Published online Apr 26, 2023. doi: 10.12998/wjcc.v11.i12.2582
Peer-review started: November 27, 2022
First decision: December 27, 2022
Revised: January 22, 2023
Accepted: March 29, 2023
Article in press: March 29, 2023
Published online: April 26, 2023
Processing time: 149 Days and 7.2 Hours
This review summarized the current controversies in the management of acute pancreatitis (AP). The controversies in management range from issues involving fluid resuscitation, nutrition, the role of antibiotics and antifungals, which analgesic to use, role of anticoagulation and intervention for complications in AP. The interventions vary from percutaneous drainage, endoscopy or surgery. Active research and emerging data are helping to formulate better guidelines. The available evidence favors crystalloids, although the choice and type of fluid resuscitation is an area of dynamic research. The nutrition aspect does not have controversy as of now as early enteral feeding is preferred most often than not. The empirical use of antibiotics and antifungals are gray zones, and more data is needed for conclusive guidelines. The choice of analgesic is being studied, and the recommendations are still evolving. The position of using anticoagulation is still awaiting consensus. The role of intervention is well established, although the modality is constantly changing and favoring endoscopy or percutaneous drai
Core Tip: The controversies in the management of acute pancreatitis are an area of dynamic research, and emerging data is assisting in guideline formulation. The current evidence favors crystalloids, although the choice and type of fluid resuscitation is an evolving research area. The empirical use of antibiotics and antifungals are gray zones and lack guidelines. The choice of analgesic lacks definite recommendations. The role of anticoagulation lacks agreement. The role of intervention is well established and favors endoscopy or percutaneous drainage rather than surgery. It is obvious that more evidence is essential for effective guidelines in these critical management issues of acute pancreatitis.
- Citation: Manrai M, Dawra S, Singh AK, Jha DK, Kochhar R. Controversies in the management of acute pancreatitis: An update. World J Clin Cases 2023; 11(12): 2582-2603
- URL: https://www.wjgnet.com/2307-8960/full/v11/i12/2582.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i12.2582
Acute pancreatitis (AP) is an acute inflammatory process involving the pancreas, frequently affecting the peripancreatic tissue and less commonly the remote organ systems. It represents a spectrum of diseases ranging from a mild, self-limited course needing only brief hospitalization to moderate disease with increased morbidity and a rapidly progressive, severe illness culminating into multiorgan dysfunction, as categorized by the revised Atlanta Classification[1].
In 2019, the countries with the greatest number of incident cases of AP were India followed by China and the United States. The global estimate of AP incidence in 2019 was 33.7/per 100000 population and is rising in the Western world. The global burden of disease estimation is 1.4 deaths per 100000[2]. Therefore the disease burden is significant and requires more data and research in optimizing therapy. Although the revised Atlanta Classification has standardized the disease severity classification, there are a few controversies in the management of AP that are still evolving and are areas of active research.
In this review, we summarized the current controversies in the management of AP. The controversies are in the following areas: (1) Fluid resuscitation; (2) Nutrition; (3) Antibiotics and antifungals; (4) Analgesics; (5) Role of anticoagulation; (6) Endoscopic retrograde cholangiopancreatography (ERCP); and (7) Drainage in local complications. Certain issues like intra-abdominal hypertension (IAH) and persistent ascites also confound the management. Therefore, despite active research in many of these areas, the consensus is lacking. The data are still emerging, and guidelines are evolving.
The pathophysiology of AP can broadly be classified into an early phase of systemic inflammatory response syndrome (SIRS), lasting 1-2 wk followed by a late phase characterized by disease sequelae and infection. There is a paucity of pharmacological options in the initial acute inflammatory phase; hence, treatment by and large remains supportive. Fluid management in the initial acute inflammatory phase becomes particularly important.
Our understanding of this vital management aspect is based on our understanding of altered pancreatic microcirculation in animal models. Studies have focused on using crystalloids as well as colloids to offset circulatory alterations. However, none of these studies conclusively established the superiority of one over the other[3,4].
Colloids (albumin, dextran, hexastarch) in animal studies have been shown to have better optimization of hemodynamic response. They have a larger molecular size and are better retained in the intravascular compartment. Their osmotic effect draws the fluid from the interstitium into the vascular compartment, thus maintaining better circulatory flow. These benefits, however, come at the cost of anaphylactic reactions, intravascular volume overload and renal impairment. Hypertonic saline, in particular, has shown promising results in animal models especially in modulating cytokine expression[5,6]. The use of balanced solutions like Ringer’s lactate (RL) has demonstrated an inflammasome-mediated anti-inflammatory effect by acting on G-protein-coupled receptor 81, which is a cell surface lactate receptor[7]. The use of colloids in human studies include a combination of dextran with albumin in varying concentrations. A study using albumin after dilution with dextran has demonstrated reduced mortality (7.7%) and reduced progression of pancreatic necrosis (15.0%)[8]. The use of hydroxyethyl starch has not shown any benefit in reducing the risk of organ failure (OF) or mortality in AP[9]. Trials combining the colloids and crystalloids in different concentrations have also shown promising results[10]. The American Gastroenterology Association recommends crystalloids as the initial fluid of choice for resuscitation in the acute inflammatory phase of AP, while it does not recommend the use of colloids like hydroxyl ethyl starch[11].
Traditionally, normal saline (NS) is the crystalloid of choice for critical illnesses like trauma or sepsis. Studies, however, have highlighted the adverse effects of NS therapy notably acute kidney injury (AKI) and non-anion gap acidosis. The landmark SMART trial provided valuable insight supporting the role of balanced crystalloids, i.e. RL and Plasma-Lyte A over NS alone in critically ill patients. Out of a total of 15802 adults admitted to intensive care units (ICUs), those receiving balanced crystalloids (n = 7942) had a lower incidence of major adverse kidney events (14.3%) vs 15.4% in patients receiving NS (n = 1211). Other notable benefits were reduced requirement of renal replacement therapy (2.5% vs 2.9%), persistent renal dysfunction (6.4% vs 6.6%), and 30 d in-hospital mortality (10.3% vs 11.1%) in the RL group compared to the NS group, respectively[12].
Researchers have strived hard to critically analyze the effects of RL vs NS in patients with AP. de Madaria et al[13] showed favorable anti-inflammatory effects of using RL vs NS in AP. Choosakul et al[14] showed a beneficial effect of using RL in reducing SIRS in the first 24 h of pancreatic injury as compared to those receiving NS. This beneficial effect, however, was not reciprocated at 48 h with no effect on disease-related mortality. This is in contrast to an earlier randomized controlled trial (RCT) by Wu et al[15] who demonstrated a statistically significant reduction in SIRS after 24 h of pancreatic injury in patients receiving RL vs those receiving NS. Karki et al[16] in their recent paper provided evidence of reduced systemic inflammation at 72 h in patients who received initial resuscitation with RL vs those who received NS (Table 1).
| Ref. | RL | NS | SIRS | CRP | ||||
| Wu et al[15], 2011 | 19 | 21 | RL | 84% at 24 h | P = 0.035 | RL | Mean CRP 51 mg/L | P = 0.018 |
| NS | 0% reduction at 24 h | NS | Mean CRP 104 mg/L | |||||
| de Madaria et al[13], 2018 | 19 | 21 | RL | Median no of SIRS criteria at 48 h: 01 (0-1) | P = 0.060 | RL | Mean CRP at 48 h: 28 mg/L | P = 0.037 |
| NS | Median no of SIRS criteria at 48 h: 01 (1-2) | NS | Mean CRP at 48 h: 166 mg/L | |||||
| Choosakul et al[14], 2018 | 23 | 24 | RL | Reduction in SIRS at 48 h: 26.1% | P = 0.02 | No difference in CRP | ||
| NS | Reduction in SIRS at 48 h: 26.1% 4.2% | |||||||
| Karki et al[16], 2022 | 26 | 25 | RL | SIRS at 24 h: 15.4% | P = 0.025 | Median CRP at 72 h: 14.2 mg/L | P < 0.001 | |
| NS | SIRS at 24 h: 44.0% | Median CRP at 72 h: 22.2 mg/L | ||||||
Four recent meta-analyses including the above-mentioned RCTs have drawn conflicting conclusions varying from reduced severity of AP, laparoscopic cholecystectomy and risk of ICU admission to no statistically significant benefit of resuscitating with RL compared to NS (Table 2)[17-20].
| Ref. | Inclusion | Conclusion |
| Zhou et al[17], 2021 | 4 RCT, 7964 abstracts, 57 full-text documents | Patients resuscitated with RL were less likely to develop moderately severe/severe AP (OR: 0.49; 95%CI: 0.25-0.97), had reduced requirement of ICU admission (OR: 0.33; 95%CI: 0.13-0.81) and had reduced local complications (OR: 0.42; 95%CI: 0.20-0.88) |
| Aziz et al[18], 2021 | 4 RCT, 2 cohort studies | Patients resuscitated with RL had a lower rate of ICU admission (RR: 0.43; 95%CI: 0.22-0.84), a lower length of hospital stay (MD: 0.77 d; 95%CI: 1.44-0.09 d) and no difference in overall mortality and SIRS at 24 h |
| Vedantam et al[19], 2022 | 6 studies | Patients resuscitated with RL had a decreased need for ICU admission and no statistical difference in the risk of developing SIRS at 24 h (pooled OR: 0.59; 95%CI: 0.22-1.62, P = 0.31) |
| Chen et al[20], 2022 | 4 RCT | Patients resuscitated with RL had a reduced incidence of ICU admission (RR: 0.39; 95%CI: 0.18-0.85; P = 0.02), no significant reduction in SIRS at 24 h, 48 h and 72 h and no reduction in risk of mortality, severe disease or local complications |
Recent human studies in AP have focused on two distinct aspects of fluid management, namely the aggressiveness of fluid therapy and the optimal fluid required for resuscitation.
Early aggressive resuscitation proposes to transfuse one-third of the body’s 72-h fluid requirement within the first 24 h of presentation. This hypothesis was subsequently challenged by other investigators. Garg et al[21] aptly described that this clinical dilemma may require the services of an ‘alchemist.’ This clinical aspect thus required critical review. RCTs comparing aggressive vs restricted fluid resuscitation in the inflammatory phase of AP have been summarized in Table 3[22-26].
| Ref. | No. of patients | Disease severity | Aggressive resuscitation | Non-aggressive resuscitation |
| Mao et al[22], 2009 | Aggressive: 36 | SAP | Mortality: 94.4% | Mortality: 10.0% |
| Non-aggressive: 40 | Mechanical ventilation: 30.6% | Mechanical ventilation: 65.0% | ||
| Mao et al[23], 2010 | Aggressive: 56 | SAP | Mortality: 33.9% | Mortality: 15.3% |
| Non-aggressive: 59 | Sepsis: 78.6% | Sepsis: 57.6% | ||
| Wu et al[15], 2011 | Aggressive: 19 | Reduction in SIRS: 58% | Reduction in SIRS: 42% | |
| Non-aggressive: 21 | ||||
| Buxbaum et al[24], 2017 | Aggressive: 27 | Mild AP | Clinical improvement: 70% | Clinical improvement: 42% |
| Non-aggressive: 33 | SIRS: 7.4% | SIRS: 21.1% | ||
| Cuellar-Monterrubeo et al[25], 2020 | Aggressive: 43 | Mild, moderately severe and severe AP | SIRS at day 7: 13.3% | SIRS at day 7: 13.9% |
| Non-aggressive: 45 | ||||
| Li et al[26], 2020 | Total number (n = 912) | Hemoconcentration hematocrit > 44% vs < 44% | In hematocrit > 44%: increased NPPV | In hematocrit < 44%: reduced risk of NPPV |
Recent systemic reviews and meta-analyses that included both RCTs and cohort studies on the use of aggressive vs restricted intravenous fluid resuscitation in the early acute phase (within the first 24 h from presentation) have weighed in favor of restrictive intravenous transfusion. This has shown that restricted intravenous fluid administration decreases the risk of AKI, pulmonary edema and the need for mechanical ventilation[27].
The recent ‘waterfall trial’ has provided valuable evidence supporting ‘moderate resuscitation, i.e. up to 1.5 mL/kg/h and bolus of 10 mL/kg only in the presence of hypovolemia[28].
To conclude, there is considerable heterogeneity in the study designs amongst various studies, the rate and type of fluids studied, study population and outcome measures. There is a paucity of evidence to recommend aggressive vs restrictive intravenous fluid administration. Most guidelines recommend RL as the initial fluid of choice intending to maintain urine output > 0.5 mL/kg[28,29]. The need of the hour is to incorporate non-invasive methods to assess the patient’s hydration status before commencing intravenous fluid administration and dynamic hemodynamic monitoring and to determine a patient-centric treatment strategy.
There has been a paradigm shift in the management of AP from surgical management to conservative support. While judicious fluid therapy is imperative in the initial inflammatory phase, the concept of “nutritional support‘’ to prevent malnutrition is widely gaining acceptance. Inflammatory cytokines, higher ‘’resting energy expenditure,” protein catabolism, ongoing pain, poor oral intake and complications like gastric outlet obstruction and ileus in combination with micronutrient deficiency have all been postulated as contributing factors that precipitate a state of malnutrition in AP[30].
The earlier concept of “pancreatic rest” (i.e. initiation of enteral feeding on the complete resolution of pain abdomen) has given way to the concept of “early enteral nutrition (EN)”. This concept is based on experimental evidence demonstrating that pancreatic enzyme secretion reduces with increased severity of AP. Thus, injured acinar cells may not respond to an increased physiological stimulus[31].
Early EN has shown a reduced incidence of bacterial translocation thus reducing systemic inflammation and maintaining gut integrity and gut microbiota composition[32-34]. The benefits of early EN have been confirmed in a meta-analysis and systemic reviews[35-38].
Table 4 highlights the meta-analysis demonstrating the benefits of early EN in AP. The newer concept of “immediate EN” vs early EN has been shown to decrease the length of hospital stay and intolerance of feeding but with no statistically significant decrease in the rate of progression to severe pancreatitis or incidence of complications[39].
| Ref. | Inclusion | Conclusion |
| Li et al[35], 2013 | 6 studies | Early EN vs delayed EN: reduced incidence of all infections (OR: 0.38; 95%CI: 0.21–0.68, P < 0.05); reduced incidence of catheter-related sepsis (OR: 0.26; 95%CI: 0.11–0.58, P < 0.05); reduced pancreatic infection (OR: 0.49; 95%CI: 0.31–0.78, P < 0.05); reduced risk of hyperglycemia (OR: 0.24; 95%CI: 0.11–0.52, P < 0.05); reduced length of hospitalization (mean difference: -2.18; 95%CI: -3.48-(-0.87); P < 0.05); reduced mortality (OR: 0.31; 95%CI: 0.14–0.71, P < 0.05); and no difference in pulmonary complications (P > 0.05) |
| Feng et al[36], 2017 | 4 RCTs, 2 retrospective studies | Early EN (within 48 h) vs delayed EN (after 48 h): reduced risk of multiple organ failure (RR: 0.67; 95%CI: 0.46-0.99; P = 0.04); decreased systemic inflammatory response syndrome but not significant (RR: 0.85; 95%CI: 0.71-1.02; P = 0.09); and no significant difference in mortality (RR: 0.78; 95%CI: 0.27-2.24; P = 0.64) |
| Qi et al[37], 2018 | 8 studies (727 patients) | Early EN vs late EN and TPN: risk of mortality (OR: 0.56; 95%CI: 0.23-1.34); multiple OF (OR: 0.40; 95%CI: 0.20-0.79); infectious complications: (OR: 0.57; 95%CI: 0.23-1.42); adverse events (OR: 0.45; 95%CI: 0.17-1.21); and pancreatitis-related infections (OR: 0.83; 95%CI: 0.59-1.18) |
| Zeng et al[38], 2019 | 17 RCTs | Early EN vs delayed EN: lower mortality (9.21% vs 11.22%) but no statistical significance between the two groups (RR: 0.86; 95%CI: 0.60-1.23; P = 0.42); reduced risk of complications (RR: 0.81; 95%CI: 0.70-0.93; P = 0.002); reduced incidence of infections (RR: 0.68; 95%CI: 0.51-0.91, P = 0.009); and no difference in risk of multi OF (RR: 0.82; 95%CI: 0.59-1.14; P = 0.23) |
Oral nutritional support is the preferred mode of feeding in mild AP[37]. The traditional approach of nasojejunal feeding is based on the premise that it bypasses the inflamed pancreas. On the other hand, it was believed that nasogastric (NG) nutrition stimulates pancreatic secretion, thereby causing an exacerbation of the inflammatory process and increasing the risk of developing aspiration pneumonia. However, there is growing evidence that establishes the safety, feasibility and tolerability of NG feeding in AP (Table 5)[40,41]. Whether NG feeding affects disease mortality or morbidity is debatable.
| Ref. | Inclusion | Conclusion |
| Zhu et al[40], 2016 | 4 RCTs | NG vs NJ feed: mortality (RR: 0.71; 95%CI: 0.38-1.32; z = 1.09; P = 0.28); infectious complications (RR: 0.77; 95%CI: 0.45-1.30; z = 0.99; P = 0.32); digestive complications (RR: 1.02; 95%CI: 0.57-1.83; z = 0.08; P = 0.93); achievement of energy balance (RR: 1.00; 95%CI: 0.97-1.03; z = 0.00; P = 1.00) |
| Dutta et al[41], 2020 | 5 RCTs | NG vs NJ feed: mortality (RR: 0.65; 95%CI: 0.36-1.17; no difference in the rate of OF, procedure-related complications, the requirement of surgical intervention and the requirement of PN |
The ESPEN guidelines recommend early initiation of oral feeding in predicted mild AP and EN in preference to parenteral nutrition in those who are unable to take an oral feed with an initial energy requirement of 15-20 kcal/kg/d and protein requirement of 1.2-1.5 g/kg/d.
Diagnosis of infection in AP and judicious use of antimicrobials is a challenge faced by clinicians with very limited tools available for decision-making. Infections and OFs are critical determinants of outcome in cases of AP[42].
Infections can be of pancreatic [infected pancreatic necrosis, infected pseudocyst and infected walled-off necrosis (WON)] or extrapancreatic origin (pneumonia, bacteremia, urinary tract infection or indwelling catheters). Etiologically, infections may be of bacterial origin, fungal origin or both may coexist. Bacterial infections can complicate 30%-50% of severe AP (SAP), and the presence of infected necrosis increases the risk of mortality by 50% vs those with sterile necrosis[43]. Bacterial infections are monomicrobial in 60%-87% of patients. Infected necrosis may harbor polymicrobial infection in 10%-40% of patients, with Gram-negative anaerobes being the most common[44].
The use of antibiotics for extrapancreatic infections is less contested. Extrapancreatic infection can complicate almost one-third of patients. Respiratory infections are the commonest; however, their impact on mortality is less clear[45,46].
Patients with SAP or moderate SAP who manage to tide over the initial onslaught of the inflammatory response may later develop an infection. This timing is variable and unpredictable; however, the incidence peaks during weeks 2 to 4 of illness, presumably secondary to increased gut translocation of bacteria and reduced immunity[47]. Tools that are readily available for diagnosis of infection are based on cultures, pancreatic necrotic aspirate or drainage samples. Cross-sectional imaging may demonstrate the presence of air in the collection. However, none provides absolute certainty. Recently there has been great emphasis on procalcitonin in guiding antibiotic treatment due to ease of applicability. Procalcitonin levels directly correlate with levels of microbial toxins and indirectly to cytokine-mediated host inflammatory response. However, cutoff values indicating infection are not standardized[48]. Recently procalcitonin-directed deescalation of antibiotics has shown efficacy in the management of infections in the setting of AP. Although, further RCTs may be required before definite conclusions can be drawn[49].
The use of antibiotics may be considered empirically in a subset of patients with pancreatic or extrapancreatic necrosis specifically in those patients who fail to improve or develop new onset OF after 7-10 d of initial hospitalization[50]. Empirical antibiotics should cover Gram-negative, Gram-positive and anaerobic microorganisms effectively, giving adequate cognizance to nosocomial infections and local antibiotics policy. The role of prophylactic antibiotics is contested routinely in clinical practice, with most of the guidelines and evidence recommending against its usage except for Japanese guidelines, which recommend prophylactic antibiotics in SAP and necrotizing pancreatitis within 72 h (Table 6)[11,46,50-53]. Prophylactic antibiotics increase the risk of multidrug resistant organisms and pancreatic fungal infection.
| Societies | Prophylactic antibiotics | Indications of therapeutic antibiotics | Probiotics |
| ACG, 2013[50] | Not recommended | Extrapancreatic infections. Cholangitis, catheter-acquired infections, bacteremia, urinary tract infection, pneumonia. Infected pancreatic necrosis | Not recommended |
| IAP/APA, 2013[46] | Not recommended | Infected pancreatic necrosis | No recommendations |
| Japanese guidelines, 2021[51] | Not recommended | Not addressed | No recommendations |
| AGA, 2018[11] | Not recommended | Not addressed | No recommendations |
| ESGE, 2018[52] | Not recommended | Infected pancreatic necrosis | Not recommended |
| World Society of Emergency Surgery, 2019[53] | Not recommended | Infected pancreatic necrosis | No recommendations |
In critically ill patients with pancreatic fungal infection, echinocandins and liposomal amphotericin are the first-line drugs. However, differentiating invasive fungal infection from colonization can be perplexing[54]. Modalities available for diagnosing fungal infection are histological (aspirate samples or perioperative samples), cultures (drain catheters or blood cultures) and biomarkers[55]. Clinical judgment should be exercised when starting antifungals based on the likely diagnosis of invasive fungal infection, whereas it should be started in all cases with a definitive diagnosis[55]. Antifungals may be added considering clinical profile and risk factors for pancreatic fungal infection, such as prolonged intensive care, antibiotic administration, total parenteral nutrition and indwelling catheters[55].
In conclusion, antibiotics in AP should be initiated whenever a definite indication exists along with source control. However, there is no role for prophylactic antibiotics. Prophylactic antifungals especially with new-onset OF requires further evaluation.
Pain is a cardinal symptom and one of the diagnostic criteria for AP[1]. It not only contributes significantly to patient distress but also prognosticates the course of disease[56,57]. Alleviation of pain is an essential component in the management of the early phase of AP. We will be restricting our discussion to the management of inflammatory pain. Most, but not all, guidelines on AP remain noncommittal on analgesic management due to the paucity of high-quality evidence[11,46,50,58]. Japanese guidelines recommend that if pain associated with AP is severe and persistent, then it requires sufficient pain control; however, they remain noncommittal on the choice of analgesic[51]. The World Society of Emergency Surgery guidelines for the management of SAP provide no evidence or recommendation about any restriction in available pain medications except that nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided in cases with AKI[53]. None of the above guidelines provide sufficient recommendations on the type, route, dose, frequency and duration of analgesics in AP.
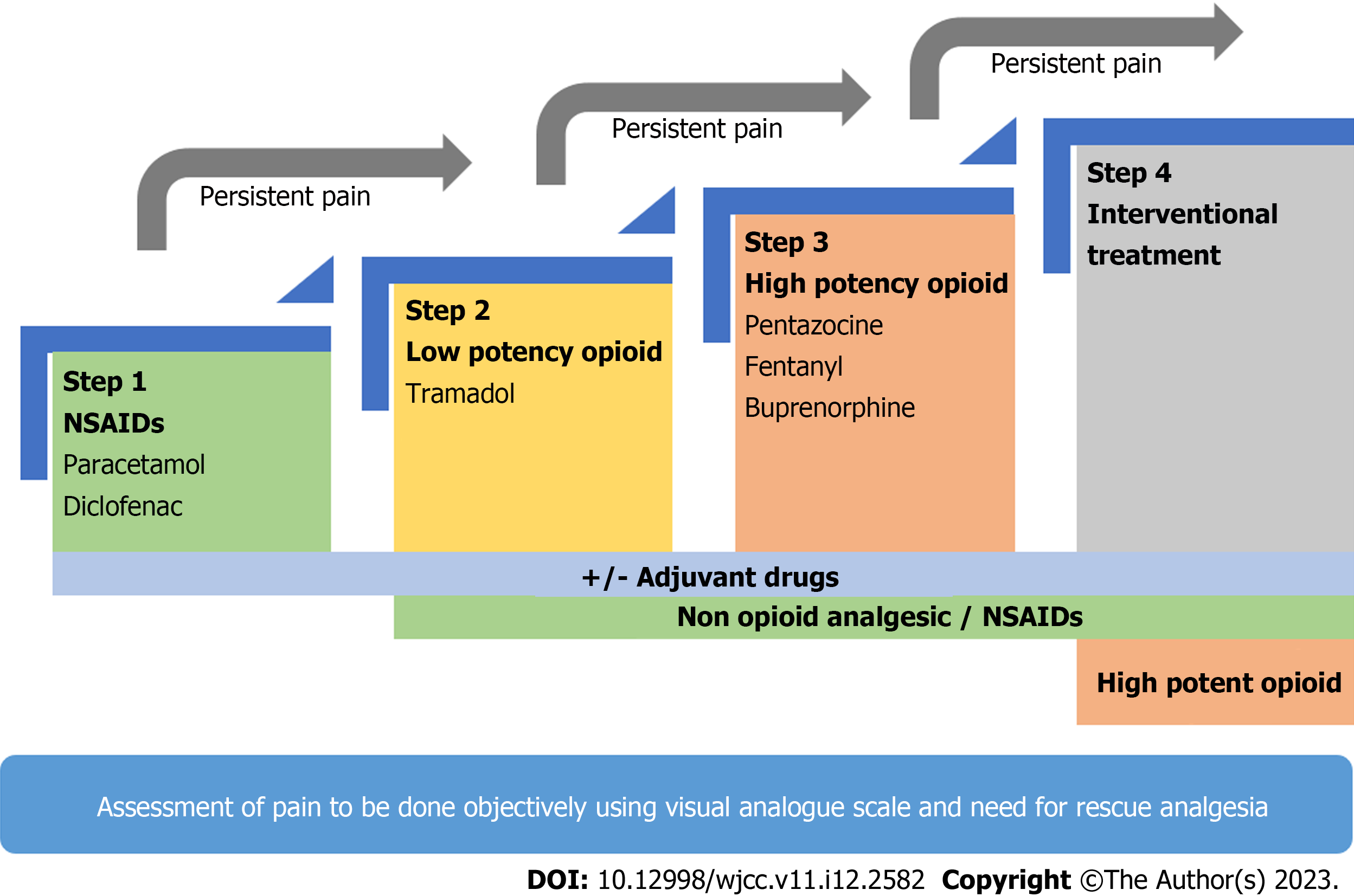
NSAIDs and opioids are the most frequently prescribed analgesic for pain in AP. Thirteen RCTs and multiple meta-analyses have failed to provide any conclusive data on the analgesic management of AP, which hinges on the World Health Organization analgesic ladder (Figure 1, Table 7)[59-61]. Opioids have been the most studied analgesic for AP in RCTs, establishing good efficacy, and are the agent of choice for rescue analgesia in all of the trials. NSAIDs have been reported to be beneficial in mitigating the inflammatory cascade thus improving outcomes. However, their analgesic potency as compared to opioids remains controversial[62]. NSAIDs have been studied in only a few RCTs where it was found to be better than placebo but similar efficacy to weak opioids[63-65].
| Ref. | Country | Comparison drugs | Study design | Patients, n | Rescue agent | Primary outcome | Results | Conclusion |
| Blamey et al[74], 1984 | United Kingdom | IM buprenorphine vs IM pethidine | RCT, blinding not mentioned | 32 | Pethidine | Pain relief at 24 h | No significant difference in pain relief at 24 h and no significant difference in pain-free period | No superiority established |
| Ebbehøj et al[75], 1985 | Denmark | Indomethacin suppository vs placebo | Placebo-controlled, double-blind RCT | 30 | Opiate not specified | Pain relief using VAS; Pain-free days | Indomethacin provided better pain control, a lesser number of painful days and lesser need for rescue analgesia | Indomethacin suppository favored over placebo |
| Jakobs et al[76], 2000 | Germany | IV buprenorphine vs IV procaine | Open-label RCT | 39 | Procaine group–pethidine; buprenorphine group–pethidine | Pain relief: VAS ever 8 hr for 3 d; rescue demand | Buprenorphine provided better pain relief on days 1 and 2 with lesser need for rescue analgesia; comparable side effects, complications, mortality | Buprenorphine favored |
| Stevens et al[77], 2002 | United States | Transdermal fentanyl IM pethidine vs placebo and IM pethidine | Double-blind placebo-controlled RCT | 32 | IM pethidine | Pain relief: Self-reported 0-5 scale; self-reported satisfaction 1-5 at discharge | Fentanyl provided no significant difference in pain relief at 24 h but better pain relief at 36 h and a shortened hospital stay | Fentanyl favored |
| Kahl et al[78], 2004 | Germany | Infusion procaine vs IV pentazocine | Open RCT | 101 | IM pethidine | Pain relief based on VAS and rescue analgesia | Pentazocine provided better pain relief until day 3 and required fewer rescue doses | Pentazocine favored |
| Peiró et al[79], 2008 | Spain | IV metamizole vs SC morphine | Open RCT | 16 | Pethidine | Pain relief based on VAS and time to pain relief | Metamizole showed better pain relief at 24 h and faster pain relief, which was nonsignificant | A favorable trend towards metamizole but a small sample size |
| Wilms et al[80], 2009 | Germany | IV procaine vs IV placebo | Double-blind placebo-controlled RCT | 42 | Buprenorphine | Pain relief and need for rescue analgesia over 3 d | Failed to show better pain relief as compared to placebo, and the need for rescue analgesia was similar in both groups | Procaine is not superior to placebo |
| Layer et al[81], 2011 | Germany | IV procaine vs IV placebo | Double-blind placebo-controlled RCT | 44 | Metamizole or buprenorphine | Pain relief at 3 d; rescue analgesia; proportion achieving > 67% drop in VAS | Procaine showed higher analgesic superiority with greater pain relief at 72 h, lesser need for rescue analgesia and more patients achieving VAS drop > 67% | Procaine favored over placebo |
| Sadowski et al[82], 2015 | Switzerland | Epidural analgesia vs PCA | Open RCT | 35 | Not applicable | Safety and efficacy of EA; pancreatic perfusion on CT; pain relief VAS | EA was safe, provided faster pain relief and increased pancreatic perfusion | EA favored over PCA |
| Gülen et al[83], 2016 | Turkey | Tramadol vs paracetamol + dexketoprofen | Single-blind RCT | 90 | Morphine | Pain relief at 30 min | No significant drop in VAS at 30 min for both agents and a similar need for rescue analgesia for both groups | No superior analgesia |
| Mahapatra et al[84], 2019 | India | IV pentazocine vs IV diclofenac | Double blind RCT | 50 | Fentanyl PCA | Pain relief; pain-free period; rescue analgesia | Higher rescue analgesia needed with diclofenac; longer pain-free period and lower need for PCA with pentazocine | Pentazocine favored |
| Kumar et al[85], 2019 | India | IV diclofenac vs IV tramadol | Double-blind RCT | 41 | IV morphine | Pain relief VAS over 7 d; painful days; rescue demand; time for significant VAS drop | No significant difference among both groups except time to a significant drop in VAS was quicker with diclofenac | No superior agent |
| Chen et al[86], 2022 | China | Hydromorphone PCA vs IM pethidine | Open-label RCT | 77 | IM dezocine | Change in VAS score over 72 h; rescue analgesia; organ failures; local complications; ICU admission LOH; mortality | No significant difference in VAS score deduction was noted with PCA as compared to pethidine, but a higher dose of hydromorphone needed for similar pain relief; need for rescue analgesia similar | No superior agent |
NSAIDs and opioids have different safety profiles. Opioids are known to cause bowel dysfunction and ileus, which may induce or exacerbate ileus in AP[66]. There is some evidence that opioid use is associated with sphincter of Oddi dysfunction as well as the risk of overuse and addiction[67]. The problem with NSAIDs is a risk of AKI and peptic ulcer disease, which should be avoided in AP with AKI[53]. Based on the better safety profile and comparable efficacy, NSAIDs may be preferred as first-line analgesia in patients with mild AP, keeping opioids as a reserve in refractory pain[62]. Monitoring of response using a visual analog scale and the need for rescue analgesia should be monitored regularly before consideration for escalation of therapy[21,68]. Lack of relevant and high-quality data on analgesics in cases of moderately SAP and SAP warrants further studies before any clear-cut recommendations can be made.
Patient-controlled analgesia (PCA) and epidural analgesia are emerging therapies in AP. PCA allows adequate pain control allowing patients to control their medication doses. Intravenous protease inhibitor nafamostat mesilate is one of the newer agents that has been used in an open label RCT. The analgesic effect was analyzed based on 24 h cumulative dose of fentanyl required and any administration of intravenous PCA. Results showed encouraging analgesic effect. An ongoing clinical trial is studying the use of PCA in AP[69,70]. Epidural analgesia has been used infrequently in patients with SAP and has shown a beneficial effect on mortality and pancreatic arterial perfusion[71,72]. However, it bears the risk of catheter-related hypotension and epidural abscess and is presently not recommended for mild to moderate AP. Further studies assessing the efficacy and safety of epidural analgesia in SAP are needed to make a definite conclusion.
In conclusion, we would suggest using the World Health Organization analgesic ladder for the management of pain in AP keeping in mind the safety profile of drugs[59-61,73]. It begins with low-potency NSAIDs (e.g., paracetamol, indomethacin and diclofenac), which is usually sufficient in mild to moderate AP. If NSAIDs are not sufficient for pain relief, then upgrading to weak opioids (e.g., tramadol and codeine) or strong opioids (e.g., pentazocine, fentanyl and buprenorphine) appears logical. PCA and epidural analgesia are promising therapies but need validation in larger cohorts and may be suited best as individualized therapy due to cost and limited availability (Figure 1). Table 7 summarizes the RCTs evaluating the role of different analgesics in management of pain in AP[74-86].
The use of anticoagulation in AP is perhaps the least studied in the literature. This is because the disease can give rise to two different complications: Splanchnic thrombosis and retroperitoneal bleeding. Management of these two opposing complications poses a unique challenge for a clinician. Pancreatitis is an acute inflammatory condition coupled with systemic response to inflammation, fluid shifts and subsequent hypovolemia. These pathophysiological mechanisms in unison precipitate a prothrombotic milieu. Thrombosis involving the splanchnic vasculature may involve the portal vein (PV), superior mesenteric vein (SMV) and splenic vein either separately or in combination.
Splanchnic vein thrombosis in AP, with a reported incidence of 1%-2%, has been poorly studied in clinical trials[87] partly because thrombosis in splanchnic vasculature is often incidentally detected on imaging. Clinical presentation of splanchnic vein thrombosis may overlap with that of AP. Our understanding of the natural history of splanchnic vein thrombosis in AP is still evolving. Some of these patients may have underlying prothrombotic risk factors that have just been unmasked because of pancreatitis. Understanding this rare complication is important because of prominent life-threatening manifestations, namely bowel gangrene, chronic portal hypertension and hepatic failure.
Thus, should we use anticoagulation in patients presenting with splanchnic vein thrombosis? Experience gained from the use of anticoagulation in patients without cirrhosis who present with acute PV thrombosis has been summarized in the European network of vascular diseases of the liver study. This study has shown the recanalization of the PV in 39% of those who were initiated on anticoagulation in the acute phase of PV thrombosis. Gastrointestinal bleeding and intestinal infarction occurred in 9.4% and 2.1% of anticoagulated patients, respectively[88]. This has led to some researchers advocating the use of anticoagulation in those with documented thrombosis of splanchnic vasculature in AP.
However, the benefits of giving anticoagulation have to be weighed in light of another potentially life-threatening complications (i.e. pseudoaneurysm-related bleeding from large vessels and retroperitoneal bleeding). Moreover, many of these patients with SAP undergo interventions (percutaneous/endoscopic drainage of collections or surgical interventions). Thus, from a clinician’s point of view, using therapeutic anticoagulation in patients with AP may be a risky proposition. The lack of RCTs on the efficacy of anticoagulation in AP needs special attention. Then splenic vein lies in close anatomical proximity to the inflamed pancreas. Researchers have shown a direct correlation between the degree of peripancreatic inflammation, direct venous compression by collections and the incidence of splanchnic vein thrombosis. Thus, drainage of collections has been postulated to be the most ideal way of treating and preventing splanchnic vein thrombosis in AP[89].
Systematic reviews have been attempted to address this pertinent management dilemma. Hajibandeh et al[90] in their systemic review of 5 observational studies and 252 patients demonstrated no significant difference in the rate of resolution of thrombus or formation of varices/collaterals. The study had a major drawback of low study heterogeneity between the anticoagulation and no anticoagulation groups. Another systemic review by Norton et al[91] included 16 studies (9 case reports, 2 case series and 5 single-center studies); among the total of 198 affected patients, 46.5% received anticoagulation therapy. The rate of venous recanalization was 14% in the anticoagulated group vs 11% in the untreated group, while 16% and 5% of patients had bleeding manifestations, respectively.
The most recent meta-analysis included 7 retrospective cohort studies (233 AP patients suffered from splanchnic venous thrombosis). Splanchnic vein thrombosis was seen in 33%-82%, PV thrombosis in 4%-32% and SMV thrombosis in 5%-9% of all patients with splanchnic vein thrombosis. A combination of splanchnic vein thrombosis, PV thrombosis and/or SMV thrombosis has also been reported in variable combinations. Moderate AP to SAP was resent in 89% of patients who had some evidence of splanchnic vein thrombosis. Several drawbacks of these systemic reviews and meta-analysis include the absence of RCTs and the serious risk of bias, imprecision and indirectness[92].
There are no guidelines on the management of splanchnic vein thrombosis in AP. Management issues have been extrapolated from existing guidelines on pulmonary embolism, extrahepatic PV obstruction and deep vein thrombosis. Low molecular weight heparin followed by vitamin K antagonist, fonda
Interventions in AP could be an emergency or may be delayed. The emergency interventions in AP include ERCP to relieve the biliary obstruction. Non-emergency or delayed interventions include percutaneous catheter drainage or endoscopic drainage of necrotic or walled-off collections.
ERCP is an invasive intervention with a complication rate of 5% to 15%[90]. The current use of ERCP in AP is limited to relief of biliary obstruction. In patients with AP who present with acute cholangitis, emergency ERCP (within 24 h) is the recommended first-line treatment[46,50]. However, the role and timing of ERCP in biliary obstruction without cholangitis in AP is not clear[46].
Multiple studies have looked at the role and timing of ERCP in these patients of acute biliary pancreatitis (ABP) without cholangitis[96]. Neoptolemos et al[97] showed that patients with predicted SAP had fewer complications with an early ERCP (within 72 h of admission) (24% vs 61%, P < 0.05). On the other hand, Fölsch et al[98] reported that early ERCP was not beneficial in patients with ABP without obstructive jaundice. Furthermore, a meta-analysis showed no significant difference in mortality rate according to the timing of ERCP (< 24 h vs < 72 h) in patients with persistent biliary obstruction without cholangitis[6]. Fölsch et al[98] also compared urgent ERCP with a conservative approach in patients with predicted severe ABP. This study showed that urgent ERCP with sphincterotomy did not reduce the major complication or mortality [38% vs 44%, risk ratio: 0.87; 95% confidence interval (CI): 0.64–1.18].
The available studies suggest that emergency ERCP (within 24 h) is indicated in patients with ABP with cholangitis or persistent cholestasis. For the rest of the patients with ABP, the role of urgent ERCP is controversial, and a conservative approach should be considered.
The management of the pancreatic and peripancreatic collections has evolved over the last two decades. The indications to drain (peri-) pancreatic collections in AP are the presence of infection and symptomatic sterile necrosis (Table 8). The choice of interventions includes percutaneous, endoscopic, minimally invasive surgery or a combined approach. The approach depends on multiple factors including the time elapsed since the onset of the disease, condition of the patient, anatomy of the collection and expertise available. An open surgical approach is no longer the preferred strategy due to the higher risk of mortality and major complications[99].
| Clinical suspicion or documented infected pancreatic collection |
| Presence of gas in the fluid collection on imaging |
| Systemic signs of infections |
| Increasing leucocytes and worsening clinical condition |
| Persistent or new onset organ failure |
| Pressure symptoms |
| Gastric outlet obstruction |
| Intestinal obstruction |
| Biliary obstruction |
| Persistent symptoms (e.g., pain, persistent unwellness) |
| Disconnected pancreatic duct (i.e. full transection of the pancreatic duct) with ongoing symptoms |
Across the world, the step-up approach remains the standard of care for the management of collections in AP. The approach involves initial conservative management, and then either percutaneous drainage or endoscopic transluminal drainage can be selected.
There are multiple dilemmas while contemplating the drainage of necrotic collection. Should the drainage be performed early (i.e. before encapsulation of the collection) or should it be delayed? Most guidelines suggest delaying drainage as much as possible and preferably until 4 wk after the disease onset to allow liquefaction and encapsulation of the collection[46,100]. The cutoff of 4 wk is arbitrary, and studies have shown variable results for early and delayed drainage.
Various studies have reported a widespread time window, varying from a median of 9 d to 75 d, between the onset of the disease and the first drainage procedure. The older studies suggested that delaying percutaneous drainage until encapsulation may improve the outcome[101-106]. Other recent studies have suggested the usefulness of early drainage in improving outcomes[107]. However, a recent multicenter randomized study (POINTER trail), which compared early vs delayed drainage in AP, did not show the superiority of early drainage[108]. The study showed similar rates of mortality (13% vs 10%, relative risk: 1.25; 95%CI: 0.42-3.68) and adverse events (76% vs 82%, relative risk: 0.94; 95%CI: 0.77-1.14) in early and delayed drainage. Studies have shown that early drainage required a higher number of reinterventions compared to a delayed strategy[108]. Trikudanathan et al[109] demonstrated that early endoscopic drainage (< 4 wk) required higher percutaneous drainage compared with patients with walled-off collections. Navalho et al[110] demonstrated the benefits of early drainage of infected pancreatic collections in patients in ICU settings[110,111]. Table 9 summarizes the studies highlighting timing of first catheter drainage and outcome in various studies of AP[99,101,103-104,110,112-122].
| Ref. | Number of days after the onset of the disease when PCD was performed, mean (range) | Patients, n | IPN, % | Mortality, % |
| Infected necrotic collection | ||||
| Freeny et al[112], 1998 | 9 (1-48) | 34 | 100 | 12 |
| Navalho et al[110], 2006 | 18 | 30 | 100 | 17 |
| Mortelé et al[113], 2009 | 12 (2-33) | 13 | 100 | 17 |
| Baril et al[114], 2000 | 24 (18-30)a | 7 | 100 | 0 |
| Bala et al[115], 2009 | 26 (18-88) | 8 | 100 | 13 |
| Baudin et al[116], 2012 | 19.8 ± 15.7 | 48 | 100 | 29 |
| Tong et al[101], 2012 | PCD only = 30.74 ± 5.67; PCD + surgery = 27.80 ± 6.00 | 34 | 100 | 0 and 7 |
| Pascual et al[117], 2013 | 28 ± 17 | 13 | 100 | 23 |
| Wroński et al[102], 2013 | PCD only = 33 (27-46); surgery = 35 (8-116) | 18 | 100 | 0 and 17 |
| Wang et al[118], 2016 | 11.7 ± 8.1 | 59 | 100 | 18.6 |
| Infected or sterile necrotic collection | ||||
| Lee et al[103], 2007 | 10 (1-58)a | 23 | 12 | 4 |
| Bruennler et al[119], 2008 | 3.5 (median 7) | 80 | 65 | 23 |
| van Santvoort et al[99], 2010 | 30 (11-71)a | 43 | 91 | 19 |
| Kumar et al[104], 2014 | 36.4 ± 7 | 12 | 67 | 8 |
| Bellam et al[120], 2019 | Median: 20 d | 51 | 33.3 | 29.4 |
| Gupta et al[121], 2020 | Median: 22 d (range: 3–267 d) | 146 | 47.9 | 20.5 |
| Lu et al[105], 2020 | 15.26 ± 7.08 | 43 | 86 | 13.9 |
| 50.86 ± 19.58 | 55 | 56.3 | 10.9 | |
| Sterile necrotic collection | ||||
| Walser et al[122], 2006 | NR | 22 | 0 | 9.1 |
The available literature suggests that the correct timing of intervention in AP requires careful clinical judgment. A subset of patients with infected collections, sepsis and persistence or new onset OF may require early drainage.
Percutaneous drainage: Percutaneous catheter drainage is an important treatment modality for acute necrotizing pancreatitis. The percutaneous procedure could be done safely under ultrasound (US) or computed tomography guidance. Percutaneous catheter drainage is important in patients where early drainage is required and the necrotic collection is not well encapsulated. Freeny et al[112] for the first time demonstrated the safety and efficacy of percutaneous drainage in AP in 1998 with a successful outcome in 47% of patients with percutaneous drainage only. Subsequently, Mortelé et al[113] and Baril et al[114] also confirmed the success of percutaneous drainage in AP.
In 2010 van Santvoort et al[99] (PANTER trial) performed an RCT of the step-up approach and primary surgery and found a significant success rate of percutaneous drainage. The first step in the step-up approach is percutaneous drainage, and it remains the standard of care for early drainage. Several studies have also confirmed the safety of early percutaneous catheter drainage in sick patients[109,110]. Table 10 summarizes the important studies and outcomes after percutaneous catheter drainage in AP.
| Ref. | Collection | n | Success |
| Lee et al[124], 2014 | WON and pseudocyst | PS = 25; FCMS = 25 | PS: 90%; FCMS: 87% |
| Mukai et al[125], 2015 | WON | PS = 27; BFMS = 43 | PS: 90.6%; FCMS: 97.7% |
| Siddiqui et al[126], 2017 | WON | PS = 106 FCMS = 121; LAMS = 86 | PS: 81%; FCMS: 95%; LAMS: 90% |
| Bapaye et al[127], 2016 | WON | PS = 61; BFMS = 72 | PS: 73.7%; BFMS: 94.0% |
| Bang et al[123], 2019 | WON | PS = 29; LAMS = 31 | PS: 96.6%; LAMS: 93.5% |
| Muktesh et al[128], 2022 | WON 108 | PS = 45; BFMS = 53 | PS: 81.8%; BFMS: 96.2% |
Endoscopic drainage: Endoscopic drainage involves the internal drainage of collection by creating a temporary fistula and placing a stent between the collection and the gastrointestinal lumen. Internal drainage carries the advantage of a lower risk of infection of collections and eliminates the risk of pancreatic-cutaneous fistula. However, these benefits come with a risk of anesthesia-related complications. Internal drainage could be completed using conventional endoscopic drainage or under endoscopic US (EUS) guidance. Though the studies have established the efficacy and safety of the conventional technique, its use is limited by a visible bulge in only 40%-50% of patients, and most endoscopists prefer EUS-guided drainage.
As with percutaneous drainage, the appropriate timing of drainage for endoscopic drainage is a matter of research. Though few studies have suggested the safety and efficacy of early endoscopic drainage for necrotic collections, most of the guidelines and reviews suggest the endoscopic drainage of collections with a well-defined wall[46,100].
The percutaneous method is a time-tested method of drainage of infected pancreatic collections. Endoscopic drainage is an alternative approach to draining such collections in AP. Compared to percutaneous drainage it carries less risk of secondary infection and pancreatic-cutaneous fistula. Recent American Gastroenterology Association guidelines also suggest that an endoscopic approach may be preferred. However, the choice of drainage method should be individualized and guided by multiple factors including the time elapsed since the onset of disease, encapsulation of the collection, location of the collection, solid contents of the collection, hemodynamic condition of the patient and available expertise. In early pancreatic collection with an ill-defined wall, sicker patients and peripherally located collections or when expertise is not available, percutaneous drainage should be considered. Endoscopic drainage is preferable for centrally located pancreatic collections in patients with a well-defined wall. A combined approach can be used for larger central collections extending into the periphery or when a single modality fails.
Endoscopic drainage of a collection could be performed with multiple plastic stents or metal stents. Historically, plastic stents were the mainstay of endoscopic drainage. However, their placement is time-consuming and challenging when multiple stents are required. On the other hand, the insertion of transmural metal stents ensures a short procedure time and wider transmural fistula and provides a more efficient way of drainage compared to plastic stents. Though the larger diameter of metal stents allows rapid drainage and facilitates endoscopic necrosectomy through the stent, the metal flanges may increase the risk of pseudoaneurysm formation[123]. Table 10 summarizes the studies for the outcome of endoscopic drainage with plastic and metal stents[123-128].
The retrospective studies comparing metal and plastic stents showed that the biflanged metal stent performed better than multiple plastic stents for draining WON[127,129]. On the other hand, two RCTs showed similar clinical efficacy with metal and multiple plastic stents for WON[123,124]. Furthermore, a meta-analysis concluded no differences in clinical success and adverse events between lumen- apposing metal stents and multiple plastic stents for symptomatic WON[130]. A recent study of EUS-guided drainage of infected WON identified that the use of metal stents was associated with higher clinical success (96.2% vs 81.8%, P = 0.04) and shorter hospital stays (6 d vs 10 d)[128].
The current evidence suggests that the choice of a stent for draining the collection is a matter of ongoing research and depends on multiple factors including the hemodynamic condition of the patients, size of the collection, solid contents of the collection and cost associated with metal stents. In patients with pseudocysts and limited solid contents, multiple plastic stents can be considered. While in patients with large collections, significant solid contents and peripherally extending collections metal stents should be preferred.
The concept of irrigating the collection to remove the solid necrotic debris is a less popular and debatable approach. It is based on the principle of chemical debridement using necrolytic agents to accelerate the drainage of pancreatic necrosis. The irrigation technique has been used for either percutaneous or endoscopic transmural drainage[43,131]. Studies have shown variable results with the use of different agents. Agents used for irrigation include NS, antibiotics, hydrogen peroxide and streptokinase. Werge et al[43] showed that local instillation of antibiotics in infected pancreatic necrosis improves the eradication of infection. Similarly, LarinoNoia et al[131] showed that the addition of local infusion of antibiotics avoids the need for necrosectomy in half of the patients with infected pancreatic necrosis not responding to drainage and systemic antibiotics. Hydrogen peroxide and streptokinase are other adjunctives for the management of necrotic collections.
Though such agents have been used with modest success to improve the outcome of AP and collections, the optimal dose, volumes, concentration and timing for use of these agents are still not known. A recent review by Trikudanathan et al[132] suggested that these agents can be used in the management of necrotic pancreatitis if there is no clinical and imaging improvement after drainage alone.
The term direct refers to the access of necrotic collection directly by endoscope through the gastric or duodenal wall. The direct endoscopic necrosectomy (DEN) forms the last step of the endoscopic step-up approach and involves direct access to the collection and debridement of the necrotic material. The step-up approach includes declogging of the blocked stent lumen, placement of a nasocystic tube and irrigation (chemical necrolysis) and DEN. Lakhtakia et al[133] showed that after initial drainage with a biflanged metal stent, 74.6% of patients had clinical success. Reintervention with a step-up approach improved the overall clinical success to 96.5% with DEN required in only 9.2% of the patients.
Several studies have confirmed the safety and efficacy of DEN in patients with infected pancreatic collections[134,135]. The PENGUIN trial compared DEN and surgical necrosectomy [video-assisted retroperitoneal debridement (VARD) or open] in patients with infected WON and showed significantly lower IL-6 levels and lower rates of complication (20% vs 80%) in the DEN group[136]. Subsequently, the TENSION trial compared the endoscopic step-up approach (EUS-guided stent placement followed by DEN) with the surgical step-up approach (percutaneous catheter drainage followed by VARD)[137]. The major complications and mortality rates were similar in both groups. However, the incidence of pancreatic fistula formation was higher with the percutaneous approach.
Though DEN has been shown to improve the outcome with a reduced need for surgical intervention. A relevant point of discussion is the timing of DEN after initial drainage. It was initially thought that performing DEN after 3-7 d would allow maturation of the cystogastrostomy/cystoenterostomy tract. However, with the advent of lumen-apposing metal stents, DEN can be performed immediately after the placement of the stent. Yan et al[138] in a multicentric study compared immediate and delayed DEN for WON. The study showed no difference in clinical success and adverse events. The study also showed the mean number of necrosectomy sessions for WON resolution was significantly lower in the immediate DEN group compared to the delayed DEN group (3.1 vs 3.9, P < 0.001).
The studies suggest that DEN remains the cornerstone of the endoscopic step-up approach with similar or lower complication rates than the percutaneous step-up approach. After initial endoscopic drainage, DEN can be performed immediately post-drainage, or delayed DEN can be considered depending on the clinical status of the patients. Post-endoscopic drainage of collection, a step-up approach of initial chemical necrolysis followed by DEN or upfront DEN can be considered depending on the available expertise, clinical status of the patient and residual collection.
The indications of surgery are limited in the setting of AP. Surgery is usually required for necrosectomy and rarely for acute compartment syndrome. As a general rule of thumb, any surgical intervention should not be done before 4 wk of the onset of the disease to enable the walling-off of the collections.
The approach for surgical necrosectomy could be minimally invasive, laparoscopic or open. In 2010 van Santvoort et al[99] (PANTER trial) compared the step-up approach with primary open surgical necrosectomy surgery. The study concluded that a minimally invasive step-up approach reduced the rate of major complications and mortality in patients with infected pancreatic necrosis. In the step-up approach, initial drainage is followed by debridement and necrosectomy using minimally invasive surgical methods. Several minimally invasive approaches are described and popularly utilized including minimally invasive retroperitoneal percutaneous necrosectomy and VARD[139,140]. Both minimally invasive retroperitoneal percutaneous and VARD retroperitoneal techniques are modifications of the open lateral approach initially described in the 1980s by Fagniez et al[141]. The aim of these minimally invasive approaches is not complete necrosectomy but to remove loosely adherent pieces of necrosis, thus minimizing the risk of hemorrhage. Open surgical necrosectomy is only indicated when a minimally invasive approach fails or in the absence of expertise.
Certain issues like the management of IAH and persistent ascites may require a multipronged approach predominantly revolving around timely drainage.
In AP, high intra-abdominal pressures (IAPs) are a common finding and occur through multiple mechanisms (i.e. pancreatic and/or peri-pancreatic inflammation, third space fluid loss and retention in the abdominal cavity and ileus). The pressure can reach the extent to produce IAH or abdominal compartment syndrome. IAH is defined as sustained IAP above 12 mmHg and occurs frequently in AP[51]. Several studies have observed poor outcomes in patients with IAH[142,143].
The management of increased abdominal pressure should follow the standard algorithm proposed by the various societies irrespective of the etiology[144,145]. The management includes the frequent monitoring of IAP, evacuation of intraluminal contents using NG or rectal tubes, improving abdominal wall compliance by use of adequate analgesia and sedation, goal-directed use of fluid and release of intra-abdominal fluid or collection using percutaneous drainage.
Singh et al[143] in a retrospective study showed that the presence of IAH increases the risk of development of multiple OF and was associated with higher mortality. At 48 h post-percutaneous drainage, the mean reduction in IAP was significantly higher (6.87 mmHg vs 3.21 mmHg, P < 0.001) in patients with baseline IAH than in patients without IAH. The study also identified that post-percutaneous drainage a pressure reduction of > 40% was associated with better survival.
Ascites are commonly described in patients with AP, but its association and effect on outcome are poorly understood. Samanta et al[146] identified that the presence of ascites was associated with higher rates of OF and increased mortality in AP. Mortality rates were four times higher in the presence of ascites compared to non-ascites patients (34.1% vs 8.4%, P = 0.001). The study showed that the presence of moderate to gross ascites was associated with IAH and higher rates of OF. Though the presence of ascites increases IAP, several unidentified mechanisms could contribute to the poor outcome in the presence of ascites in AP. Serum ascites albumin gradient (SAAG) can be used to differentiate the underlying pathophysiological process in addition to history and diligent physical examination. SAAG > 1 may indicate underlying portal hypertension, while pancreatic ascites (SAAG < 1) may require drainage and/or endoscopic placement of transpapillary pancreatic duct stent. Hence, the decision of drainage of persistent ascites should be considered before drainage of the collection.
The management of AP is still a work in progress. Even though there are several guidelines, there is a lack of consensus on certain issues. The choice and type of fluid resuscitation are still evolving. The nutrition aspect is settled with ample evidence for early enteral feeding. The judicious use of antibiotics is always debatable, and the ideal analgesic is unknown. The intervention is tending towards endoscopy or percutaneous drainage rather than surgery. With the progressive development of technology and expertise, more data is likely to emerge that may help in the formulation of more conclusive indications and guidelines.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American College of Gastroenterology, No. 51519; American Gastroenterological Association, No. 1050754; American Society for Gastrointestinal Endoscopy, No. 151100; Indian Society of Gastroenterology, No. LM001975.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li G, China; Nishida T, Japan; Xiao B, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Cai YX
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4306] [Article Influence: 358.8] [Reference Citation Analysis (45)] |
| 2. | Li CL, Jiang M, Pan CQ, Li J, Xu LG. The global, regional, and national burden of acute pancreatitis in 204 countries and territories, 1990-2019. BMC Gastroenterol. 2021;21:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 3. | Schmidt J, Ferńandez-del Castillo C, Rattner DW, Lewandrowski KB, Messmer K, Warshaw AL. Hyperoncotic ultrahigh molecular weight dextran solutions reduce trypsinogen activation, prevent acinar necrosis, and lower mortality in rodent pancreatitis. Am J Surg. 1993;165:40-4; discussion 45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Huch K, Schmidt J, Schratt W, Sinn HP, Buhr H, Herfarth C, Klar E. Hyperoncotic dextran and systemic aprotinin in necrotizing rodent pancreatitis. Scand J Gastroenterol. 1995;30:812-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Yang R, Uchiyama T, Alber SM, Han X, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate ameliorates distant organ injury in a murine model of acute necrotizing pancreatitis. Crit Care Med. 2004;32:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Shields CJ, Winter DC, Sookhai S, Ryan L, Kirwan WO, Redmond HP. Hypertonic saline attenuates end-organ damage in an experimental model of acute pancreatitis. Br J Surg. 2000;87:1336-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (1)] |
| 8. | Klar E, Foitzik T, Buhr H, Messmer K, Herfarth C. Isovolemic hemodilution with dextran 60 as treatment of pancreatic ischemia in acute pancreatitis. Clinical practicability of an experimental concept. Ann Surg. 1993;217:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Chen QJ, Yang ZY, Wang CY, Dong LM, Zhang YS, Xie C, Chen CZ, Zhu SK, Yang HJ, Wu HS, Yang C. Hydroxyethyl starch resuscitation downregulate pro-inflammatory cytokines in the early phase of severe acute pancreatitis: A retrospective study. Exp Ther Med. 2016;12:3213-3220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Chang YS, Fu HQ, Zou SB, Yu BT, Liu JC, Xia L, Lv NH. [The impact of initial fluid resuscitation with different ratio of crystalloid-colloid on prognosis of patients with severe acute pancreatitis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 555] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 12. | Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018;378:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 888] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 13. | de-Madaria E, Herrera-Marante I, González-Camacho V, Bonjoch L, Quesada-Vázquez N, Almenta-Saavedra I, Miralles-Maciá C, Acevedo-Piedra NG, Roger-Ibáñez M, Sánchez-Marin C, Osuna-Ligero R, Gracia Á, Llorens P, Zapater P, Singh VK, Moreu-Martín R, Closa D. Fluid resuscitation with lactated Ringer's solution vs normal saline in acute pancreatitis: A triple-blind, randomized, controlled trial. United European Gastroenterol J. 2018;6:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 14. | Choosakul S, Harinwan K, Chirapongsathorn S, Opuchar K, Sanpajit T, Piyanirun W, Puttapitakpong C. Comparison of normal saline vs Lactated Ringer's solution for fluid resuscitation in patients with mild acute pancreatitis, A randomized controlled trial. Pancreatology. 2018;18:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, Smith B, Banks PA, Conwell DL. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710-717.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 339] [Article Influence: 24.2] [Reference Citation Analysis (1)] |
| 16. | Karki B, Thapa S, Khadka D, Karki S, Shrestha R, Khanal A, Paudel BN. Intravenous Ringers lactate vs normal saline for predominantly mild acute pancreatitis in a Nepalese Tertiary Hospital. PLoS One. 2022;17:e0263221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Zhou S, Buitrago C, Foong A, Lee V, Dawit L, Hiramoto B, Chang P, Schilperoort H, Lee A, de-Madaria E, Buxbaum J. Comprehensive meta-analysis of randomized controlled trials of Lactated Ringer's vs Normal Saline for acute pancreatitis. Pancreatology. 2021;21:1405-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Aziz M, Ahmed Z, Weissman S, Ghazaleh S, Beran A, Kamal F, Lee-Smith W, Assaly R, Nawras A, Pandol SJ, McDonough S, Adler DG. Lactated Ringer's vs normal saline for acute pancreatitis: An updated systematic review and meta-analysis. Pancreatology. 2021;21:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Vedantam S, Tehami N, de-Madaria E, Barkin JA, Amin S. Lactated Ringers Does Not Reduce SIRS in Acute Pancreatitis Compared to Normal Saline: An Updated Meta-Analysis. Dig Dis Sci. 2022;67:3265-3274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Chen H, Lu X, Xu B, Meng C, Xie D. Lactated Ringer Solution Is Superior to Normal Saline Solution in Managing Acute Pancreatitis: An Updated Meta-analysis of Randomized Controlled Trials. J Clin Gastroenterol. 2022;56:e114-e120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Garg PK, Mahapatra SJ. Optimum Fluid Therapy in Acute Pancreatitis Needs an Alchemist. Gastroenterology. 2021;160:655-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Mao EQ, Tang YQ, Fei J, Qin S, Wu J, Li L, Min D, Zhang SD. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl). 2009;122:169-173. [PubMed] |
| 23. | Mao EQ, Fei J, Peng YB, Huang J, Tang YQ, Zhang SD. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl). 2010;123:1639-1644. [PubMed] |
| 24. | Buxbaum JL, Quezada M, Da B, Jani N, Lane C, Mwengela D, Kelly T, Jhun P, Dhanireddy K, Laine L. Early Aggressive Hydration Hastens Clinical Improvement in Mild Acute Pancreatitis. Am J Gastroenterol. 2017;112:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Cuéllar-Monterrubio JE, Monreal-Robles R, González-Moreno EI, Borjas-Almaguer OD, Herrera-Elizondo JL, García-Compean D, Maldonado-Garza HJ, González-González JA. Nonaggressive Versus Aggressive Intravenous Fluid Therapy in Acute Pancreatitis With More Than 24 Hours From Disease Onset: A Randomized Controlled Trial. Pancreas. 2020;49:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 26. | Li L, Jin T, Wen S, Shi N, Zhang R, Zhu P, Lin Z, Jiang K, Guo J, Liu T, Philips A, Deng L, Yang X, Singh VK, Sutton R, Windsor JA, Huang W, Xia Q. Early Rapid Fluid Therapy Is Associated with Increased Rate of Noninvasive Positive-Pressure Ventilation in Hemoconcentrated Patients with Severe Acute Pancreatitis. Dig Dis Sci. 2020;65:2700-2711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Gad MM, Simons-Linares CR. Is aggressive intravenous fluid resuscitation beneficial in acute pancreatitis? World J Gastroenterol. 2020;26:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (6)] |
| 28. | de-Madaria E, Buxbaum JL, Maisonneuve P, García García de Paredes A, Zapater P, Guilabert L, Vaillo-Rocamora A, Rodríguez-Gandía MÁ, Donate-Ortega J, Lozada-Hernández EE, Collazo Moreno AJR, Lira-Aguilar A, Llovet LP, Mehta R, Tandel R, Navarro P, Sánchez-Pardo AM, Sánchez-Marin C, Cobreros M, Fernández-Cabrera I, Casals-Seoane F, Casas Deza D, Lauret-Braña E, Martí-Marqués E, Camacho-Montaño LM, Ubieto V, Ganuza M, Bolado F; ERICA Consortium. Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis. N Engl J Med. 2022;387:989-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 141] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 29. | Aggarwal A, Manrai M, Kochhar R. Fluid resuscitation in acute pancreatitis. World J Gastroenterol. 2014;20:18092-18103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Lakananurak N, Gramlich L. Nutrition management in acute pancreatitis: Clinical practice consideration. World J Clin Cases. 2020;8:1561-1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (15)] |
| 31. | O'Keefe SJ, Lee RB, Li J, Stevens S, Abou-Assi S, Zhou W. Trypsin secretion and turnover in patients with acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G181-G187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Kotani J, Usami M, Nomura H, Iso A, Kasahara H, Kuroda Y, Oyanagi H, Saitoh Y. Enteral nutrition prevents bacterial translocation but does not improve survival during acute pancreatitis. Arch Surg. 1999;134:287-292. [PubMed] [DOI] [Full Text] |
| 33. | Rinninella E, Annetta MG, Serricchio ML, Dal Lago AA, Miggiano GA, Mele MC. Nutritional support in acute pancreatitis: from physiopathology to practice. An evidence-based approach. Eur Rev Med Pharmacol Sci. 2017;21:421-432. [PubMed] |
| 34. | Petrov MS, Windsor JA. Nutritional management of acute pancreatitis: the concept of 'gut rousing'. Curr Opin Clin Nutr Metab Care. 2013;16:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 35. | Li JY, Yu T, Chen GC, Yuan YH, Zhong W, Zhao LN, Chen QK. Enteral nutrition within 48 hours of admission improves clinical outcomes of acute pancreatitis by reducing complications: a meta-analysis. PLoS One. 2013;8:e64926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Feng P, He C, Liao G, Chen Y. Early enteral nutrition vs delayed enteral nutrition in acute pancreatitis: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e8648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Qi D, Yu B, Huang J, Peng M. Meta-Analysis of Early Enteral Nutrition Provided Within 24 Hours of Admission on Clinical Outcomes in Acute Pancreatitis. JPEN J Parenter Enteral Nutr. 2018;42:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Fuentes Padilla P, Martínez G, Vernooij RW, Urrútia G, Roqué I Figuls M, Bonfill Cosp X. Early enteral nutrition (within 48 hours) versus delayed enteral nutrition (after 48 hours) with or without supplemental parenteral nutrition in critically ill adults. Cochrane Database Syst Rev. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Guo QH, Tian XY, Qin YL, Han XT, Wang W. Immediate enteral nutrition can accelerate recovery and be safe in mild acute pancreatitis: A meta-analysis of randomized controlled trials. Heliyon. 2022;8:e08852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Zhu Y, Yin H, Zhang R, Ye X, Wei J. Nasogastric Nutrition versus Nasojejunal Nutrition in Patients with Severe Acute Pancreatitis: A Meta-Analysis of Randomized Controlled Trials. Gastroenterol Res Pract. 2016;2016:6430632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Dutta AK, Goel A, Kirubakaran R, Chacko A, Tharyan P. Nasogastric versusnasojejunal tube feeding for severe acute pancreatitis. Cochrane Database Syst Rev. 2020;3:CD010582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 42. | Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G, Whitcomb DC, Windsor JA; Pancreatitis Across Nations Clinical Research and Education Alliance (PANCREA). Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 43. | Werge M, Novovic S, Schmidt PN, Gluud LL. Infection increases mortality in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology. 2016;16:698-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 44. | Räty S, Sand J, Nordback I. Difference in microbes contaminating pancreatic necrosis in biliary and alcoholic pancreatitis. Int J Pancreatol. 1998;24:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Brown LA, Hore TA, Phillips AR, Windsor JA, Petrov MS. A systematic review of the extra-pancreatic infectious complications in acute pancreatitis. Pancreatology. 2014;14:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1033] [Article Influence: 86.1] [Reference Citation Analysis (6)] |
| 47. | Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CH, Schaapherder AF, Gooszen HG; Dutch Acute Pancreatitis Study Group. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 48. | Rhee C. Using Procalcitonin to Guide Antibiotic Therapy. Open Forum Infect Dis. 2017;4:ofw249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Siriwardena AK, Jegatheeswaran S, Mason JM; PROCAP investigators. A procalcitonin-based algorithm to guide antibiotic use in patients with acute pancreatitis (PROCAP): a single-centre, patient-blinded, randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 50. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-15; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1376] [Article Influence: 114.7] [Reference Citation Analysis (3)] |
| 51. | Takada T, Isaji S, Mayumi T, Yoshida M, Takeyama Y, Itoi T, Sano K, Iizawa Y, Masamune A, Hirota M, Okamoto K, Inoue D, Kitamura N, Mori Y, Mukai S, Kiriyama S, Shirai K, Tsuchiya A, Higuchi R, Hirashita T. JPN clinical practice guidelines 2021 with easy-to-understand explanations for the management of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2022;29:1057-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 52. | Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 53. | Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 419] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 54. | Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, Lass-Flörl C, Calandra T, Viscoli C, Herbrecht R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102:433-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 422] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 55. | Kochhar R, Noor MT, Wig J. Fungal infections in severe acute pancreatitis. J Gastroenterol Hepatol. 2011;26:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Phillip V, Schuster T, Hagemes F, Lorenz S, Matheis U, Preinfalk S, Lippl F, Saugel B, Schmid RM, Huber W. Time period from onset of pain to hospital admission and patients' awareness in acute pancreatitis. Pancreas. 2013;42:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Kapoor K, Repas K, Singh VK, Conwell DL, Mortele KJ, Wu BU, Banks PA. Does the duration of abdominal pain prior to admission influence the severity of acute pancreatitis? JOP. 2013;14:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Italian Association for the Study of the Pancreas (AISP); Pezzilli R, Zerbi A, Campra D, Capurso G, Golfieri R, Arcidiacono PG, Billi P, Butturini G, Calculli L, Cannizzaro R, Carrara S, Crippa S, De Gaudio R, De Rai P, Frulloni L, Mazza E, Mutignani M, Pagano N, Rabitti P, Balzano G. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis. 2015;47:532-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 59. | Thavanesan N, White S, Lee S, Ratnayake B, Oppong KW, Nayar MK, Sharp L, Drewes AM, Capurso G, De-Madaria E, Siriwardena AK, Windsor JA, Pandanaboyana S. Analgesia in the Initial Management of Acute Pancreatitis: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. World J Surg. 2022;46:878-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 60. | Ventafridda V, Saita L, Ripamonti C, De Conno F. WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 1985;7:93-96. [PubMed] |
| 61. | Cai W, Liu F, Wen Y, Han C, Prasad M, Xia Q, Singh VK, Sutton R, Huang W. Pain Management in Acute Pancreatitis: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Front Med (Lausanne). 2021;8:782151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 62. | Huang Z, Ma X, Jia X, Wang R, Liu L, Zhang M, Wan X, Tang C, Huang L. Prevention of Severe Acute Pancreatitis With Cyclooxygenase-2 Inhibitors: A Randomized Controlled Clinical Trial. Am J Gastroenterol. 2020;115:473-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 63. | Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 64. | Liu C, Zhu S, Dong Y, Shao J, Liu B, Shen J. The Potential Predictive Biomarkers for Advanced Hepatocellular Carcinoma Treated With Anti-Angiogenic Drugs in Combination With PD-1 Antibody. Front Immunol. 2022;13:930096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 65. | Bouida W, Beltaief K, Msolli MA, Ben Marzouk M, Boubaker H, Grissa MH, Zorgati A, Methamem M, Boukef R, Belguith A, Nouira S. Effect on Morphine Requirement of Early Administration of Oral Acetaminophen vs. Acetaminophen/Tramadol Combination in Acute Pain. Pain Pract. 2019;19:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 405] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 67. | Helm JF, Venu RP, Geenen JE, Hogan WJ, Dodds WJ, Toouli J, Arndorfer RC. Effects of morphine on the human sphincter of Oddi. Gut. 1988;29:1402-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Schorn S, Ceyhan GO, Tieftrunk E, Friess H, Demir IE. Pain Management in Acute Pancreatitis. Pancreapedia: Exocrine Pancreas Knowledge Base. 2015;. [DOI] [Full Text] |
| 69. | Hirota M, Shimosegawa T, Kitamura K, Takeda K, Takeyama Y, Mayumi T, Ito T, Takenaka M, Iwasaki E, Sawano H, Ishida E, Miura S, Masamune A, Nakai Y, Mitoro A, Maguchi H, Kimura K, Sanuki T, Haradome H, Kozaka K, Gabata T, Kataoka K, Hirota M, Isaji S, Nakamura R, Yamagiwa K, Kayaba C, Ikeda K. Continuous regional arterial infusion versus intravenous administration of the protease inhibitor nafamostat mesilate for predicted severe acute pancreatitis: a multicenter, randomized, open-label, phase 2 trial. J Gastroenterol. 2020;55:342-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Sunil Sheth, Beth Israel Deaconess Medical Cente. ClinicalTrials.gov Identifier: NCT04816877. |
| 71. | Harper D, McNaught CE. The role of thoracic epidural anesthesia in severe acute pancreatitis. Crit Care. 2014;18:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Jabaudon M, Belhadj-Tahar N, Rimmelé T, Joannes-Boyau O, Bulyez S, Lefrant JY, Malledant Y, Leone M, Abback PS, Tamion F, Dupont H, Lortat-Jacob B, Guerci P, Kerforne T, Cinotti R, Jacob L, Verdier P, Dugernier T, Pereira B, Constantin JM; Azurea Network. Thoracic Epidural Analgesia and Mortality in Acute Pancreatitis: A Multicenter Propensity Analysis. Crit Care Med. 2018;46:e198-e205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 73. | Basurto Ona X, Rigau Comas D, Urrútia G. Opioids for acute pancreatitis pain. Cochrane Database Syst Rev. 2013;CD009179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Blamey SL, Finlay IG, Carter DC, Imrie CW. Analgesia in acute pancreatitis: comparison of buprenorphine and pethidine. Br Med J (Clin Res Ed). 1984;288:1494-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Ebbehøj N, Friis J, Svendsen LB, Bülow S, Madsen P. Indomethacin treatment of acute pancreatitis. A controlled double-blind trial. Scand J Gastroenterol. 1985;20:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Jakobs R, Adamek MU, von Bubnoff AC, Riemann JF. Buprenorphine or procaine for pain relief in acute pancreatitis. A prospective randomized study. Scand J Gastroenterol. 2000;35:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Stevens M, Esler R, Asher G. Transdermal fentanyl for the management of acute pancreatitis pain. Appl Nurs Res. 2002;15:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Kahl S, Zimmermann S, Pross M, Schulz HU, Schmidt U, Malfertheiner P. Procaine hydrochloride fails to relieve pain in patients with acute pancreatitis. Digestion. 2004;69:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Peiró AM, Martínez J, Martínez E, de Madaria E, Llorens P, Horga JF, Pérez-Mateo M. Efficacy and tolerance of metamizole vs morphine for acute pancreatitis pain. Pancreatology. 2008;8:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Wilms B, Meffert KS, Schultes B. [Procaine infusion for pain treatment of acute pancreatitis: a randomized, placebo-controlled double-blind trial]. Dtsch Med Wochenschr. 2010;135:2290-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Layer P, Bronisch HJ, Henniges UM, Koop I, Kahl M, Dignass A, Ell C, Freitag M, Keller J. Effects of systemic administration of a local anesthetic on pain in acute pancreatitis: a randomized clinical trial. Pancreas. 2011;40:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Sadowski SM, Andres A, Morel P, Schiffer E, Frossard JL, Platon A, Poletti PA, Bühler L. Epidural anesthesia improves pancreatic perfusion and decreases the severity of acute pancreatitis. World J Gastroenterol. 2015;21:12448-12456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 83. | Gülen B, Dur A, Serinken M, Karcıoğlu Ö, Sönmez E. Pain treatment in patients with acute pancreatitis: A randomized controlled trial. Turk J Gastroenterol. 2016;27:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Mahapatra SJ, Jain S, Bopanna S, Gupta S, Singh P, Trikha A, Sreenivas V, Shalimar, Garg PK. Pentazocine, a Kappa-Opioid Agonist, Is Better Than Diclofenac for Analgesia in Acute Pancreatitis: A Randomized Controlled Trial. Am J Gastroenterol. 2019;114:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Kumar NS, Muktesh G, Samra T, Sarma P, Samanta J, Sinha SK, Dhaka N, Yadav TD, Gupta V, Kochhar R. Comparison of efficacy of diclofenac and tramadol in relieving pain in patients of acute pancreatitis: A randomized parallel group double blind active controlled pilot study. Eur J Pain. 2020;24:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Chen Z, Jiang K, Liu F, Zhu P, Cai F, He Y, Jin T, Lin Z, Li Q, Hu C, Tan Q, Yang X, Guo J, Huang W, Deng L, Xia Q. Safety and efficacy of intravenous hydromorphone patient-controlled analgesia vs intramuscular pethidine in acute pancreatitis: An open-label, randomized controlled trial. Front Pharmacol. 2022;13:962671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 88. | Plessier A, Darwish-Murad S, Hernandez-Guerra M, Consigny Y, Fabris F, Trebicka J, Heller J, Morard I, Lasser L, Langlet P, Denninger MH, Vidaud D, Condat B, Hadengue A, Primignani M, Garcia-Pagan JC, Janssen HL, Valla D; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 390] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 89. | Gonzelez HJ, Sahay SJ, Samadi B, Davidson BR, Rahman SH. Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience. HPB (Oxford). 2011;13:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 90. | Hajibandeh S, Hajibandeh S, Agrawal S, Irwin C, Obeidallah R, Subar D. Anticoagulation Versus No Anticoagulation for Splanchnic Venous Thrombosis Secondary to Acute Pancreatitis: Do We Really Need to Treat the Incidental Findings? Pancreas. 2020;49:e84-e85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Norton W, Lazaraviciute G, Ramsay G, Kreis I, Ahmed I, Bekheit M. Current practice of anticoagulant in the treatment of splanchnic vein thrombosis secondary to acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2020;19:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 92. | Sissingh NJ, Groen JV, Koole D, Klok FA, Boekestijn B, Bollen TL, van Santvoort HC, Verdonk RC, Bonsing BA, van Eijck CHJ, van Hooft JE, Mieog JSD; Dutch Pancreatitis Study Group. Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: A systematic review and meta-analysis. Pancreatology. 2022;22:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 93. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 2593] [Article Influence: 648.3] [Reference Citation Analysis (1)] |
| 94. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1460] [Article Influence: 486.7] [Reference Citation Analysis (2)] |
| 95. | Mazzolai L, Aboyans V, Ageno W, Agnelli G, Alatri A, Bauersachs R, Brekelmans MPA, Büller HR, Elias A, Farge D, Konstantinides S, Palareti G, Prandoni P, Righini M, Torbicki A, Vlachopoulos C, Brodmann M. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2018;39:4208-4218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 96. | ASGE Standards of Practice Committee; Anderson MA, Fisher L, Jain R, Evans JA, Appalaneni V, Ben-Menachem T, Cash BD, Decker GA, Early DS, Fanelli RD, Fisher DA, Fukami N, Hwang JH, Ikenberry SO, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Shergill AK, Dominitz JA. Complications of ERCP. Gastrointest Endosc. 2012;75:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 97. | Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy vs conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 470] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 98. | Fölsch UR, Nitsche R, Lüdtke R, Hilgers RA, Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997;336:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 330] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 99. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1029] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 100. | Isayama H, Nakai Y, Rerknimitr R, Khor C, Lau J, Wang HP, Seo DW, Ratanachu-Ek T, Lakhtakia S, Ang TL, Ryozawa S, Hayashi T, Kawakami H, Yamamoto N, Iwashita T, Itokawa F, Kuwatani M, Kitano M, Hanada K, Kogure H, Hamada T, Ponnudurai R, Moon JH, Itoi T, Yasuda I, Irisawa A, Maetani I. Asian consensus statements on endoscopic management of walled-off necrosis Part 1: Epidemiology, diagnosis, and treatment. J Gastroenterol Hepatol. 2016;31:1546-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 101. | Tong Z, Li W, Yu W, Geng Y, Ke L, Nie Y, Sun J, Ni H, Wang X, Ye X, Li N, Li J. Percutaneous catheter drainage for infective pancreatic necrosis: is it always the first choice for all patients? Pancreas. 2012;41:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Wroński M, Cebulski W, Karkocha D, Słodkowski M, Wysocki L, Jankowski M, Krasnodębski IW. Ultrasound-guided percutaneous drainage of infected pancreatic necrosis. Surg Endosc. 2013;27:2841-2848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Lee JK, Kwak KK, Park JK, Yoon WJ, Lee SH, Ryu JK, Kim YT, Yoon YB. The efficacy of nonsurgical treatment of infected pancreatic necrosis. Pancreas. 2007;34:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Kumar N, Conwell DL, Thompson CC. Direct endoscopic necrosectomy vs step-up approach for walled-off pancreatic necrosis: comparison of clinical outcome and health care utilization. Pancreas. 2014;43:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 105. | Lu J, Cao F, Zheng Z, Ding Y, Qu Y, Mei W, Guo Y, Feng YL, Li F. How to Identify the Indications for Early Intervention in Acute Necrotizing Pancreatitis Patients: A Long-Term Follow-Up Study. Front Surg. 2022;9:842016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 106. | Tyberg A, Karia K, Gabr M, Desai A, Doshi R, Gaidhane M, Sharaiha RZ, Kahaleh M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol. 2016;22:2256-2270. [PubMed] [DOI] [Full Text] |
| 107. | Sugimoto M, Sonntag DP, Flint GS, Boyce CJ, Kirkham JC, Harris TJ, Carr SM, Nelson BD, Bell DA, Barton JG, Traverso LW. Better Outcomes if Percutaneous Drainage Is Used Early and Proactively in the Course of Necrotizing Pancreatitis. J Vasc Interv Radiol. 2016;27:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 108. | Boxhoorn L, van Dijk SM, van Grinsven J, Verdonk RC, Boermeester MA, Bollen TL, Bouwense SAW, Bruno MJ, Cappendijk VC, Dejong CHC, van Duijvendijk P, van Eijck CHJ, Fockens P, Francken MFG, van Goor H, Hadithi M, Hallensleben NDL, Haveman JW, Jacobs MAJM, Jansen JM, Kop MPM, van Lienden KP, Manusama ER, Mieog JSD, Molenaar IQ, Nieuwenhuijs VB, Poen AC, Poley JW, van de Poll M, Quispel R, Römkens TEH, Schwartz MP, Seerden TC, Stommel MWJ, Straathof JWA, Timmerhuis HC, Venneman NG, Voermans RP, van de Vrie W, Witteman BJ, Dijkgraaf MGW, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. Immediate versus Postponed Intervention for Infected Necrotizing Pancreatitis. N Engl J Med. 2021;385:1372-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 109. | Trikudanathan G, Tawfik P, Amateau SK, Munigala S, Arain M, Attam R, Beilman G, Flanagan S, Freeman ML, Mallery S. Early (<4 Weeks) Versus Standard (≥ 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am J Gastroenterol. 2018;113:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 110. | Navalho M, Pires F, Duarte A, Gonçalves A, Alexandrino P, Távora I. Percutaneous drainage of infected pancreatic fluid collections in critically ill patients: correlation with C-reactive protein values. Clin Imaging. 2006;30:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Becker V, Huber W, Meining A, Prinz C, Umgelter A, Ludwig L, Bajbouj M, Gaa J, Schmid RM. Infected necrosis in severe pancreatitis--combined nonsurgical multi-drainage with directed transabdominal high-volume lavage in critically ill patients. Pancreatology. 2009;9:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 112. | Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998;170:969-975. [PubMed] |
| 113. | Mortelé KJ, Girshman J, Szejnfeld D, Ashley SW, Erturk SM, Banks PA, Silverman SG. CT-guided percutaneous catheter drainage of acute necrotizing pancreatitis: clinical experience and observations in patients with sterile and infected necrosis. AJR Am J Roentgenol. 2009;192:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 114. | Baril NB, Ralls PW, Wren SM, Selby RR, Radin R, Parekh D, Jabbour N, Stain SC. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg. 2000;231:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 115. | Bala M, Almogy G, Klimov A, Rivkind AI, Verstandig A. Percutaneous "stepped" drainage technique for infected pancreatic necrosis. Surg Laparosc Endosc Percutan Tech. 2009;19:e113-e118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 116. | Baudin G, Chassang M, Gelsi E, Novellas S, Bernardin G, Hébuterne X, Chevallier P. CT-guided percutaneous catheter drainage of acute infectious necrotizing pancreatitis: assessment of effectiveness and safety. AJR Am J Roentgenol. 2012;199:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Pascual I, Sabater L, Añón R, Calvete J, Pacheco G, Muñoz E, Lizarraga J, Sastre J, Peña A, Mora F, Pérez-Griera J, Ortega J, Benages A. Surgical vs nonsurgical treatment of infected pancreatic necrosis: more arguments to change the paradigm. J Gastrointest Surg. 2013;17:1627-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 118. | Wang T, Liu LY, Luo H, Dai RW, Liang HY, Chen T, Yan HT, Cui JF, Li NL, Yang W, Liu WH, Tang LJ. Intra-Abdominal Pressure Reduction After Percutaneous Catheter Drainage Is a Protective Factor for Severe Pancreatitis Patients With Sterile Fluid Collections. Pancreas. 2016;45:127-133. [PubMed] |
| 119. | Bruennler T, Langgartner J, Lang S, Wrede CE, Klebl F, Zierhut S, Siebig S, Mandraka F, Rockmann F, Salzberger B, Feuerbach S, Schoelmerich J, Hamer OW. Outcome of patients with acute, necrotizing pancreatitis requiring drainage-does drainage size matter? World J Gastroenterol. 2008;14:725-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 120. | Bellam BL, Samanta J, Gupta P, Kumar M P, Sharma V, Dhaka N, Sarma P, Muktesh G, Gupta V, Sinha SK, Kochhar R. Predictors of outcome of percutaneous catheter drainage in patients with acute pancreatitis having acute fluid collection and development of a predictive model. Pancreatology. 2019;19:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Gupta P, Bansal A, Samanta J, Mandavdhare H, Sharma V, Gupta V, Yadav TD, Dutta U, Kochhar R, Singh Sandhu M. Larger bore percutaneous catheter in necrotic pancreatic fluid collection is associated with better outcomes. Eur Radiol. 2021;31:3439-3446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 122. | Walser EM, Nealon WH, Marroquin S, Raza S, Hernandez JA, Vasek J. Sterile fluid collections in acute pancreatitis: catheter drainage vs simple aspiration. Cardiovasc Intervent Radiol. 2006;29:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 123. | Bang JY, Navaneethan U, Hasan MK, Sutton B, Hawes R, Varadarajulu S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut. 2019;68:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 124. | Lee BU, Song TJ, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. Newly designed, fully covered metal stents for endoscopic ultrasound (EUS)-guided transmural drainage of peripancreatic fluid collections: a prospective randomized study. Endoscopy. 2014;46:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 125. | Mukai S, Itoi T, Baron TH, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Tanaka R, Umeda J, Tonozuka R, Honjo M, Gotoda T, Moriyasu F, Yasuda I. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: a retrospective single-center series. Endoscopy. 2015;47:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Siddiqui AA, Kowalski TE, Loren DE, Khalid A, Soomro A, Mazhar SM, Isby L, Kahaleh M, Karia K, Yoo J, Ofosu A, Ng B, Sharaiha RZ. Fully covered self-expanding metal stents vs lumen-apposing fully covered self-expanding metal stent vs plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc. 2017;85:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 127. | Bapaye A, Dubale NA, Sheth KA, Bapaye J, Ramesh J, Gadhikar H, Mahajani S, Date S, Pujari R, Gaadhe R. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: Comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig Endosc. 2017;29:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 128. | Muktesh G, Samanta J, Dhar J, Agarwala R, Bellam BL, James D, Gupta P, Chauhan R, Yadav TD, Gupta V, Sinha SK, Kochhar R. Endoscopic Ultrasound-guided Drainage of Patients With Infected Walled-off Necrosis: Which Stent to Choose? Surg Laparosc Endosc Percutan Tech. 2022;32:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 129. | Chen YI, Yang J, Friedland S, Holmes I, Law R, Hosmer A, Stevens T, Franco MC, Jang S, Pawa R, Mathur N, Sejpal DV, Inamdar S, Trindade AJ, Nieto J, Berzin TM, Sawhney M, DeSimone ML, DiMaio C, Kumta NA, Gupta S, Yachimski P, Anderloni A, Baron TH, James TW, Jamil LH, Ona MA, Lo SK, Gaddam S, Dollhopf M, Bukhari MA, Moran R, Gutierrez OB, Sanaei O, Fayad L, Ngamruengphong S, Kumbhari V, Singh V, Repici A, Khashab MA. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: a large international multicenter study. Endosc Int Open. 2019;7:E347-E354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 130. | Mohan BP, Jayaraj M, Asokkumar R, Shakhatreh M, Pahal P, Ponnada S, Navaneethan U, Adler DG. Lumen apposing metal stents in drainage of pancreatic walled-off necrosis, are they any better than plastic stents? Endosc Ultrasound. 2019;8:82-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 131. | Lariño-Noia J, de la Iglesia-García D, González-Lopez J, Díaz-Lopez J, Macías-García F, Mejuto R, Quiroga A, Mauriz V, Jardí A, Iglesias-García J, Domínguez-Muñoz JE. Endoscopic drainage with local infusion of antibiotics to avoid necrosectomy of infected walled-off necrosis. Surg Endosc. 2021;35:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 132. | Trikudanathan G, Rana SS. Current Controversies and Challenges in Endoscopic Management of Necrotizing Pancreatitis. Clin Gastroenterol Hepatol. 2022;20:2717-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 133. | Lakhtakia S, Basha J, Talukdar R, Gupta R, Nabi Z, Ramchandani M, Kumar BVN, Pal P, Kalpala R, Reddy PM, Pradeep R, Singh JR, Rao GV, Reddy DN. Endoscopic "step-up approach" using a dedicated biflanged metal stent reduces the need for direct necrosectomy in walled-off necrosis (with videos). Gastrointest Endosc. 2017;85:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 134. | Gardner TB, Coelho-Prabhu N, Gordon SR, Gelrud A, Maple JT, Papachristou GI, Freeman ML, Topazian MD, Attam R, Mackenzie TA, Baron TH. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 135. | Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, Kreitmair C, Meining A, Wehrmann T, Rösch T. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 136. | Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM, Poley JW, van Ramshorst B, Vleggaar FP, Boermeester MA, Gooszen HG, Weusten BL, Timmer R; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 137. | van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P; Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 471] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 138. | Yan L, Dargan A, Nieto J, Shariaha RZ, Binmoeller KF, Adler DG, DeSimone M, Berzin T, Swahney M, Draganov PV, Yang DJ, Diehl DL, Wang L, Ghulab A, Butt N, Siddiqui AA. Direct endoscopic necrosectomy at the time of transmural stent placement results in earlier resolution of complex walled-off pancreatic necrosis: Results from a large multicenter United States trial. Endosc Ultrasound. 2019;8:172-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 139. | Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 267] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 140. | Horvath KD, Kao LS, Wherry KL, Pellegrini CA, Sinanan MN. A technique for laparoscopic-assisted percutaneous drainage of infected pancreatic necrosis and pancreatic abscess. Surg Endosc. 2001;15:1221-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 141. | Fagniez PL, Rotman N, Kracht M. Direct retroperitoneal approach to necrosis in severe acute pancreatitis. Br J Surg. 1989;76:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 142. | Chen H, Li F, Sun JB, Jia JG. Abdominal compartment syndrome in patients with severe acute pancreatitis in early stage. World J Gastroenterol. 2008;14:3541-3548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 93] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 143. | Singh AK, Samanta J, Dawra S, Gupta P, Rana A, Sharma V, Kumar-M P, Sinha SK, Kochhar R. Reduction of intra-abdominal pressure after percutaneous catheter drainage of pancreatic fluid collection predicts survival. Pancreatology. 2020;20:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, Sugrue M, Cheatham M, Ivatury R, Ball CG, Reintam Blaser A, Regli A, Balogh ZJ, D'Amours S, Debergh D, Kaplan M, Kimball E, Olvera C; Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 841] [Cited by in RCA: 852] [Article Influence: 71.0] [Reference Citation Analysis (2)] |
| 145. | Kirkpatrick AW, Roberts DJ, Jaeschke R, De Waele JJ, De Keulenaer BL, Duchesne J, Bjorck M, Leppäniemi A, Ejike JC, Sugrue M, Cheatham ML, Ivatury R, Ball CG, Reintam Blaser A, Regli A, Balogh Z, D'Amours S, De Laet I, Malbrain ML. Methodological background and strategy for the 2012-2013 updated consensus definitions and clinical practice guidelines from the abdominal compartment society. Anaesthesiol Intensive Ther. 2015;47 Spec No:s63-s77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 146. | Samanta J, Rana A, Dhaka N, Agarwala R, Gupta P, Sinha SK, Gupta V, Yadav TD, Kochhar R. Ascites in acute pancreatitis: not a silent bystander. Pancreatology. 2019;19:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (1)] |