Published online Apr 26, 2023. doi: 10.12998/wjcc.v11.i12.2670
Peer-review started: January 29, 2023
First decision: February 17, 2023
Revised: March 10, 2023
Accepted: March 27, 2023
Article in press: March 27, 2023
Published online: April 26, 2023
Processing time: 86 Days and 1.2 Hours
For thousands of years, medicinal cannabis has been used for pain treatment, but its use for pain management is still controversial. Meta-analysis of the literature has shown contrasting results on the addition of cannabinoids to opioids compared with placebo/other active agents to reduce pain. Clinical studies are mainly focused on medicinal cannabis use in chronic pain management, for which the analgesic effect has been proven in many studies. This review focuses on the potential use of medical cannabis for acute pain management in preclinical studies, studies on healthy subjects and the few pioneering studies in the clinical setting.
Core Tip: Medicinal cannabis use for pain management is still controversial. Meta-analysis of the literature has shown contrasting results on the addition of cannabinoids to opioids to in reducing pain. Clinical studies are mainly focused on medicinal cannabis use in chronic pain management, for which the analgesic effect has been proven in many studies. This present review focuses on the potential application of medical cannabis for acute pain, exploring the physiopathology of the endocannabinoid system, preclinical studies, studies on healthy subjects and the few pioneering studies in the clinical setting.
- Citation: Fiore M, Alfieri A, Di Franco S, Petrou S, Damiani G, Pace MC. Medicinal cannabis products for the treatment of acute pain. World J Clin Cases 2023; 11(12): 2670-2676
- URL: https://www.wjgnet.com/2307-8960/full/v11/i12/2670.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i12.2670
The definition “medicinal cannabis” identifies the prescription-based cannabis and cannabinoids recognized to treat, modulate or even extinguish signs and symptoms of disease[1].
At the moment its prescription is limited to certain rare clusters of patients such as forms of drug-resistant adult or pediatric epilepsy, third-line antiemetic agents in chemotherapy-treated patients, and spasms in the context of multiple sclerosis (MS). There is some evidence medical cannabis can help certain types of pain, though this evidence is not yet strong enough to recommend it for pain relief[2]. Pain is “an unpleasant sensory and emotional experience associated with, or resembling that associated with actual or potential tissue damage”, the International Association for the Study of Pain in the last revision of the pain definition pointed out the concept of the protective role of acute pain in contrast with the maladaptive nature of chronic pain[3]. Acute pain serves as a warning sign of disease or threat to the body; It happens suddenly, starts sharp or intense, is caused by injury, surgery, illness, trauma, or painful medical procedures and generally lasts from a few minutes to less than six months. Acute pain usually disappears whenever the underlying cause is treated or healed[4].
The burden of acute pain worldwide is remarkable and covers 4 out of 10 patients admitted yearly to the Emergency Department (ED)[5]. Their improper/inefficient therapeutic management is capable to cause several hospital new admission and even consolidate a life-long chronic pain status[6]. Their evolution toward pain chronicity is also testified by the opioid use/misuse of these patients in the previous three 3 months, quantified in a recent report regarding 1 out of 5 patients[7].
Remarkably, patients with chronic pain treated with opioids have a higher risk of opioid overdose[8], in particular, the United States population with opioid use disorder was quantified in 1.6 million last year[9]. Even more dramatic are the 30 years of survey data that found 564 thousand patients perished from opioid (medical and illicit) fatal overdoses[10]. In this respect, a multimodal approach to acute pain management with the use of non-opioids medication should be mandatory. This review aims to evaluate medicinal cannabis products for the treatment of acute pain from the physio-pathological rationale to the more recent clinical practice.
The endocannabinoid system (ECS) plays a vital role in managing pain by adjusting the activity of various neurotransmitters and receptors that are involved in the sensation of pain. The ECS is composed of endocannabinoids (such as anandamide and 2-arachidonoylglycerol), their receptors (CB1 and CB2), and numerous enzymes that are responsible for both the synthesis and the degradation of endocannabinoids.
The ECS can modulate multiple pathways involved in the perception of pain. Two primary endocannabinoid receptors, CB1 and CB2, have been identified as being involved in the pathophysiology of acute pain.
The CB1 receptor, which belongs to the family of receptors that are coupled to G-proteins, transmits signals through the release of Gβγ proteins and the decrease of cAMP orchestrated by Gαi in the regulation of neurotransmission, neurodevelopment, and synaptic plasticity[11].
Once activated and dissociated these proteins are modulated by a kinase through a series of interactions mediated by β-arrestins[12].
CB1 receptors are expressed in higher concentration in the central nervous system (CNS), and modulate the release of neurotransmitters, acting as gatekeepers at the pre-synaptic terminals of GABAergic and glutamatergic neurons[13].
Furthermore, these receptors are dispersed throughout the cell, including areas such as lipid rafts, endosomes, and mitochondria. They can also be found in cells such as astrocytes and oligodendrocytes, as well as in various non-neural tissues such as the heart, lungs, and bones. These peripheral receptors are involved in the control and modulation of nociceptive sensitivity, peristalsis, reproduction, energy and muscle metabolism[14].
CB1 receptors have a vital function in controlling the experience of pain by managing the conversion of harmful peripherical stimuli into pain signals at the spinal cord level. They are capable of both diminishing or intensifying the transmission of pain signals to the CNS.
CB1 receptors located in higher brain regions (periaqueductal gray matter and rostral ventromedial medulla) are linked to the perception of pain, initiating the descending inhibition of pain signals or hindering the descending facilitation to the nociceptive circuit of the spinal cord[15].
Furthermore, CB1 is highly expressed in the brain regions related to the emotional and affective aspects of pain in humans, like the frontal-limbic circuits. It's mainly located on the presynaptic terminals of neurons and concentrated in the perisynaptic zone where it can regulate the neurotransmitter release. This mechanism aligns with CB1's role in retrograde neurotransmission, and it's becoming an increasingly important area of research in pain management[16].
CB1 receptors are coupled negatively, by Gi/o proteins, to the enzyme adenylate cyclase.
When activated, the CB1 receptors inhibit the calcium channels and activate the potassium channels, which decreases neurotransmitter release[17].
However, this is a synthetic description of the role of CB1 receptors. In vivo, the overall effect of CB1 receptors activation differs within the neural network according to the areas of the brain and the type of presynaptic cell involved in the pain pathway.
CB1 receptors have been found even in B immunity cells and the CNS's glial cells. In the CNS astroglial CB1 receptors play a crucial role in regulating behaviour and plasticity[18].
The CB2 receptor is mostly linked to Gi/o proteins and is involved in signalling pathways that regulate intracellular calcium concentration. Unlike CB1 receptors, the CB2 receptor is not commonly found in the CNS, but it is highly expressed in immune system cells both peripheral (macrophages, lymphocytes, and mast cells).
The activation of CB2 receptors reduces especially inflammatory pain states by anti-inflammatory effect and the reduction of hyperalgesia[17].
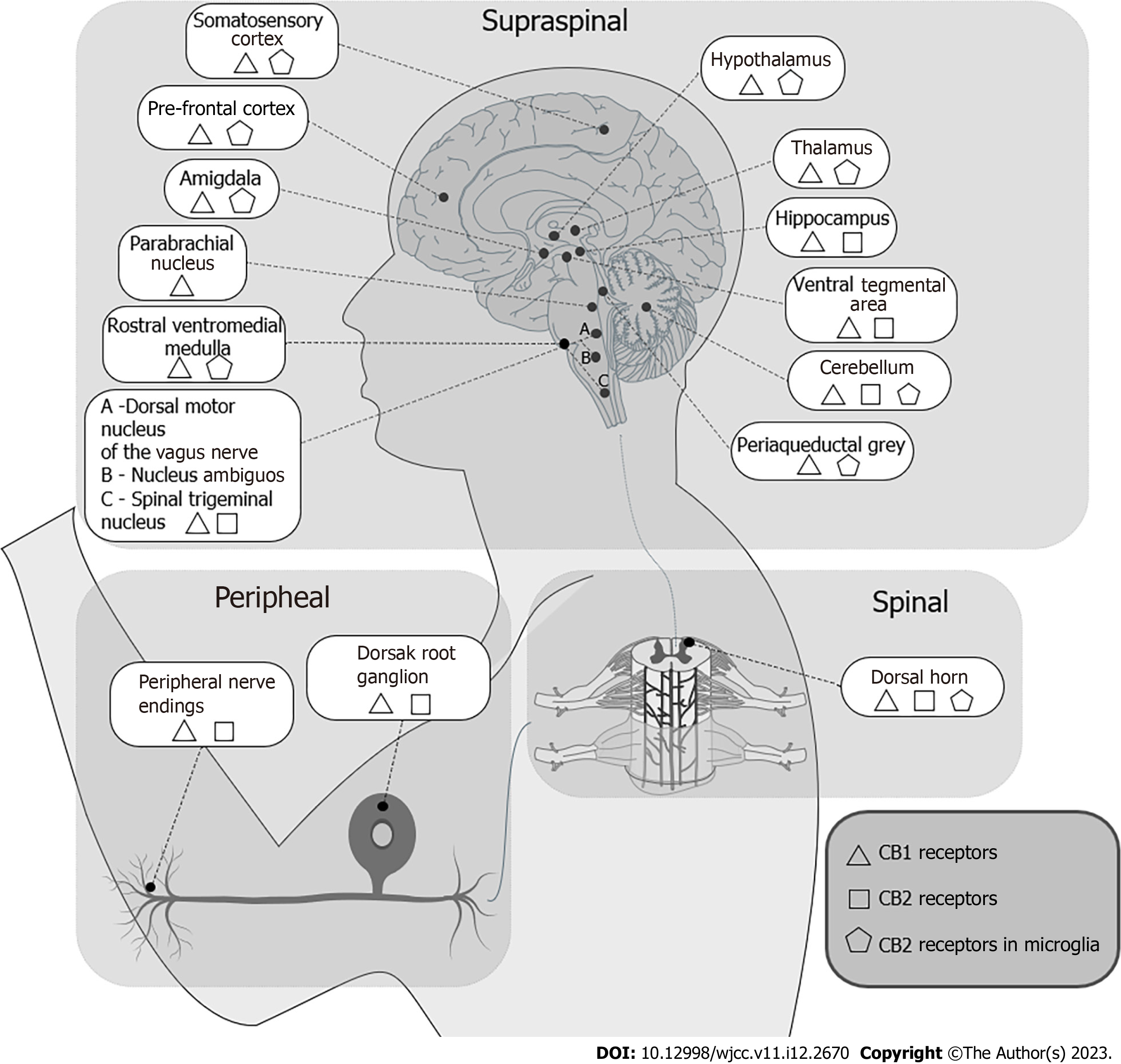
There is an ongoing debate about the presence of active CB2 receptors in the CNS, especially in astrocytes and microglia, but the evidence remains inconclusive. Figure 1 offers a synthesis of the distribution of the ECS receptors in the CNS, in the spinal cord and the peripheral nervous system. The receptors expressed in the CNS (mostly CB1) are involved in the modulation of the descending control of nociception and in the emotive perception of pain.
The receptors located in the dorsal horns of the spinal cord have a role in pain ascendent sensitive nociception pathways. The receptors expressed in the peripheral nervous system contribute to the excitability of nervous termination and modulate the immunity system through the immunity cells sensible to endocannabinoids.
For half a century cannabinoids have been evaluated for their analgesic effect in animal models[19]. In 1973 Sofia et al[19] evaluated the analgesic properties of tetrahydrocannabinol (THC) in mice and rats using four different models of experimentally induced pain (Acetic acid-induced abdominal constriction, Haffner’s tail pinch, Hot plane test, Randall-Selitto test); THC, in all the models studied, showed greater analgesic activity than aspirin. In 2002 Conti et al[20] evaluated the anti-inflammatory action of the synthetic cannabinoid nabilone in a model of acute inflammation (AI) in male Wistar rats: The anti-inflammatory activity and thermal hyperalgesia were explored respectively by measuring oedema and paw withdrawal latencies, using the radiant heat method, after the injection of 0.1 mL carrageenan into the right paw. Paw oedema and thermal hyperalgesia were modulated by nabilone in a dose-dependent manner. Likewise, the CB2 antagonist (SR 144528) pre-administered neutralized the nabilone effect. The authors concluded the effect of nabilone is mediated by an uncharacterized CB2-like cannabinoid receptor[20]. More recently, Rock et al[21] evaluated the effect of cannabidiolic acid (CBDA) and Δ9-THC in a similar rodent model of carrageenan-induced AI in the rat hind paw. Contrary to the previous study the authors used a CB1 cannabinoid receptor antagonist (SR141716) to block the anti-hyperalgesia effects of THC while CBDA’s effects were blocked by a competitive and selective vanilloid receptor 1 (AMG9810). Consistent with previous literature (Conti et al[20]), Rock et al[21] demonstrated that THC produced dose-dependent anti-hyperalgesia and anti-inflammatory effects and they are further synergic use of CBDA. CBDA contrasts hyperalgesia via vanilloid receptor 1, as testified by its selective inhibition with AMG9810, conversely THC acts on central and peripheral CB1 receptors, as testified by its selective inhibitor SR141716[21].
Cannabinoids improve pain in pre-clinical models of traumatic spinal cord injury (SCI)[22]. SCI causes Neuropathic pain (NP) with different mechanisms, one of which involves oxidative stress. Baron-Flores et al[23] established the biomechanism by which CBD may exert a powerful analgesic effect in acute NP: In fact, CBD empowers the natural anti-oxidative cellular defence such as glutathione concentration in a dose-dependent manner. Thus, cells experienced decreased lipid peroxidation (Table 1).
Unlike preclinical studies that showed the efficacy of cannabinoids in acute pain, the results on healthy volunteers (HVs) do not appear to confirm the potential analgesic effect of medicinal cannabis products for the treatment of acute pain. Kraft et al[24] demonstrated that THC has no analgesic and antihyperalgesic effects in two human models of physically-induce and chemically induced pain.
In line with the previous study, also Schindler et colleagues that did not find any THC analgesic and antihyperalgesic effect of THC administered intravenously[25].
In a randomized, placebo-controlled, double-blinded, crossover study (CANAB I), Schneider et al[26] colleagues did not find any effect of oral CBD on hyperalgesia and allodynia electrically induced, results conformed in the CANAB II trial doubling CBD dose used in CANAB I trial[27] (Table 2).
| Ref. | Number | Cannabinoid (dose) | Model(s) | Outcome(s) | Assessment |
| Kraft et al[24], 2008 | 18 | THC 20 (mg PO) | Sunburn; Capsaicin ID | Heat and electrical thresholds | 1, 2, 2.5, 3, 4, 5, 6, 7, 8 h |
| Schindler et al[25], 2020 | 11 | THC (0.01 mg/Kg or 0.03 mg/Kg IV) | Capsaicin ID | VAS; HA; Heat and electrical thresholds | 0.3; 2 h |
| Schneider et al[26], 2022 | 20 | CBD (800-mg PO) | IES | NRS; vFF; DCS | 0, 1, 2.1 h |
| Dieterle et al[27], 2022 | 24 | CBD (1600-mg PO) | IES | NRS; HA; AA | 1 h |
Beaulieu et al[28] colleagues performed a double-blind, randomized, placebo-controlled, parallel-group pilot trial enrolling forty-one patients undergoing major surgery, mainly gynecologic (46%) or orthopaedic (44%). Patients were randomly assigned to four distinct groups (nabilone 1 mg/die Vs nabilone 2 mg/die Vs ketoprofen 50 mg/die Vs placebo). Despite no differences were detected in terms of 24-h morphine request, interestingly the pain control was statistically significant only in the nabilone 2 mg/die.
Conversely, Ostenfeld et al[29] did not find a statistically significant analgesic effect of a single dose of GW842166 (non-cannabinoid CB2 agonist) in patients that underwent dental surgery (third molar surgery). Furthermore, this randomized controlled trial (RCT) only re-affirm the high effectivity of ibuprofen Versus placebo[29]. Furthermore, Bebee et al[30] colleagues in their RCT focused on low back pain in patients admitted to ED and evaluated the potential efficacy of 400 mg oral CBD as a combination therapy with the department's standard of care. Pain assessment was performed with a verbal scale rating from 0 to 10 their residual pain after two hours from the therapies. This RCT failed to find a clinically relevant decrease in pain comparing patients that underwent CBD+pain killer vs pain killer alone (Table 3).
| Ref. | Patient number | Cannabinoid (dose) | Participants | Conclusion |
| Beaulieu[28], 2006 | 41 | Nabilone (1 or 2 mg PO) | Major surgery | Pain higher in 2 mg group |
| Ostenfeld et al[29], 2011 | 123 | GW842166 100 and 800 mg | Extractive surgery | Not superiority to placebo |
| Bebee et al[30], 2021 | 100 | CBD (400 mg) | LBP | Not superiority to placebo |
This is the first review of the literature exploring cannabis products use from preclinical to clinical studies. Mice models have highlighted the analgesic properties of cannabinoids[20,21,23] capable to interrupt pain through partially unknown mechanisms targeting the ECS.
In light of the fact that all studies conducted on HVs failed to show the significant efficacy of medicinal cannabis products for acute pain management[24-27], it seems paradoxical that studies have been conducted on patients[28-30]. To date, the use of cannabis products in the treatment of acute pain is clearly disadvantageous compared to available therapeutic alternatives.
Methodologically phase II clinical trials should follow phase I studies when these latest show efficacies. In the case of studies published on the use of medicinal cannabis products for acute pain induced in HVs, no one showed superiority to a placebo so there is no support to conduct phase II clinical trials. Acute pain management in this Era of the “Opioid Crisis” should not deprive itself of a potential therapeutic option[31]. The knowledge of this review should be taken into consideration by researchers before proceeding further with phase II clinical trials, the researchers should take a “step back” to identify effective models for the treatment of acute pain in phase I studies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand; Ji X, China S-Editor: Liu XF L-Editor: A P-Editor: Yu HG
| 1. | Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | National Health Service (NHS). Medical cannabis (and cannabis oils). 2022. Available from: https://www.nhs.uk/conditions/medical-cannabis/. |
| 3. | Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2257] [Cited by in RCA: 2248] [Article Influence: 449.6] [Reference Citation Analysis (1)] |
| 4. | International Association for the Study of Pain (IASP). Acute Pain. 2023. Available from: https://www.iasp-pain.org/resources/topics/acute-pain/. |
| 5. | Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 580] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 6. | Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med. 2010;11:1859-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 363] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 7. | Dahlhamer JM, Connor , Bose J, Lucas J W, Zelaya Carla E, National Center for Health Statistics (US). Prescription opioid use among adults with chronic pain: United States, 2019. In: National Health Statistics Reports. [DOI] [Full Text] |
| 8. | Hartz SM, Culverhouse RC, Mintz CM, Ellis MS, Kasper ZA, Cavazos-Rehg P, Grucza RA, Bierut LJ, Cicero TJ. Association between recent overdose and chronic pain among individuals in treatment for opioid use disorder. PLoS One. 2022;17:e0271379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 9. | O'Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL. Vital Signs: Characteristics of Drug Overdose Deaths Involving Opioids and Stimulants - 24 States and the District of Columbia, January-June 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1189-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 10. | Centers for Disease Control and Prevention (CDC). Understanding the Opioid Overdose Epidemic. 2022. Available from: https://www.cdc.gov/opioids/basics/epidemic.html. |
| 11. | Lu HC, Mackie K. An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry. 2016;79:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 787] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 12. | Nogueras-Ortiz C, Yudowski GA. The Multiple Waves of Cannabinoid 1 Receptor Signaling. Mol Pharmacol. 2016;90:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol. 2005;168:327-365. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Ilyasov AA, Milligan CE, Pharr EP, Howlett AC. The Endocannabinoid System and Oligodendrocytes in Health and Disease. Front Neurosci. 2018;12:733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Nyilas R, Gregg LC, Mackie K, Watanabe M, Zimmer A, Hohmann AG, Katona I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci. 2009;29:1964-1978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Luongo L, Maione S, Di Marzo V. Endocannabinoids and neuropathic pain: focus on neuron-glia and endocannabinoid-neurotrophin interactions. Eur J Neurosci. 2014;39:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Soethoudt M, Grether U, Fingerle J, Grim TW, Fezza F, de Petrocellis L, Ullmer C, Rothenhäusler B, Perret C, van Gils N, Finlay D, MacDonald C, Chicca A, Gens MD, Stuart J, de Vries H, Mastrangelo N, Xia L, Alachouzos G, Baggelaar MP, Martella A, Mock ED, Deng H, Heitman LH, Connor M, Di Marzo V, Gertsch J, Lichtman AH, Maccarrone M, Pacher P, Glass M, van der Stelt M. Cannabinoid CB(2) receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun. 2017;8:13958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 18. | Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, Lichtman AH. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology. 2018;43:52-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 19. | Sofia RD, Nalepa SD, Harakal JJ, Vassar HB. Anti-edema and analgesic properties of delta9-tetrahydrocannabinol (THC). J Pharmacol Exp Ther. 1973;186:646-655. [PubMed] |
| 20. | Conti S, Costa B, Colleoni M, Parolaro D, Giagnoni G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br J Pharmacol. 2002;135:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Rock EM, Limebeer CL, Parker LA. Effect of cannabidiolic acid and ∆(9)-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology (Berl). 2018;235:3259-3271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Bhatti FI, Mowforth OD, Butler MB, Bhatti AI, Adeeko S, Akhbari M, Dilworth R, Grodzinski B, Osunronbi T, Ottewell L, Teh JQ, Robinson S, Suresh G, Waheed U, Walker B, Kuhn I, Smith L, Bartlett RD, Davies BM, Kotter MRN. Systematic review of the impact of cannabinoids on neurobehavioral outcomes in preclinical models of traumatic and nontraumatic spinal cord injury. Spinal Cord. 2021;59:1221-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Baron-Flores V, Diaz-Ruiz A, Manzanares J, Rios C, Burelo M, Jardon-Guadarrama G, Martínez-Cárdenas MLÁ, Mata-Bermudez A. Cannabidiol attenuates hypersensitivity and oxidative stress after traumatic spinal cord injury in rats. Neurosci Lett. 2022;788:136855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, Kress HG. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology. 2008;109:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Schindler EAD, Schnakenberg Martin AM, Sewell RA, Ranganathan M, DeForest A, Pittman BP, Perrino A Jr, D'Souza DC. In an exploratory randomized, double-blind, placebo-controlled, cross-over study, psychoactive doses of intravenous delta-9-tetrahydrocannabinol fail to produce antinociceptive effects in healthy human volunteers. Psychopharmacology (Berl). 2020;237:3097-3107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Schneider T, Zurbriggen L, Dieterle M, Mauermann E, Frei P, Mercer-Chalmers-Bender K, Ruppen W. Pain response to cannabidiol in induced acute nociceptive pain, allodynia, and hyperalgesia by using a model mimicking acute pain in healthy adults in a randomized trial (CANAB I). Pain. 2022;163:e62-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Dieterle M, Zurbriggen L, Mauermann E, Mercer-Chalmers-Bender K, Frei P, Ruppen W, Schneider T. Pain response to cannabidiol in opioid-induced hyperalgesia, acute nociceptive pain, and allodynia using a model mimicking acute pain in healthy adults in a randomized trial (CANAB II). Pain. 2022;163:1919-1928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anaesth. 2006;53:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Ostenfeld T, Price J, Albanese M, Bullman J, Guillard F, Meyer I, Leeson R, Costantin C, Ziviani L, Nocini PF, Milleri S. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain. 2011;27:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Bebee B, Taylor DM, Bourke E, Pollack K, Foster L, Ching M, Wong A. The CANBACK trial: a randomised, controlled clinical trial of oral cannabidiol for people presenting to the emergency department with acute low back pain. Med J Aust. 2021;214:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Vučković S, Srebro D, Vujović KS, Vučetić Č, Prostran M. Cannabinoids and Pain: New Insights From Old Molecules. Front Pharmacol. 2018;9:1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |