors, including primary and draining lymph nodes. Although the lesion can be removed during surgery, complete removal is still difficult in patients with extensive local disease. For patients with advanced CRC, the tumor size is relatively large, with high vascular variation, and the visual field of laparotomy is poor, making the surgery difficult. In recent years, laparoscopy has emerged as an auxiliary operation with the advantages of a small surgical wound, an open operation field, and rapid postoperative recovery. It has been gradually applied to radical resection of CRC and has achieved an ideal clinical effect.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poa-Li C, United States; Strong VE, United States S-Editor: Liu XF L-Editor: A P-Editor: Cai YX
| 1. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (2)] |
| 2. | Jin K, Ren C, Liu Y, Lan H, Wang Z. An update on colorectal cancer microenvironment, epigenetic and immunotherapy. Int Immunopharmacol. 2020;89:107041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Davis CH, Shirkey BA, Moore LW, Gaglani T, Du XL, Bailey HR, Cusick MV. Trends in laparoscopic colorectal surgery over time from 2005-2014 using the NSQIP database. J Surg Res. 2018;223:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Kashihara H, Shimada M, Yoshikawa K, Higashijima J, Tokunaga T, Nishi M, Takasu C, Yoshimoto T. The influence and countermeasure of obesity in laparoscopic colorectal resection. Ann Gastroenterol Surg. 2021;5:677-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | Harr JN, Luka S, Kankaria A, Juo YY, Agarwal S, Obias V. Robotic-assisted colorectal surgery in obese patients: a case-matched series. Surg Endosc. 2017;31:2813-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Merkow RP, Bilimoria KY, McCarter MD, Bentrem DJ. Effect of body mass index on short-term outcomes after colectomy for cancer. J Am Coll Surg. 2009;208:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Gorgun E, Ozben V, Costedio M, Stocchi L, Kalady M, Remzi F. Robotic vs conventional laparoscopic rectal cancer surgery in obese patients. Colorectal Dis. 2016;18:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | He Y, Wang J, Bian H, Deng X, Wang Z. BMI as a Predictor for Perioperative Outcome of Laparoscopic Colorectal Surgery: a Pooled Analysis of Comparative Studies. Dis Colon Rectum. 2017;60:433-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1617] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 10. | Hannan E, Troy A, Feeney G, Ullah MF, Ryan C, McNamara E, Coffey JC, Peirce C. The impact of body mass index on outcomes in robotic colorectal surgery: a single-centre experience. J Robot Surg. 2022;16:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Lagares-Garcia J, O'Connell A, Firilas A, Robinson CC, Dumas BP, Hagen ME. The influence of body mass index on clinical short-term outcomes in robotic colorectal surgery. Int J Med Robot. 2016;12:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, Kuroyanagi H, Yamaguchi T. Effect of body mass index on short-term outcomes of patients undergoing laparoscopic resection for colorectal cancer: a single institution experience in Japan. Surg Laparosc Endosc Percutan Tech. 2011;21:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Choi BJ, Jeong WJ, Kim SJ, Lee SC. Impact of obesity on the short-term outcomes of single-port laparoscopic colectomy for colorectal cancer in the Asian population: A retrospective cohort study. Medicine (Baltimore). 2017;96:e6649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Zhang Q, Liu Q, Chen J, Mei S, Liang J, Wang Z. Short-Term Outcomes for Laparoscopic Surgery for BMI≥30 Patients with Rectal Cancer. Asian Pac J Cancer Prev. 2021;22:3705-3709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yamashita M, Tominaga T, Nonaka T, Fukda A, Moriyama M, Oyama S, Tanaka K, Hamada K, Araki M, Sumida Y, Takeshita H, Hisanaga M, Fukuoka H, Wada H, Tou K, Sawai T, Nagayasu T. Impact of obesity on short-term outcomes of laparoscopic colorectal surgery for Japanese patients with colorectal cancer: A multicenter study. Asian J Endosc Surg. 2021;14:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Tachikawa J, Chiba H, Okada N, Arimoto J, Ashikari K, Kuwabara H, Nakaoka M, Higurashi T, Goto T, Nakajima A. Impact of obesity in colorectal endoscopic submucosal dissection: single-center retrospective cohort study. BMC Gastroenterol. 2021;21:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Bège T, Lelong B, Francon D, Turrini O, Guiramand J, Delpero JR. Impact of obesity on short-term results of laparoscopic rectal cancer resection. Surg Endosc. 2009;23:1460-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Hede P, Sörensson MÅ, Polleryd P, Persson K, Hallgren T. Influence of BMI on short-term surgical outcome after colorectal cancer surgery: a study based on the Swedish national quality registry. Int J Colorectal Dis. 2015;30:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Hirpara DH, O'Rourke C, Azin A, Quereshy FA, Wexner SD, Chadi SA. Impact of BMI on Adverse Events After Laparoscopic and Open Surgery for Rectal Cancer. J Gastrointest Cancer. 2022;53:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Makino T, Trencheva K, Shukla PJ, Rubino F, Zhuo C, Pavoor RS, Milsom JW. The influence of obesity on short- and long-term outcomes after laparoscopic surgery for colon cancer: a case-matched study of 152 patients. Surgery. 2014;156:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Bamboat ZM, Kinnier C, Dursun A, Ferrone CR, Shellito PC, Berger DL, Bordeianou L. Short-term outcomes in obese patients after colectomy for adenocarcinoma at a bariatric center. J Gastrointest Surg. 2012;16:1923-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Miyamoto Y, Ishii T, Tashiro J, Satoh T, Watanabe M, Baba H, Yamaguchi S. Effects of obesity on the outcome of laparoscopic surgery for colorectal cancer. Surg Today. 2014;44:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Bayraktar O, Aytaç E, Özben V, Atasoy D, Bilgin İA, Erenler Bayraktar İ, Baca B, Hamzaoğlu İ, Karahasanoğlu T. Does Robot Overcome Obesity-related Limitations of Minimally Invasive Rectal Surgery for Cancer? Surg Laparosc Endosc Percutan Tech. 2018;28:e8-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Poulsen M, Ovesen H. Is laparoscopic colorectal cancer surgery in obese patients associated with an increased risk? J Gastrointest Surg. 2012;16:1554-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Bell S, Kong JC, Wale R, Staples M, Oliva K, Wilkins S, Mc Murrick P, Warrier SK. The effect of increasing body mass index on laparoscopic surgery for colon and rectal cancer. Colorectal Dis. 2018;20:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Peacock O, Limvorapitak T, Hu CY, Bednarski BK, Tillman MM, Kaur H, Taggart MW, Dasari A, Holliday EB, You YN, Chang GJ. Robotic rectal cancer surgery: comparative study of the impact of obesity on early outcomes. Br J Surg. 2020;107:1552-1557. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Hussein AH, El-Baaly A, Ghareeb WM, Madbouly K, Gabr H. Outcome and quality of life in obese patients underwent laparoscopic vs. open appendectomy. BMC Surg. 2022;22:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Mao D, Flynn DE, Yerkovich S, Tran K, Gurunathan U, Chandrasegaram MD. Effect of obesity on post-operative outcomes following colorectal cancer surgery. World J Gastrointest Oncol. 2022;14:1324-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Bizzoca C, Zupo R, Aquilino F, Castellana F, Fiore F, Sardone R, Vincenti L. Video-Laparoscopic vs Open Surgery in Obese Patients with Colorectal Cancer: A Propensity Score Matching Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Conti C, Pedrazzani C, Turri G, Gecchele G, Valdegamberi A, Ruzzenente A, Zamboni GA, Lippi G, Guglielmi A. Visceral obesity enhances inflammatory response after laparoscopic colorectal resection. Int J Clin Pract. 2021;75:e14795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Li H, Boakye D, Chen X, Jansen L, Chang-Claude J, Hoffmeister M, Brenner H. Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer. Gastroenterology. 2022;162:1088-1097.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 32. | Panteleimonitis S, Pickering O, Abbas H, Harper M, Kandala N, Figueiredo N, Qureshi T, Parvaiz A. Robotic rectal cancer surgery in obese patients may lead to better short-term outcomes when compared to laparoscopy: a comparative propensity scored match study. Int J Colorectal Dis. 2018;33:1079-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |