Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.193
Peer-review started: September 25, 2022
First decision: December 13, 2022
Revised: December 20, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 6, 2023
Processing time: 102 Days and 2.1 Hours
Heterotopic ossification (HO) refers to the formation of new bone in non-skeletal tissues such as muscles, tendons or other soft tissues. Severe muscle and soft tissue injury often lead to the formation of HO. However, anterior HO of the ankle is rarely reported.
We report a patient with massive HO in front of the ankle joint for 23 years. In 1998, the patient was injured by a falling object on the right lower extremity, which gradually formed a massive heterotopic bone change in the right calf and dorsum of the foot. The patient did not develop gradual ankle function limitations until nearly 36 mo ago, and underwent resection of HO. Even after 23 years and resection of HO, the ankle joint was still able to move.
It is recommended that the orthopedist should be aware of HO and distinguish it from bone tumor.
Core Tip: Here we report an adult patient with 23 years of heterotopic ossification at the ankle. After the ankle was injured by a falling object (red brick), a huge bony mass gradually appeared from the ankle to the middle of the calf. The ankle joint remained 30 degree move range after surgery and the range maintained 30 degree in his 1 year follow up, and the radiology showed that there is no recurrence of heterotopic ossification.
- Citation: Xu Z, Rao ZZ, Tang ZW, Song ZQ, Zeng M, Gong HL, Wen J. Post-traumatic heterotopic ossification in front of the ankle joint for 23 years: A case report and review of literature. World J Clin Cases 2023; 11(1): 193-200
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/193.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.193
Heterotopic ossification (HO) refers to the formation of new bone in non-skeletal tissues such as muscles, tendons or other soft tissues. HO was first described by Patin in 1692[1]. Subsequently, many scholars have carried out in-depth research on HO. It is generally believed that HO can be divided into two categories[2]: Congenital HO and acquired HO. The former is less common, and mainly caused by gene mutation; there are mainly two diseases: Fibrodysplasia ossificans progressiva (ACVR1 gene mutation) and progressive osseous hyperplasia (GNAS1 gene heterozygous inactivating mutation)[3]. The latter is more common and related to trauma, often due to neurological trauma such as brain and spinal cord injury, severe soft tissue trauma such as burn, explosion injury, musculoskeletal trauma such as fracture, joint dislocation, and surgical trauma such as hip replacement, knee replacement, etc.[1,4,5].
Here we report an adult patient with 23 years of HO at the ankle. Following injury to the ankle due to a falling object (red brick), a huge bony mass gradually appeared from the ankle to the middle of the calf.
A 67-year-old male patient was admitted in our department due to a mass in front of the ankle joint for 23 years.
In 1998, his right foot was accidentally injured by a brick that fell from a height. He developed pain and swelling in the front of his right foot and calf. He went to a local hospital and was diagnosed with a soft tissue infection. The patient developed a hard mass on the right ankle 3 mo after the injury, about the size of an egg, with no limited ankle joint movement. He then went to the local hospital for treatment and surgery was suggested. The patient did not undergo surgery as the mass did not cause obvious pain and did not affect his joint function.
Three years ago, the patient experienced an accidental sprain of the right ankle with ankle immobility, and was admitted to our department for surgical treatment.
The patient had no remarkable medical history, no history of hypertension, diabetes, hepatitis, tuberculosis, drugs, or food allergies.
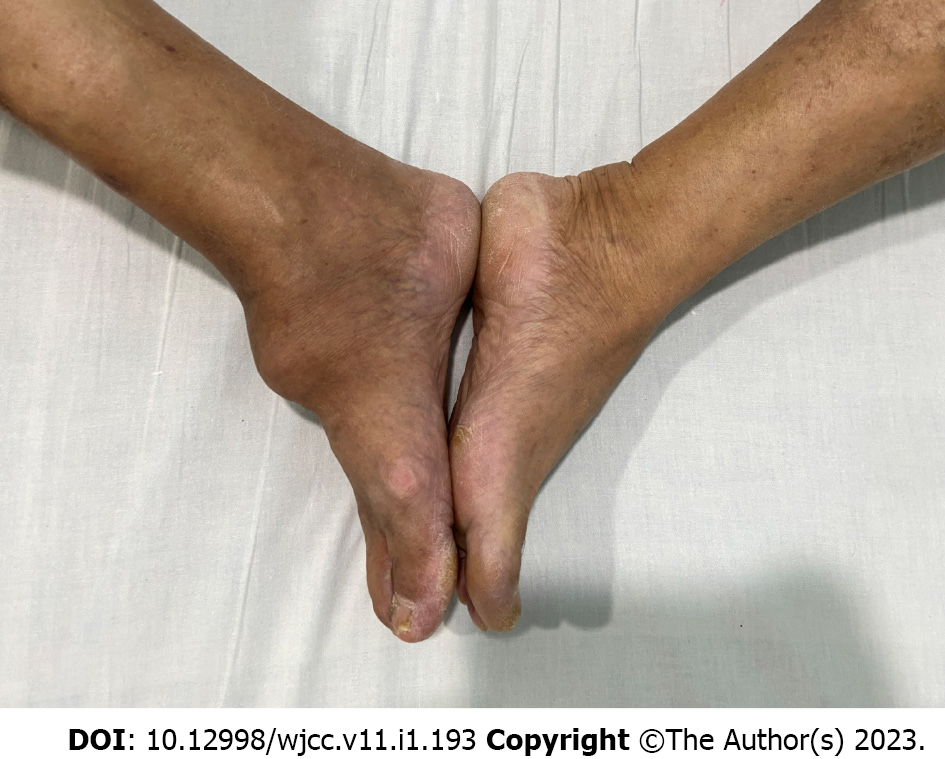
A lump was seen in front of the dorsum of the right foot and the middle and lower part of the calf, which was obvious on the right dorsum; the right calf was slightly swollen, the skin was slightly translucent, there was no obvious tenderness, and the skin temperature was not elevated. The mass on the right dorsum was approximately 6 cm × 7 cmin size (Figure 1) and non-movable, with a hard texture, unclear boundary, unsmooth surface, no obvious tenderness, no obvious boundary with surrounding tissues, and there was no right ankle joint movement. The blood supply, sensation and movement of the extremity were good.
Routine blood tests showed that C-reactive protein, erythrocyte sedimentation rate, alkaline phosphatase, blood sugar, and blood trioxypurine were normal.
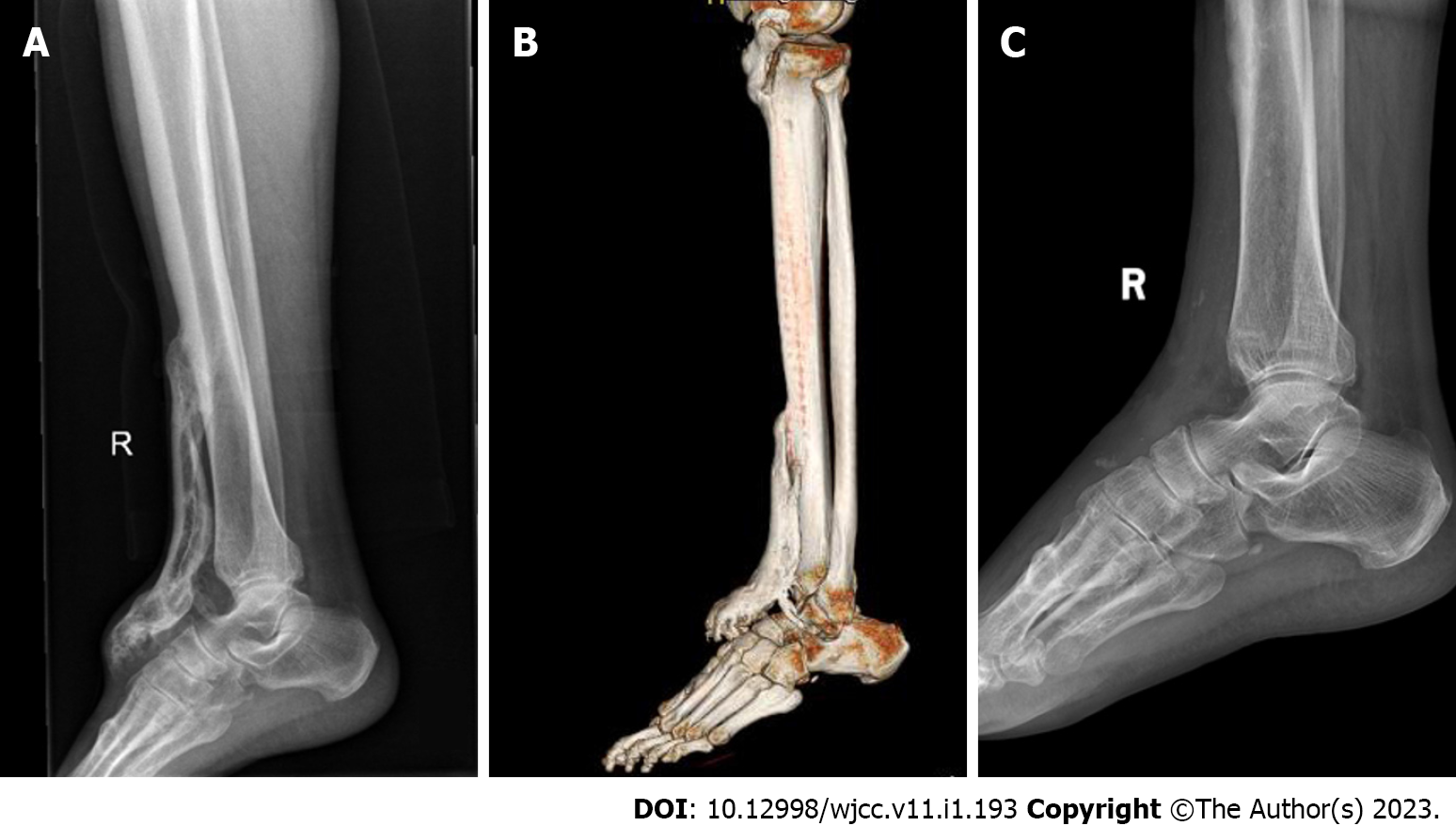
X-ray (Figure 2A) showed a bone mass in front of the right tibia. Computed tomography (CT) (Figure 2B) showed multiple patchy bone masses with uneven bone density in front of the upper segment of the right tibia and the dorsum of the right foot. The edge of the right ankle joint was osteosclerotic with adequate joint space.
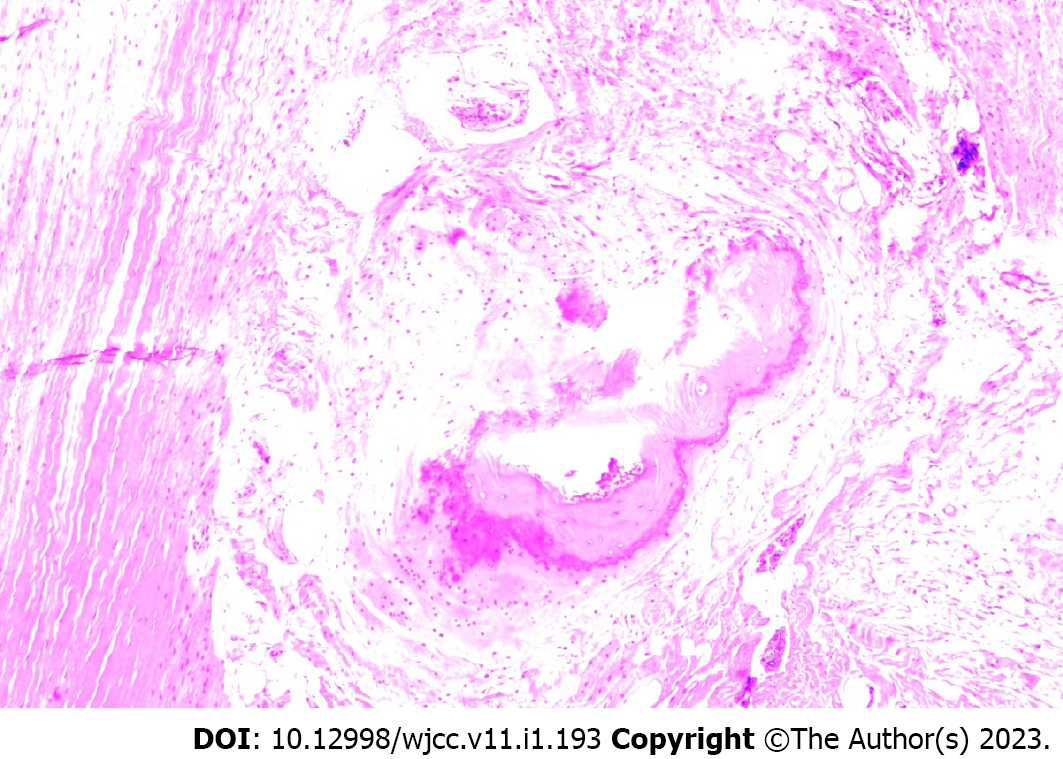
Pathological examination of fibrous connective tissue and bone tissue from the mass on the right dorsum of the foot and the anterior tibia of the calf was performed (Figure 3). Ossification was seen in the muscle tissue in some areas, which was consistent with the changes of myositis ossificans when combined with the clinical and imaging findings. The final diagnosis was HO.
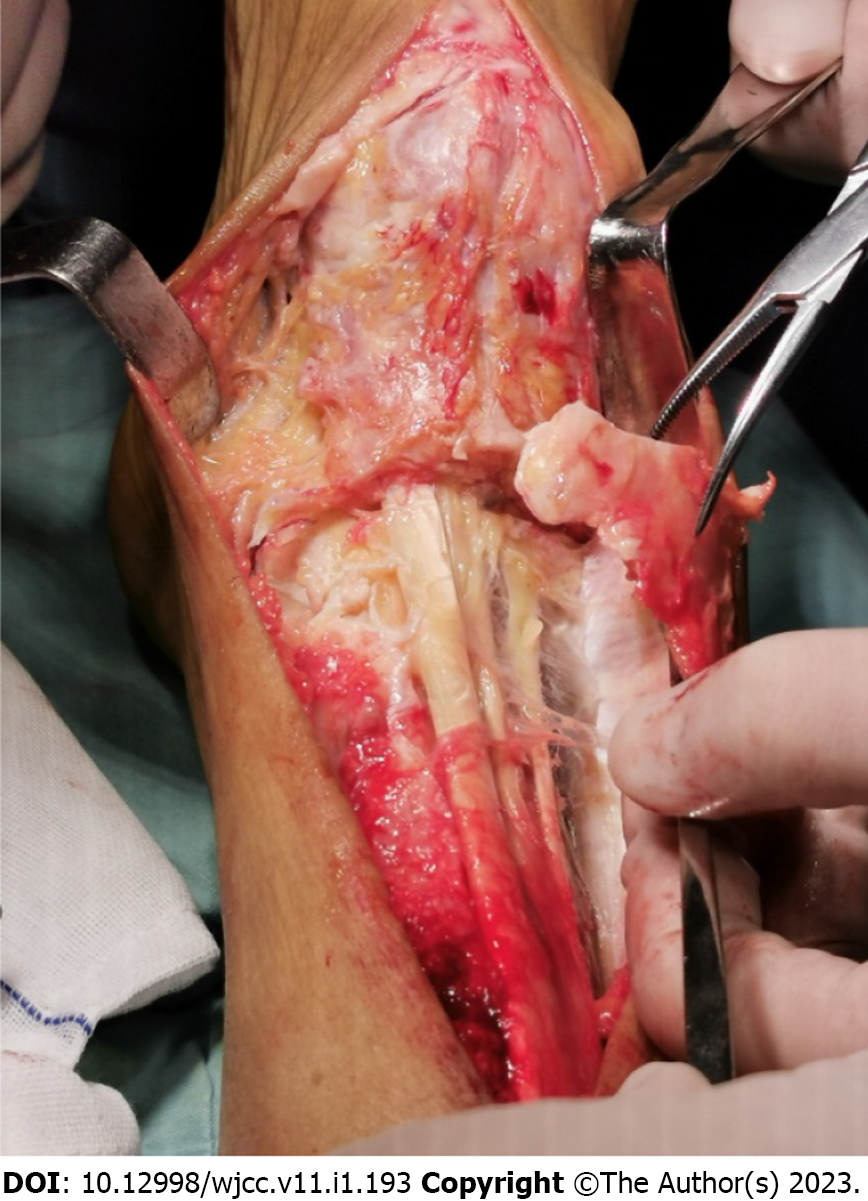
The patient underwent surgery, and the following procedures were performed: The patient was placed in the supine position on the operating table. After general anesthesia induction and routine disinfection, a sterile drape was placed. When the anterior medial bulge of the right ankle joint was seen, a longitudinal incision of approximately 6 cm was made, the skin and subcutaneous tissue were incised, and the mass was dissociated. The mass was bony, hard and could not be pushed. It started from the middle and lower part of the tibia down to the midfoot. There was a small gap between some parts and the surrounding bone. Ankle joint activity was absent. The bone was first split horizontally. The bone mass surrounded the extensor muscle. The bone mass in front of the ankle joint was excised. The ankle joint was cleaned, the adhering tendons were released, the wound was closed, and the front of the ankle joint was covered with VAC device. The excised mass was sent for medical examination. The operation was successful, and anesthesia was satisfactory. There was little intraoperative bleeding and blood transfusion was not required.
The patient wore an Ankle Foot Othosis for 4 wk to avoid recurrence of HO, and was allowed to stand and walk 2 wk after surgery. The ankle joint reached a 30 degree range of motion after surgery and this range was maintained during the first year of follow-up. The AOFAS-AH score was 85 after surgery and was maintained at 89 during the first year of follow-up. Radiology showed that there was no recurrence of HO (Figure 2C).
Heterotopic ossification refers to the formation of new bone in areas where the bone does not usually appear, such as muscle and soft tissue. It is different from calcification caused by metabolic diseases such as hypercalcemia and malnutrition in tumors, which forms mature lamellar new bone. Histologically, it is indistinguishable from the callus formed by fracture healing. Post-traumatic HO is mainly formed by endochondral osteogenesis. Its formation includes inflammation, chondrogenesis, osteogenesis and ectopic bone maturation[6].
Although the cause of post-traumatic HO is still unclear, it is currently believed that three conditions are necessary for the formation of HO: osteogenic precursor cells, osteogenic-inducing factors, and local promotion of the osteogenic microenvironment. Many multifunctional cells recruit and aggregate at the wound site, such as common mesenchymal stem cells, endothelial cells of mesenchymal stem cells, residual muscle quiescent stem cells (satellite cells) or myogenic progenitor cells, circulating precursor osteoblasts, etc.[7-12]. In the process of HO, more and more scholars believe that the local microenvironment is crucial[13,14]. There are two main types of local microenvironment: (1) Inflammatory environment: Many inflammatory cells accumulate in the wound[15], forming an inflammatory response. Current studies have shown that the inflammatory environment is important in forming traumatic HO. Kraft et al[16] summarized the role of various immune cells in forming HO, such as neutrophils, macrophages, mast cells, T and B lymphocytes and natural killer cells. Huang et al[6] and others believed that in traumatic tissue, monocyte-macrophages are polarized into M1-M2 macrophages, making mesenchymal cells secreted by the expression of Oncostatin M, transforming growth factor beta1 (TGF-β1), bone morphogenetic protein (BMP), ActA, surfactant protein, vascular endothelial growth factor (VEGF) and other factors. Stem cells differentiate towards osteogenesis through pathways such as the TGF-β1-Smad2/3 and BMP-Smad1/5/8 pathways, eventually forming HO. In addition, a large number of inflammatory markers are closely related to the formation of HO, such as interleukin (IL)-3, IL-6, IL-10, IL-12p70, effluent IL-3 and effluent IL-13, MCP-1, effluent IL-10 and MIP-1-alpha[17-19]; and (2) Hypoxic environment: Tissue hypoxia usually occurs simultaneously after tissue injury. The hypoxic microenvironment is mainly due to the release of inflammatory mediators after severe trauma, leading to the blockage of constrictive blood vessels, resulting in the disorder of tissue blood circulation. Huang et al[20] and others believed that the existence of a hypoxic microenvironment leads to the activation of HIF-1α, which up-regulates the expression of bone morphogenetic proteins and VEGF and down-regulates the expression of neurothelin-1 (NRP-1), resulting in angiogenesis and the proliferation and differentiation of chondrocytes and osteoblasts, eventually forming HO.
Nonetheless, HO is an extremely complex process. Trauma is insufficient to induce HO; other factors such as genetic mutations and neurogenic injury are also required. For example, the induction of neurogenic HO requires traumatic brain injury or spinal cord injury and muscle damage. Pure blast injury cannot cause HO, but severe HO can be induced when combined with a fracture or wound infection, such as a bacterial infection[6]. In addition, severe trauma can often induce HO[21]. It is well known that the accumulation of stem cells and the infiltration of inflammatory cells such as macrophages after tissue injury are beneficial in tissue repair[22]. Recent studies have also suggested that depletion of monocytes/macrophages can lead to the differentiation of recruited endothelial cells towards chondrogenesis[23]. Some scholars believe that severe trauma leads to a severe local and systemic inflammatory response, which makes the repair process out of balance and activates the critical threshold of the BMP signaling pathway to form HO[24]. Therefore, we believe that during the repair of the severe wound on the right calf in this case, due to changes in the local microenvironment, such as severe hypoxia, massive inflammatory cell infiltration, and the consumption of a large number of monocyte-macrophages, the stem cells tend to differentiate into the osteoblast and cartilage, and ultimately form a huge area of HO.
In the early inflammatory stage of the disease course, HO manifests as local pain, swelling, elevated skin temperature, and even fever and erythema, which is similar to cellulitis, thrombophlebitis, and osteomyelitis[1]. As the bone tissue matures in advanced stages, the swelling becomes more localized and firm and may limit movement when close to the joint[15]. The diagnosis of HO in its early stages is difficult due to nonspecific signs and symptoms. This case was misdiagnosed as a soft tissue infection at an early stage, and anti-infective treatment was given, and a huge bony mass gradually formed on the anterior tibia and muscle surface of the right calf and in the subcutaneous tissue of the dorsum of the foot, which eventually led to ankle stiffness. Therefore, we believe that considering the possibility of HO in patients with severe soft tissue trauma, oral non-steroidal anti-inflammatory drugs can be given as an early preventive treatment.
It is currently considered that plain X-ray and three-dimensional CT examination are the gold standards for diagnosing HO, but plain X-ray cannot detect calcification for at least 6 wk after trauma. Although 3D CT is the most sensitive method for detecting HO, it also provides the earliest detection at 2.5 wk after trauma[25]. Bartlett et al[26] used a rabbit HO model for early diagnosis and treatment and found that changes in prostaglandins were earlier than bone formation, for example, prostaglandin E2 (PGE2) secretion was significantly increased, reaching a peak at 10 d. Schurch et al[27] also found that increasing PGE2 in urine at 24 h suggested HO formation.
The most common sites of post-traumatic HO are the elbow joint in the upper extremity and the hip joint in the lower extremity, especially after total hip arthroplasty[5]. HO occurs relatively rarely at the ankle joint. More recently, as total ankle arthroplasty (TAA) has become a common surgical treatment option for end-stage ankle arthritis, HO at the ankle has been increasingly reported[14]. However, HO is most frequently reported posterior to the ankle joint and rarely anterior to the ankle joint[30]. Although HO is formed after TAA, it does not affect the joint function in patients, and most of them do not require reoperation[14,28]. In this case, a huge HO was formed in the front of the ankle, but it was not until the previous 3 years that the ankle joint movement was gradually limited. The lesion was removed during the operation as HO increased after the ankle sprain and formed a bony connection. Following excision, ankle function recovered. Moreover, during the operation, we found that the HO had wrapped the extensor digitorum tendon and formed a smooth surface with the tendon, which did not affect the free movement of the tendons such as the extensor hallucis longus tendon which formed a tunnel in the HO (Figure 4).
The malignant transformation of HO is rarely reported. In 1981, Eckardt et al[29] reported a 32-year-old patient with dermatitis. HO formed on the back of the left thigh when the patient was 8 years old. When the patient was 31 years old, the mass suddenly increased with pain, and the final pathological examination showed that the mass had transformed into a well-differentiated malignant osteosarcoma. Subsequently, in 1999, Aboulafia et al[30] reported a 44-year-old female patient who developed diffuse HO in the soft tissue of the forearm due to electrical burns, which transformed into osteosarcoma 10 years later. Although there are relatively few reports of malignant transformation of myositis ossificans, there are sporadic cases[31]. Although our patient had HO for 23 years, he had no symptoms of malignant transformation, and the postoperative pathological report showed that the mass had no malignant signs.
HO affecting joint function is an absolute indication for surgical resection. However, there was recurrence after surgery. Agarwal et al[32] and other studies have found that postoperative recurrence has nothing to do with unresected HO, not the enlargement of residual lesions, and the same mechanism as the original HO. In the current study, following HO resection, the ankle joint was still able to move, which indicated that the earlier the HO resection is performed, the better the joint function will be.
It is recommended that the orthopedist should be aware of HO and distinguish it from bone tumor.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Indino C, Italy S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Juarez JK, Wenke JC, Rivera JC. Treatments and Preventative Measures for Trauma-Induced Heterotopic Ossification: A Review. Clin Transl Sci. 2018;11:365-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Edwards DS, Clasper JC. Heterotopic ossification: a systematic review. J R Army Med Corps. 2015;161:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Edwards DS, Kuhn KM, Potter BK, Forsberg JA. Heterotopic Ossification: A Review of Current Understanding, Treatment, and Future. J Orthop Trauma. 2016;30 Suppl 3:S27-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Barfield WR, Holmes RE, Hartsock LA. Heterotopic Ossification in Trauma. Orthop Clin North Am 2017; 48: 35-46 . [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Huang Y, Wang X, Zhou D, Zhou W, Dai F, Lin H. Macrophages in heterotopic ossification: from mechanisms to therapy. NPJ Regen Med. 2021;6:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Kan C, Chen L, Hu Y, Ding N, Li Y, McGuire TL, Lu H, Kessler JA, Kan L. Gli1-labeled adult mesenchymal stem/progenitor cells and hedgehog signaling contribute to endochondral heterotopic ossification. Bone. 2018;109:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Medici D, Olsen BR. The role of endothelial-mesenchymal transition in heterotopic ossification. J Bone Miner Res. 2012;27:1619-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Piera-Velazquez S, Jimenez SA. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol Rev. 2019;99:1281-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 420] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 11. | Lemos DR, Eisner C, Hopkins CI, Rossi FMV. Skeletal muscle-resident MSCs and bone formation. Bone. 2015;80:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Oishi T, Uezumi A, Kanaji A, Yamamoto N, Yamaguchi A, Yamada H, Tsuchida K. Osteogenic differentiation capacity of human skeletal muscle-derived progenitor cells. PLoS One. 2013;8:e56641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Kan C, Chen L, Hu Y, Lu H, Li Y, Kessler JA, Kan L. Microenvironmental factors that regulate mesenchymal stem cells: lessons learned from the study of heterotopic ossification. Histol Histopathol. 2017;32:977-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, Penton CM, Montanaro F, Roux S, Faucheux N, Grenier G. BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res. 2011;26:1166-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, Sono T, McCarthy E, Levi B, James AW. Heterotopic Ossification: A Comprehensive Review. JBMR Plus. 2019;3:e10172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 16. | Kraft CT, Agarwal S, Ranganathan K, Wong VW, Loder S, Li J, Delano MJ, Levi B. Trauma-induced heterotopic bone formation and the role of the immune system: A review. J Trauma Acute Care Surg. 2016;80:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res. 2014;472:2845-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Evans KN, Forsberg JA, Potter BK, Hawksworth JS, Brown TS, Andersen R, Dunne JR, Tadaki D, Elster EA. Inflammatory cytokine and chemokine expression is associated with heterotopic ossification in high-energy penetrating war injuries. J Orthop Trauma. 2012;26:e204-e213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Sung Hsieh HH, Chung MT, Allen RM, Ranganathan K, Habbouche J, Cholok D, Butts J, Kaura A, Tiruvannamalai-Annamalai R, Breuler C, Priest C, Loder SJ, Li J, Li S, Stegemann J, Kunkel SL, Levi B. Evaluation of Salivary Cytokines for Diagnosis of both Trauma-Induced and Genetic Heterotopic Ossification. Front Endocrinol (Lausanne). 2017;8:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Huang Y, Wang X, Lin H. The hypoxic microenvironment: a driving force for heterotopic ossification progression. Cell Commun Signal. 2020;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Scala P, Rehak L, Giudice V, Ciaglia E, Puca AA, Selleri C, Della Porta G, Maffulli N. Stem Cell and Macrophage Roles in Skeletal Muscle Regenerative Medicine. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Tirone M, Giovenzana A, Vallone A, Zordan P, Sormani M, Nicolosi PA, Meneveri R, Gigliotti CR, Spinelli AE, Bocciardi R, Ravazzolo R, Cifola I, Brunelli S. Severe Heterotopic Ossification in the Skeletal Muscle and Endothelial Cells Recruitment to Chondrogenesis Are Enhanced by Monocyte/Macrophage Depletion. Front Immunol. 2019;10:1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Li L, Tuan RS. Mechanism of traumatic heterotopic ossification: In search of injury-induced osteogenic factors. J Cell Mol Med. 2020;24:11046-11055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Mujtaba B, Taher A, Fiala MJ, Nassar S, Madewell JE, Hanafy AK, Aslam R. Heterotopic ossification: radiological and pathological review. Radiol Oncol. 2019;53:275-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Bartlett CS, Rapuano BE, Lorich DG, Wu T, Anderson RC, Tomin E, Hsu JF, Lane JM, Helfet DL. Early changes in prostaglandins precede bone formation in a rabbit model of heterotopic ossification. Bone. 2006;38:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Schurch B, Capaul M, Vallotton MB, Rossier AB. Prostaglandin E2 measurements: their value in the early diagnosis of heterotopic ossification in spinal cord injury patients. Arch Phys Med Rehabil. 1997;78:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Jung HG, Lee SH, Shin MH, Lee DO, Eom JS, Lee JS. Anterior Heterotopic Ossification at the Talar Neck After Total Ankle Arthroplasty. Foot Ankle Int. 2016;37:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Eckardt JJ, Ivins JC, Perry HO, Unni KK. Osteosarcoma arising in heterotopic ossification of dermatomyositis: case report and review of the literature. Cancer. 1981;48:1256-1261. [PubMed] [DOI] [Full Text] |
| 30. | Aboulafia AJ, Brooks F, Piratzky J, Weiss S. Osteosarcoma arising from heterotopic ossification after an electrical burn. A case report. J Bone Joint Surg Am. 1999;81:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Savant D, Kenan S, Kahn L. Extraskeletal osteosarcoma arising in myositis ossificans: a case report and review of the literature. Skeletal Radiol. 2017;46:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Agarwal S, Loder S, Cholok D, Li J, Breuler C, Drake J, Brownley C, Peterson J, Li S, Levi B. Surgical Excision of Heterotopic Ossification Leads to Re-Emergence of Mesenchymal Stem Cell Populations Responsible for Recurrence. Stem Cells Transl Med. 2017;6:799-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |