Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.47
Peer-review started: September 7, 2022
First decision: October 30, 2022
Revised: November 14, 2022
Accepted: December 15, 2022
Article in press: December 15, 2022
Published online: January 6, 2023
Processing time: 119 Days and 12.7 Hours
Inflammatory bowel disease (IBD) is a chronic, recurrent, and debilitating disorder, and includes Crohn’s disease and ulcerative colitis. The pathogenesis of IBD is closely associated with intestinal dysbiosis, but has not yet been fully clarified. Genetic and environmental factors can influence IBD patients’ gut microbiota and metabolism, disrupt intestinal barriers, and trigger abnormal immune responses. Studies have reported the alteration of gut microbiota and metabolites in IBD, providing the basis for potential therapeutic options. Intestinal microbiota-based treatments such as pre/probiotics, metabolite supplementation, and fecal microbiota transplantation have been extensively studied, but their clinical efficacy remains controversial. Repairing the intestinal barrier and promoting mucosal healing have also been proposed. We here review the current clinical trials on intestinal microecology and discuss the prospect of research and practice in this field.
Core Tip: The intestinal microecological system plays an important role in the pathogenesis of inflammatory bowel disease (IBD). Reconstructing healthy intestinal microecology is a promising therapeutic strategy, but has not been widely accepted in the routine management of IBD. We herein discuss the progress and prospects of studies on IBD treatment targeting the intestinal microecological system, including disordered gut microbiota, metabolites, and intestinal epithelium.
- Citation: Yan XX, Wu D. Intestinal microecology-based treatment for inflammatory bowel disease: Progress and prospects. World J Clin Cases 2023; 11(1): 47-56
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/47.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.47
The intestinal microecological system is an organic and dynamic balancing state involving the intestinal epithelial barrier, mucosal immune barrier, gut microbiota, nutrition, metabolites, and other factors. Numerous studies have shown that disrupted intestinal homeostasis is closely related to inflammatory disorders, as well as metabolic, cardiovascular, allergic, psychological, and malignant diseases[1]. Inflammatory bowel disease (IBD) is a heterogeneous group of chronic recurrent inflammatory diseases, historically subdivided into two main subtypes, Crohn’s disease (CD) and ulcerative colitis (UC)[2,3]. IBD is a complex gastrointestinal disorder resulting from an inappropriate immune response to altered intraluminal microbiota in a genetically susceptible host.
Gut microbes and their metabolites help maintain intestinal homeostasis through signal transduction, immune system modulation, endocrine regulation, and other mechanisms[4]. With the development and application of multi-omics research techniques, studies have shown dramatic changes in the gut microbiome and metabolome in IBD patients. The loss of microbial diversity and metabolic diversity was observed. In addition, a longitudinal analysis showed decreased stability of the gut microbiome in IBD patients[5]. For example, facultative anaerobes such as Escherichia coli are increased in IBD patients. Obligate anaerobic producers of short-chain fatty acids (SCFAs) including Faecalibacterium prausnitzii and Roseburia hominis are decreased[5]. With regard to metabolite features, remarkable differences between IBD patients and non-IBD controls were identified. Enrichments of primary bile acids, polyunsaturated fatty acids, sphingolipids, and acylcarnitines, and depletions of secondary bile acids, triacylglycerols, tetrapyrroles, SCFAs, and vitamins have also been documented[5,6].
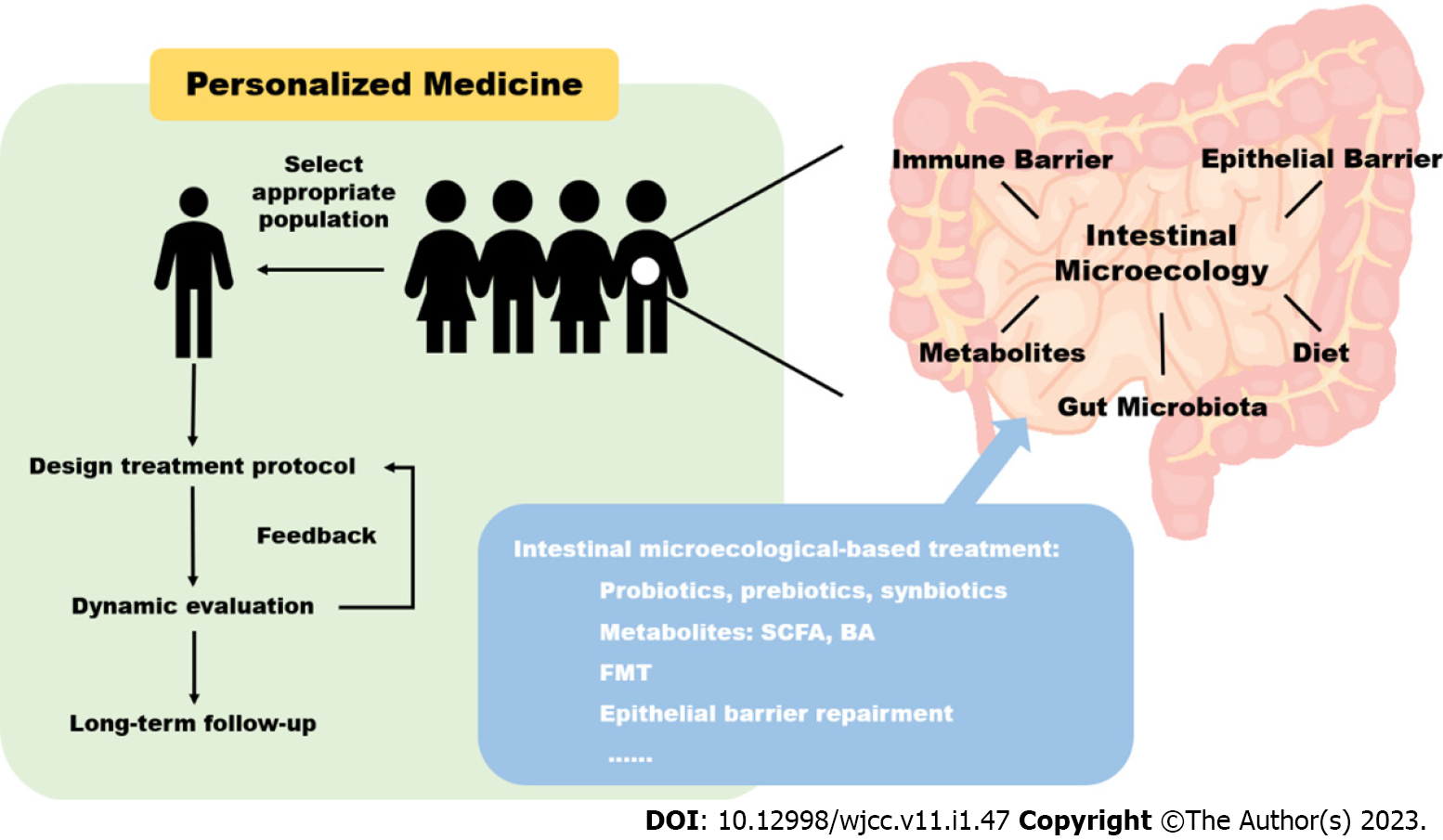
These findings shed light on the pathogenesis of IBD, providing more targets for potential diagnostic and therapeutic research. Reconstructing healthy intestinal microecology is a promising therapeutic approach using pre/probiotics supplementation, fecal microbiota transplantation, and immune and signal molecule regulation. However, these therapeutic strategies are far from established and have not been widely accepted in the routine management of IBD. In this review, we summarize the progress of studies on IBD treatment targeting intestinal microecology, especially the latest evidence from clinical trials. The limitations of current studies and prospects for future studies are also discussed (Figure 1).
Probiotics, prebiotics, and synbiotics are microecological agents used as alternative or complementary therapy for IBD, but the results of clinical trials are inconsistent. In a recent systematic review and meta-analysis including 38 randomized controlled trials (RCTs), probiotics, prebiotics, and synbiotics showed benefits in inducing/maintaining remission and reducing disease activity, especially in UC patients[7].
Probiotics are live microorganisms that are beneficial to the host by directly regulating gut microbiota. Commonly used probiotic microorganisms include Lactobacillus, Bifidobacterium, Escherichia coli Nissle 1917 (EcN), Enterococcus, and Saccharomyces boulardii. Probiotics containing Lactobacillus and Bifidobacterium or VSL#3 were effective in inducing remission and reducing disease activity in UC according to pooled results of meta-analyses[8,9]. In addition, the use of a mixture of strains had higher effects than using one single strain[7]. Probiotics were also used in combination with conventional medications. Compared with routine medical treatment, the use of probiotics combined with glucocorticoid or mesalazine was more effective in protecting mucosa, improving microflora composition, and inhibiting inflammatory cytokines[10-12].
Prebiotics refer to some organic substances (mainly oligosaccharides) that are not digested and absorbed by the host but can selectively promote the metabolism and proliferation of beneficial bacteria. Fructooligosaccharide (FOS) has been investigated in several RCTs. In a placebo-controlled trial, FOS showed no clinical benefit in active CD[13]. However, oligofructose-enriched inulin was associated with early fecal calprotectin normalization in active UC, decreased levels of Ruminococcus gnavus, and increased levels of butyrate and acetaldehyde in CD[14-16]. Wilson et al[17] investigated the effect of prebiotic galactooligosaccharide (GOS) supplementation in 17 active UC patients. GOS did not reduce clinical scores or inflammation. With regard to microbiota composition, the proportions of Bifidobacterium and Christensenellaceae were increased in patients with less active disease, indicating the correlation between disease activity and the prebiotic effect. It is reasonable to hypothesize that less-active or early-stage IBD patients may have a less-pathological gut environment and are more likely to respond to microecological agents.
Synbiotics are combined applications of probiotics and prebiotics, which are theoretically more effective than probiotics and prebiotics due to the synergistic effect[7]. Compared with placebo, synbiotics Lactocare® (seven strains with FOS) significantly decreased disease activity and achieved a higher response rate in UC patients[18]. Another study similarly supported synbiotic therapy (six strains with FOS) in reducing the inflammatory reaction, and clinical and endoscopic activity of UC[19]. For CD patients, synbiotic consumption decreased disease activity and proliferated mucosal Bifidobacterium, but did not prolong the time to relapse when given as an adjunct to standard therapy[20,21].
Although probiotics and prebiotics are generally considered safe, there are still some risks such as systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals, gene transfer and gastrointestinal side effects[22]. These risks may increase in children, immunosuppressed individuals and the critically ill[23]. For severe active IBD patients with impaired mucosal barrier, translocation of bacteria and secondary bacteremia have been reported in cases and should be carefully surveilled[24-26].
It should be noted that these published studies of probiotics, prebiotics, and synbiotics were heterogeneous. There is no consensus or guidelines on the application strategies to guide the resource, composition, and dosage of strains. The same probiotics may have different effects on different patients due to factors such as age, lifestyle, and disease severity. Moreover, the intestinal environment itself is extremely complex. The interaction between the supplementary bacteria and the original gut microbiota, and among different probiotics have not been thoroughly elucidated. Therefore, future research and application of microecological treatment should follow the principle of individualized medicine. With the help of omics technology, hopefully, changes in the composition of pathological gut microbiota can be analyzed to find targets for complementary therapy.
There are bidirectional interactions between gut microbiota and metabolites. Changes in metabolites either contribute to or result from intestinal dysbiosis. A recent study examined fecal metagenomics and metabolomic changes in IBD patients. A total of 135 metabolites were differentially expressed between IBD and control patients. Pathways including primary bile acid biosynthesis, vitamin digestion and absorption, and carbohydrate metabolism were affected[27]. Through treatments targeted at abnormal intestinal metabolism, gut-related symptoms may be alleviated, and hopefully, gut microbiota structure may be reconstructed.
SCFAs are mainly derived from the fermentation of indigestible carbohydrates by gut bacteria. SCFAs are signaling molecules that mediate the interaction between diet, gut microbiota, and the host, playing important roles in regulating immunity, metabolism, and the endocrine system. A meta-analysis reported a decrease in acetate, valerate, and total SCFAs in UC patients compared with healthy controls. In addition, a decrease in acetate, butyrate, and valerate was also observed in CD patients. The change in certain SCFA concentrations might have associations with disease activity[28]. These findings provide a potential therapeutic option for IBD by regulating SCFA metabolism.
SCFA enemas were used for UC treatment more than 20 years ago[29,30]. There is no consistent conclusion regarding the efficacy of SCFAs, and the optimal dose and indication are still unknown. The response rate ranged from 33% to 70%, but most studies showed no significant difference between butyrate administration and placebo[31-35]. Recently, a RCT evaluated the effect of oral supplementation with exogenous butyrate on the gut microbiota of IBD patients. The results showed that SCFA-producing bacteria increased both in UC (Lachnospiraceae spp.) and CD patients (Butyricicoccus). However, the effect on clinical activity was not observed in both sodium-butyrate or placebo groups[36]. Similarly, in children and adolescents with newly diagnosed IBD, 12 wk of oral sodium butyrate showed no effectiveness in remission rate and disease activity[37]. The actual clinical efficacy of butyrate supplementation still needs further investigation.
Bile acid malabsorption (BAM) occurs in CD due to the involvement of the ileum and the possible requirement of small bowel resection (type I BAM)[38]. BAM-associated diarrhea is a typical symptom in CD patients and can be relieved by bile acid sequestrant (BAS). In a randomized, double-blind, placebo-controlled study, colesevelam treatment reduced liquid stools and improved diarrhea in CD patients[39]. Colesevelam also improved patients’ life quality in a CD cohort[40]. Further studies are needed to determine which group of patients are likely to respond to BAS treatment.
There is a proven bidirectional interaction between bile acids (BAs) and gut microbiota. Gut microbiota metabolizes BAs secreted into the gut, and in turn, BAs affect the growth and composition of gut microbiota. In IBD patients, abnormal gut microbial structure and reduced microbial enzymatic activity result in dysmetabolism in the gut, especially depleted synthesis of secondary BAs. Ursodeoxycholic acid (UDCA) is a secondary BA with both cytoprotective and anti-inflammation effects, and can also modify patients’ microbiome[41,42]. The supplementation of secondary BAs is a potential therapeutic approach to correct gut dysbiosis in IBD, but no clinical trials have yet been published[43]. Apart from direct supplementation, exclusive enteral nutrition, diet intervention, and FMT can also restore the abnormal gut microbial structure and secondary BA depletion[44-46].
FMT is a direct approach to restoring the intestinal environment. It has been recommended for the treatment of Clostridium difficile infection (CDI)[47]. Experience in a large-volume European FMT center showed the effectiveness and safety of FMT in IBD patients with recurrent CDI. Not only did the Clostridium difficile toxin turn negative, but IBD disease activity also improved[48]. Increasing evidence has driven the implementation of FMT for IBD treatment.
A recent systematic review and meta-analysis included six double-blind RCTs and reported a pooled clinical and endoscopic remission rate of 30.43% in the FMT group[49]. In 2015, the first RCT evaluating the efficacy of FMT for active UC found a significantly higher remission rate in the FMT group than in the placebo group, and there was no difference in adverse events. In addition, according to the results of 16S rRNA sequencing, the FMT group had a greater increase in microbial diversity[50]. In another RCT, no statistically significant difference in clinical and endoscopic remission was observed between FMT from healthy donors or autologous fecal microbiota[51].
Given that UC is a chronic disease with a high relapse rate, the long-term outcome of FMT requires attention. However, only pilot studies with a small sample size have been conducted with a long-term perspective. Sood et al[52] evaluated maintenance FMT (every eight weeks, for 48 wk) in UC patients in clinical remission. Maintenance of steroid-free clinical remission was achieved in 27/31 patients receiving FMT and in 20/30 receiving placebo. Endoscopic and histological remission rates were also significantly higher in the FMT group. In a study with a follow-up period of 24 mo after one FMT treatment, one of nine FMT responders relapsed after six months and five maintained clinical and mucosal remission at month 24[53]. More long-term trials with adequate powers are expected to evaluate the efficacy, safety, changes in the gut environment, and effect on life quality.
Compared to UC, there is limited evidence regarding FMT in CD. Challenges include frequent involvement of the small bowel and the heterogeneity of CD phenotypes. Yang et al[54] included 27 CD patients who were randomized to receive FMT by either gastroscopy or colonoscopy. Two-thirds showed clinical remission two weeks after FMT and there was no difference between the two groups. No significant endoscopic response or remission was observed in either group. Another sham-controlled pilot trial showed a higher steroid-free clinical remission rate at week 10 in the FMT group but the difference was not statistically significant. Endoscopic activity was significantly decreased in the FMT group. This study used FMT after corticosteroid-induced clinical remission, suggesting the potential role of FMT in remission maintenance[55].
Apart from clinical and endoscopic remission, quality of life should also be addressed in IBD patients. FMT significantly improved the quality of life in IBD patients as measured by the IBD Questionnaire[56]. FMT also seems to be a cost-effective treatment. Compared with conventional therapy, FMT was 73% and 75% likely to be cost-effective from the healthcare and societal perspectives, respectively[57].
Furthermore, FMT may be a particularly effective, safe, and well-tolerated treatment for pediatric IBD. Early age of onset indicates long-term impairment and the cumulative burden of conventional treatment. Furthermore, the pediatric microbiome and immune system are highly dynamic and developing, which may be more responsive to FMT[58]. In a prospective trial, 21 IBD patients (median age 12 years) refractory to medical therapy received a single FMT. The clinical response rate at one and six months was 57% and 28%, respectively. Two CD patients were in remission at six months[59]. In another trial with a two-week FMT course, clinical response was observed in nine of ten patients, and five achieved remission[60]. Side effects in both trials were mild to moderate and self-limited[52,53]. Popov et al[61] interviewed pediatric patients and their parents regarding their experiences on receiving FMT. The efficacy and naturality of FMT were accepted, encouraging future trials involving children.
FMT for IBD treatment still has a long way from clinical trials to routine practice. Donor selection, preparation methodology, delivery route, frequency, and dose of FMT were different and controversial in previous studies. Intensive-dosing multi-donor FMT, anaerobically prepared pooled donor FMT, daily oral frozen encapsulated FMT, and oral lyophilized FMT have been assessed in RCTs, and the remission rate ranged from 27% to 53%[62-65].
Although few FMT-related serious adverse events were reported in previous studies, the FDA warned about the risk of multidrug-resistant organisms and theoretical COVID-19 transmission via FMT[66,67]. Therefore, the process from patient and donor selection, FMT preparation and administration, to long-term follow-up requires continuous standardization and optimization.
The intestinal epithelium, as the barrier between the inner and outer environment, is under rapid regeneration. It is also the interface between pathogenic factors of IBD including genetic factors, environmental factors, microbiota, and immune reactions. With the increased understanding of the epithelial barrier in IBD pathogenesis, the therapeutic targets have been upgraded to “mucosal healing”, which is related to long-term remission and a lower risk of receiving surgical therapy[68].
Glutamine is one of the major nutrients for the small-bowel mucosa which helps maintain mucosal integrity and prevent bacterial translocation. However, two earlier studies found no significant effect of glutamine supplementation on small intestinal permeability or disease activity in CD[69,70]. Benjamin et al[71] conducted a RCT in 2011 and reported that intestinal permeability and morphology in CD patients improved significantly in both the oral glutamine and active control groups.
To achieve mucosal healing, promoting epithelial regeneration and repair is a more direct method. Previous studies have proposed the use of growth factors, such as epidermal growth factor, and another notable area is stem cell therapy[72]. Autologous hematopoietic stem cell transplantation has been found to be feasible and effective in treatment-refractory CD, although with a higher security risk and higher requirements[73-76]. Furthermore, mesenchymal stroma cells (MSCs) have been successfully used for the treatment of complex perianal fistula in CD patients. In a long-term phase 3 trial comprising 212 patients, local injection of adipose-derived stem cells achieved a significantly greater proportion of closed external openings compared with placebo (56.3% vs 38.6%)[77]. Forty participants in this trial entered an extended follow-up to week 104. The results showed that clinical remission might be sustained in the long term and no apparent new safety signals were identified[78]. In the latest phase I-II pilot trial, local MSC injection may also help resolve CD strictures[79]. However, using MSCs to promote ulcer healing and repair the epithelial barrier has not been confirmed by clinical trials and more studies are awaited.
Reconstruction of intestinal homeostasis is an important therapeutic target. In order to identify relevant treatments, a deeper understanding of the pathogenesis is required.
Currently, the strategies targeting intestinal microecology are considered to be part of complementary and alternative medicines or in preclinical trials, which are still outside conventional treatment. The first step in promoting routine application is to select an appropriate population. According to previous studies, pediatric or less severe patients may benefit from gut microbiota-related interventions, and stem cell treatment is more beneficial for CD patients. Further trials are needed to confirm detailed indications, especially based on subtypes and severity of IBD. Another important topic is the exploration of optimal treatment recommendations, such as the preparation, delivery route, frequency, and dose of microbiota agents. For therapeutic response evaluation, long-term, dynamic and systematic observation results are required.
There is a high degree of heterogeneity between IBD patients and during disease courses. Therefore, the principle of individualized treatment should be emphasized in trial designs, pre-treatment assessment, treatment protocol designation and adjustment, and dynamic evaluation. Multi-omics sequencing technology may be a promising tool allowing for individualized assessment and prediction of therapeutic response in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sabelnikova EA, Russia; Sahin Y, Turkey; Wang Q S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Lu D, Huang Y, Kong Y, Tao T, Zhu X. Gut microecology: Why our microbes could be key to our health. Biomed Pharmacother. 2020;131:110784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2747] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 3. | Verstockt B, Bressler B, Martinez-Lozano H, McGovern D, Silverberg MS. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology. 2022;162:1370-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | McHardy IH, Goudarzi M, Tong M, Ruegger PM, Schwager E, Weger JR, Graeber TG, Sonnenburg JL, Horvath S, Huttenhower C, McGovern DP, Fornace AJ Jr, Borneman J, Braun J. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome. 2013;1:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, Casero D, Courtney H, Gonzalez A, Graeber TG, Hall AB, Lake K, Landers CJ, Mallick H, Plichta DR, Prasad M, Rahnavard G, Sauk J, Shungin D, Vázquez-Baeza Y, White RA 3rd; IBDMDB Investigators, Braun J, Denson LA, Jansson JK, Knight R, Kugathasan S, McGovern DPB, Petrosino JF, Stappenbeck TS, Winter HS, Clish CB, Franzosa EA, Vlamakis H, Xavier RJ, Huttenhower C. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1868] [Reference Citation Analysis (0)] |
| 6. | Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, Vatanen T, Hall AB, Mallick H, McIver LJ, Sauk JS, Wilson RG, Stevens BW, Scott JM, Pierce K, Deik AA, Bullock K, Imhann F, Porter JA, Zhernakova A, Fu J, Weersma RK, Wijmenga C, Clish CB, Vlamakis H, Huttenhower C, Xavier RJ. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4:293-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 888] [Cited by in RCA: 1228] [Article Influence: 204.7] [Reference Citation Analysis (0)] |
| 7. | Zhang XF, Guan XX, Tang YJ, Sun JF, Wang XK, Wang WD, Fan JM. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: a systematic review and meta-analysis. Eur J Nutr. 2021;60:2855-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 8. | Astó E, Méndez I, Audivert S, Farran-Codina A, Espadaler J. The Efficacy of Probiotics, Prebiotic Inulin-Type Fructans, and Synbiotics in Human Ulcerative Colitis: A Systematic Review and Meta-Analysis. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 10. | Su H, Kang Q, Wang H, Yin H, Duan L, Liu Y, Fan R. Effects of glucocorticoids combined with probiotics in treating Crohn's disease on inflammatory factors and intestinal microflora. Exp Ther Med. 2018;16:2999-3003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Huang M, Chen Z, Lang C, Chen J, Yang B, Xue L, Zhang Y. Efficacy of mesalazine in combination with bifid triple viable capsules on ulcerative colitis and the resultant effect on the inflammatory factors. Pak J Pharm Sci. 2018;31:2891-2895. [PubMed] |
| 12. | Fan H, Du J, Liu X, Zheng WW, Zhuang ZH, Wang CD, Gao R. Effects of pentasa-combined probiotics on the microflora structure and prognosis of patients with inflammatory bowel disease. Turk J Gastroenterol. 2019;30:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Hart AL, Kamm MA, Sanderson JD, Knight SC, Forbes A, Stagg AJ, Whelan K, Lindsay JO. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn's disease. Gut. 2011;60:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 14. | Casellas F, Borruel N, Torrejón A, Varela E, Antolin M, Guarner F, Malagelada JR. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Joossens M, De Preter V, Ballet V, Verbeke K, Rutgeerts P, Vermeire S. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn's disease: results from a double-blinded randomised controlled trial. Gut. 2012;61:958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | De Preter V, Joossens M, Ballet V, Shkedy Z, Rutgeerts P, Vermeire S, Verbeke Phd K. Metabolic profiling of the impact of oligofructose-enriched inulin in Crohn's disease patients: a double-blinded randomized controlled trial. Clin Transl Gastroenterol. 2013;4:e30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Wilson B, Eyice Ö, Koumoutsos I, Lomer MC, Irving PM, Lindsay JO, Whelan K. Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Amiriani T, Rajabli N, Faghani M, Besharat S, Roshandel G, Akhavan Tabib A, Joshaghani H. Effect of Lactocare® Synbiotic on Disease Severity in Ulcerative Colitis: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Middle East J Dig Dis. 2020;12:27-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Kamarlı Altun H, Akal Yıldız E, Akın M. Effects of synbiotic therapy in mild-to-moderately active ulcerative colitis: A randomized placebo-controlled study. Turk J Gastroenterol. 2019;30:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Steed H, Macfarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV, Cummings JH, Macfarlane S. Clinical trial: the microbiological and immunological effects of synbiotic consumption - a randomized double-blind placebo-controlled study in active Crohn's disease. Aliment Pharmacol Ther. 2010;32:872-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, Goldin B, Hartigan L, Kugathasan S, Levy J, Murray KF, Oliva-Hemker M, Rosh JR, Tolia V, Zholudev A, Vanderhoof JA, Hibberd PL. A randomized, double-blind trial of Lactobacillus GG vs placebo in addition to standard maintenance therapy for children with Crohn's disease. Inflamm Bowel Dis. 2005;11:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60 Suppl 2:S129-S134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 23. | Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 839] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 24. | Farina C, Arosio M, Mangia M, Moioli F. Lactobacillus casei subsp. rhamnosus sepsis in a patient with ulcerative colitis. J Clin Gastroenterol. 2001;33:251-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Vahabnezhad E, Mochon AB, Wozniak LJ, Ziring DA. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J Clin Gastroenterol. 2013;47:437-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Meini S, Laureano R, Fani L, Tascini C, Galano A, Antonelli A, Rossolini GM. Breakthrough Lactobacillus rhamnosus GG bacteremia associated with probiotic use in an adult patient with severe active ulcerative colitis: case report and review of the literature. Infection. 2015;43:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Xu X, Ocansey DKW, Hang S, Wang B, Amoah S, Yi C, Zhang X, Liu L, Mao F. The gut metagenomics and metabolomics signature in patients with inflammatory bowel disease. Gut Pathog. 2022;14:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 28. | Zhuang X, Li T, Li M, Huang S, Qiu Y, Feng R, Zhang S, Chen M, Xiong L, Zeng Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1751-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 29. | Steinhart AH, Brzezinski A, Baker JP. Treatment of refractory ulcerative proctosigmoiditis with butyrate enemas. Am J Gastroenterol. 1994;89:179-183. [PubMed] |
| 30. | Vernia P, Marcheggiano A, Caprilli R, Frieri G, Corrao G, Valpiani D, Di Paolo MC, Paoluzi P, Torsoli A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment Pharmacol Ther. 1995;9:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 159] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Patz J, Jacobsohn WZ, Gottschalk-Sabag S, Zeides S, Braverman DZ. Treatment of refractory distal ulcerative colitis with short chain fatty acid enemas. Am J Gastroenterol. 1996;91:731-734. [PubMed] |
| 32. | Scheppach W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig Dis Sci. 1996;41:2254-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 134] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Steinhart AH, Hiruki T, Brzezinski A, Baker JP. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther. 1996;10:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Breuer RI, Soergel KH, Lashner BA, Christ ML, Hanauer SB, Vanagunas A, Harig JM, Keshavarzian A, Robinson M, Sellin JH, Weinberg D, Vidican DE, Flemal KL, Rademaker AW. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997;40:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Jamka M, Kokot M, Kaczmarek N, Bermagambetova S, Nowak JK, Walkowiak J. The Effect of Sodium Butyrate Enemas Compared with Placebo on Disease Activity, Endoscopic Scores, and Histological and Inflammatory Parameters in Inflammatory Bowel Diseases: A Systematic Review of Randomised Controlled Trials. Complement Med Res. 2021;28:344-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Facchin S, Vitulo N, Calgaro M, Buda A, Romualdi C, Pohl D, Perini B, Lorenzon G, Marinelli C, D'Incà R, Sturniolo GC, Savarino EV. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol Motil. 2020;32:e13914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 37. | Pietrzak A, Banasiuk M, Szczepanik M, Borys-Iwanicka A, Pytrus T, Walkowiak J, Banaszkiewicz A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases-Randomized Placebo-Controlled Multicenter Trial. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 38. | Fromm H, Malavolti M. Bile acid-induced diarrhoea. Clin Gastroenterol. 1986;15:567-582. [PubMed] |
| 39. | Beigel F, Teich N, Howaldt S, Lammert F, Maul J, Breiteneicher S, Rust C, Göke B, Brand S, Ochsenkühn T. Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn's disease: a randomized, double-blind, placebo-controlled study. J Crohns Colitis. 2014;8:1471-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Kumar A, Galbraith N, Al-Hassi HO, Jain M, Phipps O, Butterworth J, Steed H, McLaughlin J, Brookes MJ. The impact of treatment with bile acid sequestrants on quality of life in patients with bile acid diarrhoea. BMC Gastroenterol. 2022;22:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 41. | Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, Zheng Y, Longman RS, Rastinejad F, Devlin AS, Krout MR, Fischbach MA, Littman DR, Huh JR. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 914] [Article Influence: 152.3] [Reference Citation Analysis (0)] |
| 42. | Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C, Kasper DL. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature. 2020;577:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 701] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 43. | Pearson T, Caporaso JG, Yellowhair M, Bokulich NA, Padi M, Roe DJ, Wertheim BC, Linhart M, Martinez JA, Bilagody C, Hornstra H, Alberts DS, Lance P, Thompson PA. Effects of ursodeoxycholic acid on the gut microbiome and colorectal adenoma development. Cancer Med. 2019;8:617-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | Diederen K, Li JV, Donachie GE, de Meij TG, de Waart DR, Hakvoort TBM, Kindermann A, Wagner J, Auyeung V, Te Velde AA, Heinsbroek SEM, Benninga MA, Kinross J, Walker AW, de Jonge WJ, Seppen J. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn's disease. Sci Rep. 2020;10:18879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 45. | Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC, Paramsothy R, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Lin E, Borody TJ, Wilkins MR, Colombel JF, Mitchell HM, Kaakoush NO. Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis. Gastroenterology. 2019;156:1440-1454.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 46. | Wang S, Martins R, Sullivan MC, Friedman ES, Misic AM, El-Fahmawi A, De Martinis ECP, O'Brien K, Chen Y, Bradley C, Zhang G, Berry ASF, Hunter CA, Baldassano RN, Rondeau MP, Beiting DP. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome. 2019;7:126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 47. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A; European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 784] [Article Influence: 98.0] [Reference Citation Analysis (1)] |
| 48. | Ianiro G, Bibbò S, Porcari S, Settanni CR, Giambò F, Curta AR, Quaranta G, Scaldaferri F, Masucci L, Sanguinetti M, Gasbarrini A, Cammarota G. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: experience of a large-volume European FMT center. Gut Microbes. 2021;13:1994834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 49. | El Hage Chehade N, Ghoneim S, Shah S, Chahine A, Mourad FH, Francis FF, Binion DG, Farraye FA, Hashash JG. Efficacy of Fecal Microbiota Transplantation in the Treatment of Active Ulcerative Colitis: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Inflamm Bowel Dis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 50. | Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102-109.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1087] [Article Influence: 108.7] [Reference Citation Analysis (1)] |
| 51. | Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, Löwenberg M, van den Brink GR, Mathus-Vliegen EM, de Vos WM, Zoetendal EG, D'Haens GR, Ponsioen CY. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. 2015;149:110-118.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 684] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 52. | Sood A, Mahajan R, Singh A, Midha V, Mehta V, Narang V, Singh T, Singh Pannu A. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients With Ulcerative Colitis: A Pilot Study. J Crohns Colitis. 2019;13:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 53. | Fang H, Fu L, Li X, Lu C, Su Y, Xiong K, Zhang L. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study. Microb Cell Fact. 2021;20:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 54. | Yang Z, Bu C, Yuan W, Shen Z, Quan Y, Wu S, Zhu C, Wang X. Fecal Microbiota Transplant via Endoscopic Delivering Through Small Intestine and Colon: No Difference for Crohn's Disease. Dig Dis Sci. 2020;65:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 55. | Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, Bourrier A, Le Gall G, Lalande V, De Rougemont A, Kirchgesner J, Daguenel A, Cachanado M, Rousseau A, Drouet É, Rosenzwajg M, Hagege H, Dray X, Klatzman D, Marteau P; Saint-Antoine IBD Network, Beaugerie L, Simon T. Fecal microbiota transplantation to maintain remission in Crohn's disease: a pilot randomized controlled study. Microbiome. 2020;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (1)] |
| 56. | Wei Y, Zhu W, Gong J, Guo D, Gu L, Li N, Li J. Fecal Microbiota Transplantation Improves the Quality of Life in Patients with Inflammatory Bowel Disease. Gastroenterol Res Pract. 2015;2015:517597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Zhang T, Xiang J, Cui B, He Z, Li P, Chen H, Xu L, Ji G, Nie Y, Wu K, Fan D, Huang G, Bai J, Zhang F. Cost-effectiveness analysis of fecal microbiota transplantation for inflammatory bowel disease. Oncotarget. 2017;8:88894-88903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr Res. 2014;76:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 59. | Goyal A, Yeh A, Bush BR, Firek BA, Siebold LM, Rogers MB, Kufen AD, Morowitz MJ. Safety, Clinical Response, and Microbiome Findings Following Fecal Microbiota Transplant in Children With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24:410-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 60. | Karolewska-Bochenek K, Grzesiowski P, Banaszkiewicz A, Gawronska A, Kotowska M, Dziekiewicz M, Albrecht P, Radzikowski A, Lazowska-Przeorek I. A Two-Week Fecal Microbiota Transplantation Course in Pediatric Patients with Inflammatory Bowel Disease. Adv Exp Med Biol. 2018;1047:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Popov J, Hartung E, Hill L, Chauhan U, Pai N. Pediatric Patient and Parent Perceptions of Fecal Microbiota Transplantation for the Treatment of Ulcerative Colitis. J Pediatr Gastroenterol Nutr. 2021;73:684-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Paramsothy R, Xuan W, Lin E, Mitchell HM, Borody TJ. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 876] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 63. | Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C, Rosewarne CP, Bickley C, Peters C, Schoeman MN, Conlon MA, Roberts-Thomson IC, Andrews JM. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2019;321:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 621] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 64. | Crothers JW, Chu ND, Nguyen LTT, Phillips M, Collins C, Fortner K, Del Rio-Guerra R, Lavoie B, Callas P, Velez M, Cohn A, Elliott RJ, Wong WF, Vo E, Wilcox R, Smith M, Kassam Z, Budd R, Alm EJ, Mawe GM, Moses PL. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 2021;21:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 65. | Haifer C, Paramsothy S, Kaakoush NO, Saikal A, Ghaly S, Yang T, Luu LDW, Borody TJ, Leong RW. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2022;7:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 177] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 66. | DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl J Med. 2019;381:2043-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 834] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 67. | Nicholson MR, Hourigan SK, Conrad M, Goyal A, Jensen K, Kelsen J, Kennedy M, Weatherly M, Kahn SA. Current Challenges in Fecal Microbiota Transplantation for Clostridioides difficile Infection in Children. Am J Gastroenterol. 2021;116:1954-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 664] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 69. | Den Hond E, Hiele M, Peeters M, Ghoos Y, Rutgeerts P. Effect of long-term oral glutamine supplements on small intestinal permeability in patients with Crohn's disease. JPEN J Parenter Enteral Nutr. 1999;23:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn's disease. J Pediatr Gastroenterol Nutr. 2000;30:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Benjamin J, Makharia G, Ahuja V, Anand Rajan KD, Kalaivani M, Gupta SD, Joshi YK. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn's disease: a randomized controlled trial. Dig Dis Sci. 2012;57:1000-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 73. | Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N, Kletzel M, Whitington PF, Burt RK. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 74. | Jauregui-Amezaga A, Rovira M, Marín P, Salas A, Pinó-Donnay S, Feu F, Elizalde JI, Fernández-Avilés F, Martínez C, Gutiérrez G, Rosiñol L, Carreras E, Urbano A, Lozano M, Cid J, Suárez-Lledó M, Mensa J, Rimola J, Rodríguez S, Masamunt MC, Comas D, Ruíz I, Ramírez-Morros A, Gallego M, Ordás I, Panés J, Ricart E. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn's disease. Gut. 2016;65:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Lindsay JO, Allez M, Clark M, Labopin M, Ricart E, Rogler G, Rovira M, Satsangi J, Farge D, Hawkey CJ; ASTIC trial group; European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party; European Crohn's and Colitis Organisation. Autologous stem-cell transplantation in treatment-refractory Crohn's disease: an analysis of pooled data from the ASTIC trial. Lancet Gastroenterol Hepatol. 2017;2:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 76. | López-García A, Rovira M, Jauregui-Amezaga A, Marín P, Barastegui R, Salas A, Ribas V, Feu F, Elizalde JI, Fernández-Avilés F, Martínez C, Gutiérrez G, Rosiñol L, Carreras E, Urbano A, Lozano M, Cid J, Suárez-Lledó M, Masamunt MC, Comas D, Giner A, Gallego M, Alfaro I, Ordás I, Panés J, Ricart E. Autologous Haematopoietic Stem Cell Transplantation for Refractory Crohn's Disease: Efficacy in a Single-Centre Cohort. J Crohns Colitis. 2017;11:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 77. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Diez MC, Tagarro I, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2018;154:1334-1342.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 329] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 78. | Garcia-Olmo D, Gilaberte I, Binek M, D Hoore AJL, Lindner D, Selvaggi F, Spinelli A, Panés J. Follow-up Study to Evaluate the Long-term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn's Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis Colon Rectum. 2022;65:713-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 79. | Vieujean S, Loly JP, Boutaffala L, Meunier P, Reenaers C, Briquet A, Lechanteur C, Baudoux E, Beguin Y, Louis E. Mesenchymal Stem Cell Injection in Crohn's Disease Strictures: A Phase I-II Clinical Study. J Crohns Colitis. 2022;16:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |