Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.65
Peer-review started: September 19, 2022
First decision: October 21, 2022
Revised: November 12, 2022
Accepted: December 15, 2022
Article in press: December 15, 2022
Published online: January 6, 2023
Processing time: 107 Days and 8.2 Hours
Diabetes mellitus (DM) is a complicated, globally expanding disease that is influenced by hereditary and environmental variables. Changes in modern society's food choices, physical inactivity, and obesity are significant factors in the development of type 2 DM (T2DM). The association between changes in intestinal flora and numerous disorders, including obesity, diabetes, and cardiovascular diseases, has been studied in recent years. The purpose of this review is to analyze the mechanisms underlying the alteration of the diabetic patients' intestinal flora, as well as their therapeutic choices. Also included is a summary of the anti-diabetic benefits of natural compounds demonstrated by studies. The short-chain fatty acids theory, the bile acid theory, and the endotoxin theory are all potential methods by which intestinal flora contributes to the establishment and progression of T2DM. Due to an intestinal flora imbalance, abnormalities in short-chain fatty acids and secondary bile acids have been found in diabetic patients. Additionally, metabolic endotoxemia with altering flora induces a systemic inflammatory response by stimulating the immune system via bacterial translocation. The agenda for diabetes treatment includes the use of short-chain fatty acids, probiotics, prebiotics in the diet, fecal bacteria transplantation, and antibiotics. Animal studies have proven the antidiabetic benefits of numerous bioactive substances, including Flavonoids, Alkaloids, Saponin, and Allicin. However, further research is required to contribute to the treatment of diabetes.
Core Tip: It is thought that intestinal flora may have a role in the development of type 2 diabetes mellitus. This has been demonstrated in the treatment of type 2 diabetes mellitus and that the course of the disease might differ depending on the medications used for intestinal flora imbalance.
- Citation: Aydin OC, Aydın S, Barun S. Role of natural products and intestinal flora on type 2 diabetes mellitus treatment. World J Clin Cases 2023; 11(1): 65-72
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/65.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.65
Excess salt, sugar, and fat consumption in the diet, combined with a sedentary lifestyle, contribute to an increase in chronic diseases such as obesity and diabetes in modern society. Hyperglycemia is a hallmark of diabetes, a chronic metabolic condition characterized by elevated blood sugar levels. Long-term hyperglycemia can cause problems in numerous organs, including multiorgan failure and death[1]. Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and concomitant hyperglycemia in insulin-sensitive tissues such as adipose tissue and skeletal muscle. Obesity is frequently associated with insulin resistance[2]. T2DM accounts for more than 90% of all diabetes patients. Given that T2DM is a significant public health issue and economic burden, it is critical to research methods for its prevention and treatment[3]. Recent research has shown that, in addition to other etiological factors, intestinal flora disruption can lead to diabetes by affecting intestinal permeability, inflammation, the immune system, and energy metabolism. However, the mechanisms associated with T2DM are still not fully understood. In this review, we will summarize the probable mechanisms of intestinal flora that play a role in the formation and progression of T2DM, as well as treatment methods and natural products that may be effective.
The intestinal flora has been recognized as a novel organ constituted of 500-1000 species and 1014 bacteria. This is equivalent to tenfold the number of human cells. The normal intestinal flora contains six classes of bacteria, all of which are anaerobic bacteria: Firmicutes (Lactobacillus, Enterococcus, Clostridium), Bacteroidetes, Proteobacteria (Enterobacteria), Actinobacteria (Bifidobacterium), Fusobacteria, and Verrucomicrobia are some examples. The first four groups account for 98% of total intestinal flora[3].The intestinal flora is a component of the intricate and dynamic process known as the intestinal barrier, which is formed by the functional interaction of the distinct layers[4].
These microorganisms play important roles in the human body. Among these are the synthesis of some vitamins and cofactors, the digestion of complex polysaccharides and their degradation to short-chain fatty acids, the regulation of the gastrointestinal system motility and vascularization, the effect of fatty acid composition of the retina and eye lens, the effect of bone mineral density, and the development of adaptive immunity[5].
Publications indicating that the microbiota may play a role in the onset of various diseases in the human body, particularly diabetes and obesity, have recently increased[6,7].
Overnutrition has a devastating effect on the diversity and stability of the microflora, decreasing beneficial microflora and increasing pathogenic microflora, causing low-grade inflammation in the gut that can lead to insulin resistance and T2DM. The precise mechanism of intestinal flora involvement in the genesis and progression ofT2DM remains unknown. The short-chain fatty acid theory, the bile acid theory, and the endotoxin theory are provided as potential processes[3].
Short-chain fatty acids (SCFAs) are organic carboxylic acids that have one-six carbon atom. They are primarily produced by bacteria in the intestine. Bacteroides, Clostridium, Bifidobacterium, Eubacterium, Streptococcus, Peptostreptococcus, and others are typical SCFA-producing bacteria[3]. The most important SCFAs consist of acetic, propionic, butyric, valeric, and caproic acids. The most frequent SCFAs and anions in the colon are acetate (C2), propionate (C3), and butyrate (C4), respectively. Depending on the fiber content of the diet, the large intestine produces between 500 and 600 mmol/L SCFA daily. Fermentation of fiber into SCFAs in the colon decreases pH, increases fecal acidity, and promotes the proliferation and variety of gut microbiota. SCFAs serve as mediators in numerous pathways involving local, immunological, and endocrine impacts, as well as microbiota-gut-brain interactions. After intestinal bacteria break down dietary fibers, colonocytes absorb SCFAs via passive diffusion or active transport mediated by H+-linked monocarboxylate transporters. At the cellular level, SCFAs regulate the homeostasis and function of intestinal epithelial cells generating complex and integrated effects[4] (Table 1).
| Effects of SCFAs |
| Energy substrate for ATP production |
| Receptor activation, mainly G protein coupled receptors |
| Maintaining barrier function and integrity |
| Modulation of immunity and control of inflammation, Treg differentiation, modulation of inflammation mediators |
| Modulation of intracellular permeability |
| Epigenetic effects by inhibition of histone deacetylases, hyperacetylation of histones and modulation of gene expression |
Recent research has revealed that there is an abnormality in the bacteria that make short-chain fatty acids in people with diabetes, resulting in abnormal short-chain fatty acid production. Short-chain fatty acids can help the colon's acidic environment, restrict the growth of pathogenic bacteria, maintain water and electrolyte balance, and avoid intestinal dysfunction. Due to a decrease in short-chain fatty acids, the intestinal tract is less able to develop an anti-inflammatory response, leading to intestinal inflammation[3].
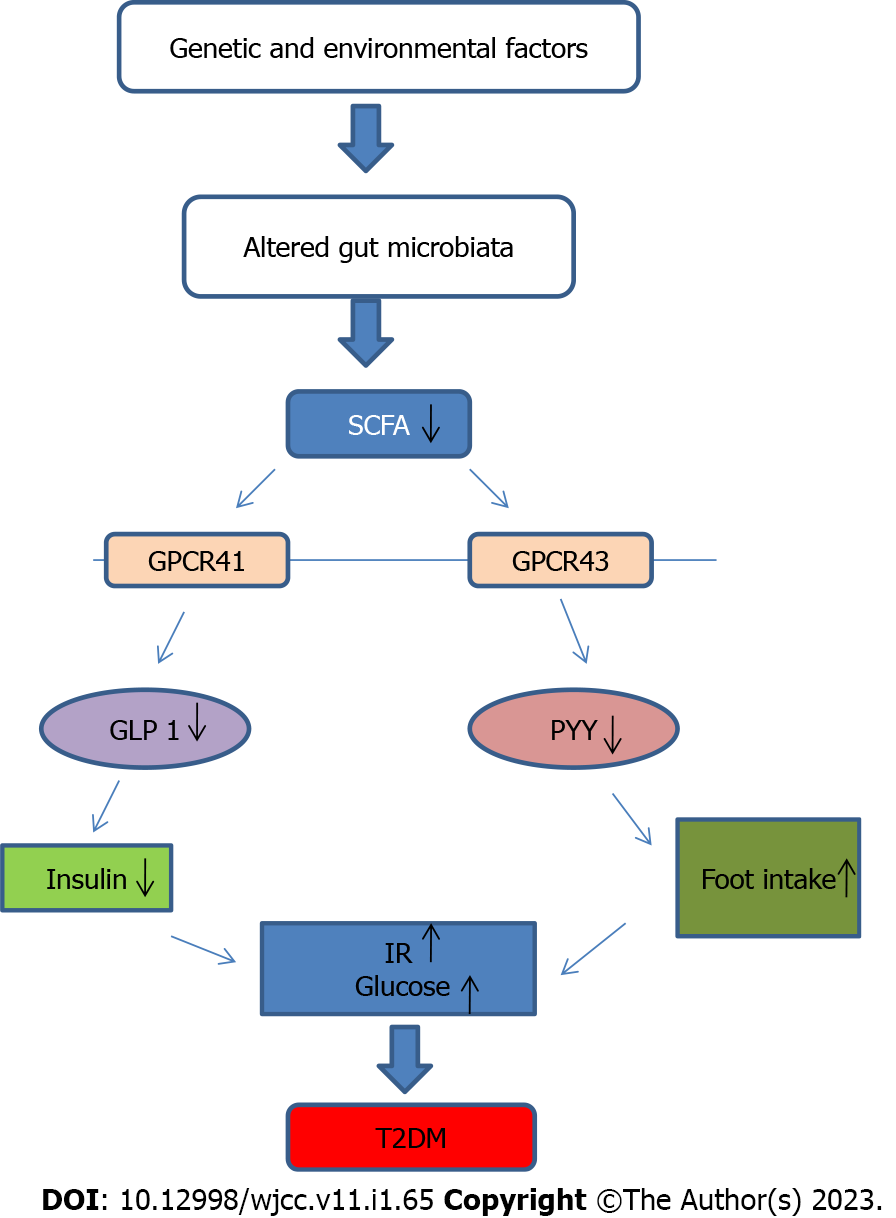
G protein-coupled receptors (GPR41 and GPR43) activated by SCFA provide essential regulatory properties for fat and glucose metabolism[8].SCFAs can promote the activation of peptide tyrosine-tyrosine (PYY) and glucagon-like peptide-1 (GLP-1) from intestinal enteroendocrine L cells by activating GPR41 and GPR43. The neuroendocrine hormone PPY affects food intake and energy balance. Reduced GLP-1 secretion in T2DM results in decreased insulin levels and poor glucose and energy metabolism[9](Figure 1). In dysbacteriosis, low SCFA production and impaired activation of SCFA receptors are seen in the intestinal tract. This leads to irregularities in lipid and glucose metabolism, which all contribute to the development of T2DM[8].
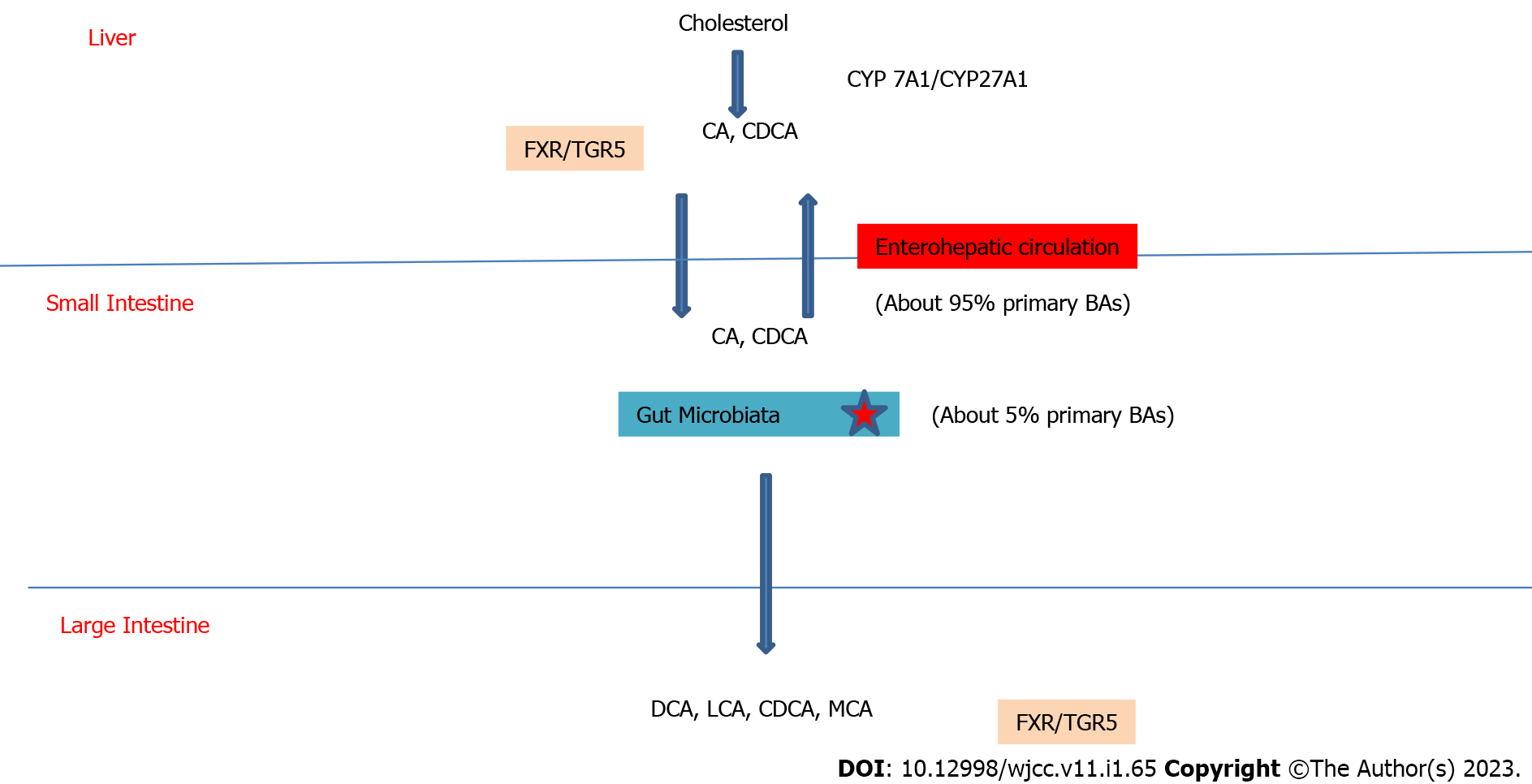
The primary functional components of bile are bile acids. They are produced from cholesterol in hepatocytes via the classic or alternative pathway, stored in the gallbladder, and subsequently released into the small intestine. The cholesterol 7a-hydroxylase enzyme is the rate-limiting enzyme in the synthesis of bile acids via the so-called classical pathway. Sterol 27 hydroxylase is active in the alternative pathway. 95% of cholic acid (CA) and chenodeoxycholic CA, the two principal bile acids, enter the enterohepatic circulation. The gut bacteria convert 5% of primary bile acids into secondary bile acids. In humans, these are deoxycholic CA(DCA), lithocholic CA (LCA), and ursodesoxycholic CA, whereas in mice, they are DCA, LCA, muricholic CA, hyodeoxycholic acid, and murideoxycholic acid[10-12].They then influence lipid, glucose, and energy metabolism by activating a series of nuclear receptors [farnesoid X receptor (FXR) in liver and intestine, G protein-coupled bile acid receptor 5 (TGR5) in enteroendocrine cells and pancreatic B cells] involved in the production of liver bile acids and intestinal bile acid reabsorption[13] (Figure 2).
Intestinal flora disruption reduces secondary bile acid production and bile acid receptor activation, resulting in impaired glucose metabolism and T2DM[3]. GLP-1 release from L cells is stimulated by the activation of TGR5 by secondary BAs, which increases insulin secretion and glucose tolerance. As a result of the decreased TGR5 stimulation with the changing BA content, the released GLP-1 decreases. This causes insulin resistance and increased glucose[9]. Bacterial diseases result in decreased bile acid activation and diminished FXR activation, which contribute in a variety of ways to the development of T2DM. As a result of FXR activation insulin sensitivity and glycogen synthesis decrease, an increase happens in hepatic gluconeogenesis and blood sugar. In addition to these changes, the levels of fibroblast growth factor 15 (FGF15), FGF21, FGF19, energy consumption, and insulin sensitivity decrease; on the other hand, body weight increases. As another result, the expression of a transcription factor which plays a role in glucose regulation, Krueppel-like factor (KLF11), decreases. The decrease in the ability of KLF11's to support insulin gene transcription results in lower insulin levels[3,9].
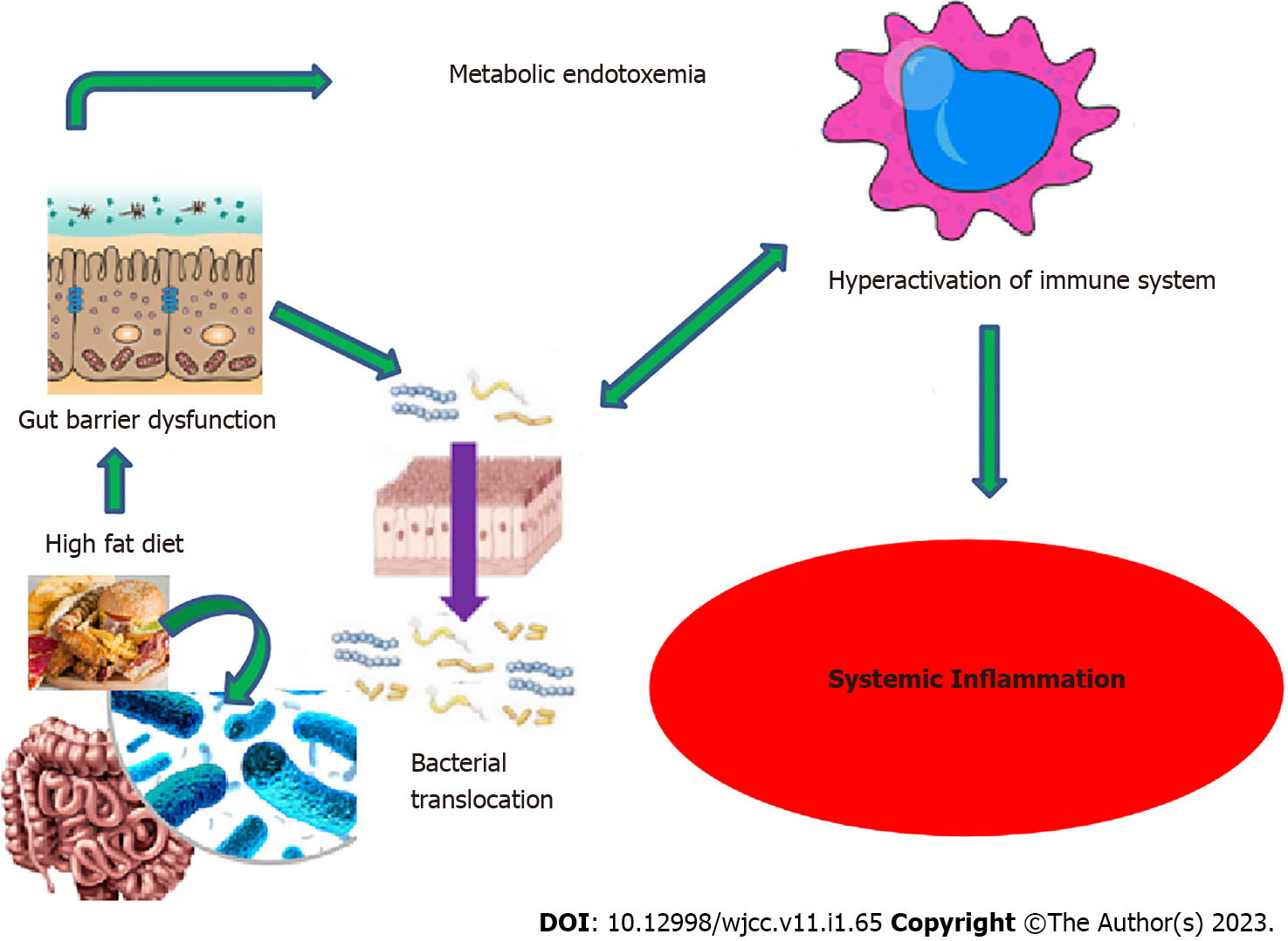
Fatty diet and the other above-mentioned factors decrease the number of helpful bacteria, including bifidobacterium and lactobacillus. As a result, gut flora deteriorates. The prevalence of gram-negative bacteria rises, and the resulting endotoxemia increases intestinal wall permeability. Gram-negative bacteria have an outermost cell wall layer which is called lipopolysaccharide (LPS) and endotoxin is a part of this layer[3,14]. Through the upregulation of inflammatory signaling pathways and proinflammatory cytokine production, a large amount of LPS generated in the gut (metabolic endotoxemia) may induce persistent low-grade inflammation in T2DM patients. On the surface of monocyte macrophages, LPS binds to the toll-like receptor 4 (TLR-4) and creates a complex with the glucose phosphate isomerase-associated protein CD14. TLRs cause insulin resistance and inflammation in adipocytes and skeletal muscle cells by activating mitogen-activated protein kinase[3,14] (Figure 3).
According to research, controlling gut flora can improve insulin resistance, boost insulin production, and play a crucial role in regulating blood sugar. SCFAs have been shown in clinical studies to maintain gut architecture and function while also having a therapeutic impact. The identification and modulation of SCFAs in the gut tract of humans could be an effective treatment for type 2 diabetes. SCFA levels in T2DM patients' stools can be measured using gas chromatography and mass spectrometry. SCFA synthesis in the intestinal tract can be increased in diabetic patients by adding fiber or direct SCFA to the diet, increasing the beneficial bacteria content in the intestinal tract, and energy metabolism is regulated to improve T2DM symptoms[3]. SCFA supplementation in T2DM patients boosted butyrate-producing bacteria, GLP-1, and hemoglobin A1c levels[15].
Probiotics are live microorganisms that when taken in adequate amounts, promote health by controlling the microbial balance in the intestines of the host. Prebiotics, on the other hand, are fermented food components that either stimulate or inhibit the growth and/or activity of gastrointestinal microorganisms in the individual's intestinal microbiota[16]. A study found that probiotic treatment in diabetic patients can improve the host's gut microenvironment and, to some extent, diminish symptoms such as abnormal glucose tolerance and insulin resistance[17]. Prebiotics and probiotics have specific effects such as preventing the development of autoimmune diabetes, regulating the flora to improve metabolism, improving the intestinal mucosal barrier function, increasing insulin sensitivity, and regulating neurologic activities related to glucose metabolism. T2DM symptoms may be alleviated by drinking beverages containing probiotics and prebiotics[3].
The demonstration of a link between intestinal microbiota and diabetes suggested that fecal bacteria transplantation could be used to treat diabetes. The process of suspending a healthy donor's stool and transferring it to the recipient's digestive system for treatment is known as fecal bacterial transplantation[18]. A study found that transplanting fecal bacteria to patients with metabolic disorders can improve insulin sensitivity[19]. Blood glucose levels in people with diabetes who have received fecal microorganism transplantation have been shown to be stable[20].
Oral antibiotics are another option for decreasing inflammation in the body and improving the T2DM phenotype. Antibiotics, on the other hand, can disrupt the intestinal flora by harming beneficial bacteria in the digestive tract. This can have a negative impact on diabetic patients. As a result, diabetic patients must be carefully evaluated, along with their current condition and etiology, and treatment must be carefully monitored[3].
Natural products (bioactive compounds) are phytochemicals found in plants, fruits, and vegetables such as polyphenols, anthocyanins, flavonoids, carotenoids, alkaloids, and tannins[21]. After meal consumption, the intestinal flora modulates the synthesis, bioavailability, and bioactivities of natural products with high molecular weight polyphenols. In addition, the intestinal flora converts bioactive chemicals into metabolites such as short-chain fatty acids and bile acids, which can influence host health and intestinal ecology by participating in various metabolic pathways[22]. Some research has recently focused on the modification of intestinal flora by bioactive substances and the treatment of metabolic diseases such as diabetes and obesity[23,24]. Bioactive compounds either have a prebiotic effect on pathogenic bacteria in the gut or have an antimicrobial effect on the microflora composition. Modulation of the colonic microbiota may aid in the management of T2DM. Table 2 lists some natural products that have an effect on diabetes by modulating the colonic microbiota[22].
| Natural products | Sources | Antidiabetic mechanisms |
| Allicin | Garlic | Maintains glucose homeostasis; Maintains insulin sensitivity |
| Saponin | Polygonatum kingianum | Lowers blood sugar and lipopolysaccharide; Improves glucose metabolism |
| Flavanoids | Enteromorphaprolifera | Decreased blood glucose levels; Improve glucose tolerance |
| Anthocyanin | Strawberry | Improved lipid, glucose metabolism, insulin resistance, gut barrier function; Reduce low grade inflammation and stimulate the host immune system |
| Alkoloids | Rhizomz coptidis | Improved insulin sensitivity, glucose homeostasis, blood lipids |
| Catechin | Rhododendron groenlandicum | Reduced intestinal endotoxin levels; Improve mucosal barrier function; Lower serum glucose |
| Flavanols | Green tea leaves, red wine, cocoa, cranberry | Increase glucose tolerance; Increase insulin secretion |
Dietary polyphenols are naturally occurring chemicals found in numerous plant foods, including fruits and vegetables. In an in vivo study evaluating the antidiabetic mechanisms of polyphenols-rich vinegar extract, vinegar extract reduced blood glucose and lipemia and reduced inflammation by inhibiting TLR4/nuclear factor kappa B signaling pathway. In addition, it has been shown to regulate intestinal microbiota dysbiosis and increase short-chain fatty acid content in diabetic mice[25]. The plant-based polyphenol-rich extract TOTUM 63 (a patented blend of plant-based biomolecules) underwent a randomized, double-blind, placebo-controlled research to enhance glucose homeostasis in multiple preclinical models of obesity and T2DM. In individuals with impaired fasting glycemia and glucose intolerance, TOTUM63 improved several metabolic syndrome characteristics with a favorable safety and tolerability profile and decreased fasting blood glucose[26]. In a randomized, double-blind, placebo-controlled, parallel group research, it was discovered that Resveratrol supplementation improved glycemic control by decreasing insulin resistance in T2DM patients taking oral hypoglycemic medications. It has a considerable positive effect on diabetes patients' chronic inflammation, oxidative stress, and microRNA expression. This has been taken to suggest that a combination of oral hypoglycemic medications may be advantageous for minimizing problems associated with diabetes[27]. In a randomized, double-blind, placebo-controlled study examining the potential of pomegranate peel extract (PoPEx) to counteract inflammation and oxidative stress in T2DM patients, PoPEx administration for 8 wk had beneficial effects on the inflammatory status and oxidative stress biomarkers in diabetic patients. In addition, the PoPEx group had a substantial improvement in lipid profile[28].
Nonalcoholic fatty liver disease (NAFLD) is significantly connected with insulin resistance disorders, such as T2DM and obesity. In a study examining the effect of curcumin supplementation on NAFLD, low-dose curcumin supplementation (250 mg daily) for 2 mo significantly decreased hepatic steatosis and enzymes compared to placebo. However, longer length and higher dose investigations are required[29].
Another study found that peanut skin procyanidins could alleviate T2DM symptoms by reducing the inflammatory response, modulating the gut microbiota, and improving gut integrity in mice with streptozotocin-induced T2DM[30].
The effect of intestinal flora on the etiology of diabetes, one of the metabolic diseases whose prevalence has increased with modern society, has been studied in recent years. The short-chain fatty acid theory, bile acid theory, and endotoxin theory are three possible mechanisms by which intestinal flora is effective in the formation and development of T2DM, which is more common. Diabetes treatment based on these etiological reasons includes the use of short-chain fatty acids, probiotics, prebiotics in the diet, fecal bacteria transplantation, and antibiotics. Bioactive compounds have been shown in preclinical and clinical studies to improve a variety of symptoms in people with diabetes. A diabetes treatment that regulates intestinal flora could be an innovative step in the prevention and treatment of diabetes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Cigrovski Berkovic M, Croatia; Herold Z, Hungary; Mishra R, India; Nong X, China; Shuang W, China S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3402] [Article Influence: 486.0] [Reference Citation Analysis (0)] |
| 2. | DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 1327] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 3. | Ma Q, Li Y, Li P, Wang M, Wang J, Tang Z, Wang T, Luo L, Wang C, Zhao B. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed Pharmacother. 2019;117:109138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 4. | Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, Khalil M, Wang DQ, Sperandio M, Di Ciaula A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 470] [Article Influence: 156.7] [Reference Citation Analysis (0)] |
| 5. | Yetkin İ, Yetiş H, Satiş NK. Bağırsakmikrobiyotasınıninsülindirenci, diabetes mellitus veobeziteileilişkisi. TürkiyeDiyabetveObeziteDergisi. 2017;2:1-8. |
| 6. | Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Krämer M, Gummesson A, Perkins R, Bergström G, Bäckhed F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab 2020; 32: 379-390. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 7. | Sircana A, Framarin L, Leone N, Berrutti M, Castellino F, Parente R, De Michieli F, Paschetta E, Musso G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr Diab Rep. 2018;18:98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 8. | Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1616] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 9. | Wang D, Liu J, Zhou L, Zhang Q, Li M, Xiao X. Effects of Oral Glucose-Lowering Agents on Gut Microbiota and Microbial Metabolites. Front Endocrinol (Lausanne). 2022;13:905171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. 2017;56:54-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 11. | Xia F, Wen LP, Ge BC, Li YX, Li FP, Zhou BJ. Gut microbiota as a target for prevention and treatment of type 2 diabetes: Mechanisms and dietary natural products. World J Diabetes. 2021;12:1146-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (4)] |
| 12. | Xie C, Huang W, Young RL, Jones KL, Horowitz M, Rayner CK, Wu T. Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 13. | Rajani C, Jia W. Bile acids and their effects on diabetes. Front Med. 2018;12:608-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34:392-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1543] [Article Influence: 220.4] [Reference Citation Analysis (68)] |
| 16. | Şanlier N. Probiyotikler, Prebiyotiklerve Diabetes Mellitus. KlinikTıp Aile Hekimliği. 2019;11:63-70. |
| 17. | Dolatkhah N, Hajifaraji M, Abbasalizadeh F, Aghamohammadzadeh N, Mehrabi Y, Abbasi MM. Is there a value for probiotic supplements in gestational diabetes mellitus? J Health Popul Nutr. 2015;33:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Borody TJ, Campbell J. Fecal microbiota transplantation: techniques, applications, and issues. Gastroenterol Clin North Am. 2012;41:781-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913-6. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2016] [Article Influence: 155.1] [Reference Citation Analysis (0)] |
| 20. | Cai TT, Ye XL, Yong HJ, Song B, Zheng XL, Cui BT, Zhang FM, Lu YB, Miao H, Ding DF. Fecal microbiota transplantation relieve painful diabetic neuropathy: A case report. Medicine (Baltimore). 2018;97:e13543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Science and Human Wellness. 2014;3:136-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 22. | Sharma BR, Jaiswal S, Ravindra PV. Modulation of gut microbiota by bioactive compounds for prevention and management of type 2 diabetes. Biomed Pharmacother. 2022;152:113148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Eid HM, Wright ML, Anil Kumar NV, Qawasmeh A, Hassan STS, Mocan A, Nabavi SM, Rastrelli L, Atanasov AG, Haddad PS. Significance of Microbiota in Obesity and Metabolic Diseases and the Modulatory Potential by Medicinal Plant and Food Ingredients. Front Pharmacol. 2017;8:387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Brahe LK, Astrup A, Larsen LH. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv Nutr. 2016;7:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Xia T, Zhang Z, Zhao Y, Kang C, Zhang X, Tian Y, Yu J, Cao H, Wang M. The anti-diabetic activity of polyphenols-rich vinegar extract in mice via regulating gut microbiota and liver inflammation. Food Chem. 2022;393:133443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 26. | Sirvent P, Chavanelle V, Otero YF, Bargetto M, Le Joubioux F, Boisseau N, Maugard T, Cazaubiel M, Pereira B, Guigas B, Hadjadj S, Peltier SL, Marette A, Bard JM. TOTUM-63, a plant-based polyphenol-rich extract, improves glycaemic control in subjects with prediabetes or early stage newly-diagnosed type 2 diabetes in a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2022;24:2331-2340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 27. | Mahjabeen W, Khan DA, Mirza SA. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement Ther Med. 2022;66:102819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 28. | Rachid AP, Moncada M, Mesquita MF, Brito J, Bernardo MA, Silva ML. Effect of Aqueous Cinnamon Extract on the Postprandial Glycemia Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Mirhafez SR, Azimi-Nezhad M, Dehabeh M, Hariri M, Naderan RD, Movahedi A, Abdalla M, Sathyapalan T, Sahebkar A. The Effect of Curcumin Phytosome on the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Adv Exp Med Biol. 2021;1308:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Liu M, Huang B, Wang L, Lu Q, Liu R. Peanut skin procyanidins ameliorate insulin resistance via modulation of gut microbiota and gut barrier in type 2 diabetic mice. J Sci Food Agric. 2022;102:5935-5947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |