Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.143
Peer-review started: August 12, 2022
First decision: October 12, 2022
Revised: November 1, 2022
Accepted: December 9, 2022
Article in press: December 9, 2022
Published online: January 6, 2023
Processing time: 145 Days and 17.7 Hours
Thermal injuries on free transferred or replanted tissues resulting from loss of sensibility are an infrequent occurrence. They require immediate and appropriate management before they progress to an irreversible condition. Although negative pressure wound therapy (NPWT) can prevent wound progression by increasing microcirculation, the inappropriate application of NPWT on complication-threatened transferred and replanted tissues can induce an adverse effect.
A 48-year-old woman who underwent immediate breast reconstruction with a deep inferior epigastric artery perforator free flap. While applying a heating pad directly to the flap site, she sustained a deep second to third-degree contact burn over 30% of the transferred flap on postoperative 7 d. As the necrotic changes had progressed, we applied an NPWT dressing over the burned area after en-bloc debridement of the transferred tissues on postoperative 21 d. After 4 d of NPWT application, the exposed fatty tissues of the flap changed to dry and brown-colored necrotic tissues. Upon further debridement, we noted that the wound gradually reached total necrosis with a collapsed vascular pedicle of deep inferior epigastric artery.
Although NPWT has been shown to be successful for treating various wound types, the significant risk of NPWT application in short-lasting reconstructed flap wounds after thermal injury should be reminded.
Core Tip: Negative pressure wound therapy (NPWT) is the effective promotion of survival in free tissue transfer, but special attention is required in cases of burn injury over the transferred flap site. Microsurgeons should be cautious about location of the pedicle, pressure and mode when using NPWT at burned site over transferred free flap tissue.
- Citation: Lim S, Lee DY, Kim B, Yoon JS, Han YS, Eo S. Devastating complication of negative pressure wound therapy after deep inferior epigastric perforator free flap surgery: A case report. World J Clin Cases 2023; 11(1): 143-149
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/143.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.143
A significant complication of autologous breast reconstruction is immediate postoperative burn injury. This is caused by transient skin insensitivity and autonomic denervation at the flap site[1-5]. Transferred flap tissues are vulnerable to thermal injury even at low temperatures because of the inability to detect and dissipate heat[2,3].Non-hazardous heat sources, such as heating pads, sunlamps, and hot water bottles can cause thermal injury in the transferred flap tissues, which may lead to reconstructive failure[1,4]. Therefore, several precautions and treatment modalities have been proposed after postoperative burn injuries to address this critical drawback[6-9].
Negative pressure wound therapy (NPWT) is known as one of the most efficient dressing modalities[9,10]. Because its clinical application has been well documented for various types of wounds, including chronic infected wounds and ischemia-perfusion injuries[11], researchers have investigated whether the application of NPWT can be expanded to different flap surgeries[12-16]. Despite the successful results of NPWT applications, the safety of NPWT remains unclear[11]. Improper placement and high external negative pressure at the flap with compromised perfusion increases the possibility of flap failure by compressing the main pedicle and microvessels[11,17-19]. The purpose of this article was to provide a warning against the use of NPWT for treating thermal injury at the short-term free flap site in breast reconstruction, based on our notable case.
A 48-year-old woman underwent a skin-sparing mastectomy for ductal carcinoma of the right breast.
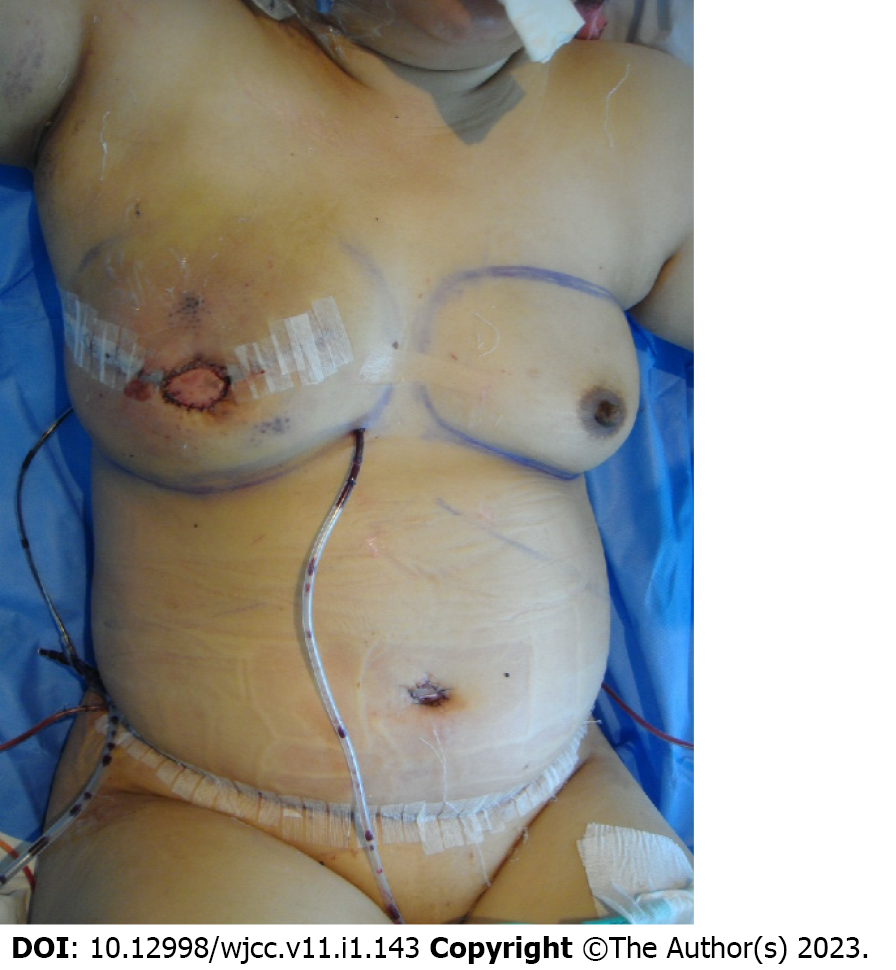
We immediately reconstructed the breast using a deep inferior epigastric artery perforator (DIEP) free flap from the lower abdomen. The pedicle of the recipient was the thoracodorsal artery and its venae comitantes, which are commonly used in breast reconstruction. The surgery was successfully performed, and the patient was on the usual course without any postoperative complications (Figure 1). However, on postoperative day 7, she suffered a deep second- to third-degree contact burn over 30 percent of the reconstructed right breast due to incorrect application of a heating pad at the flap site (Figure 2A).
Ductal carcinoma of the right breast.
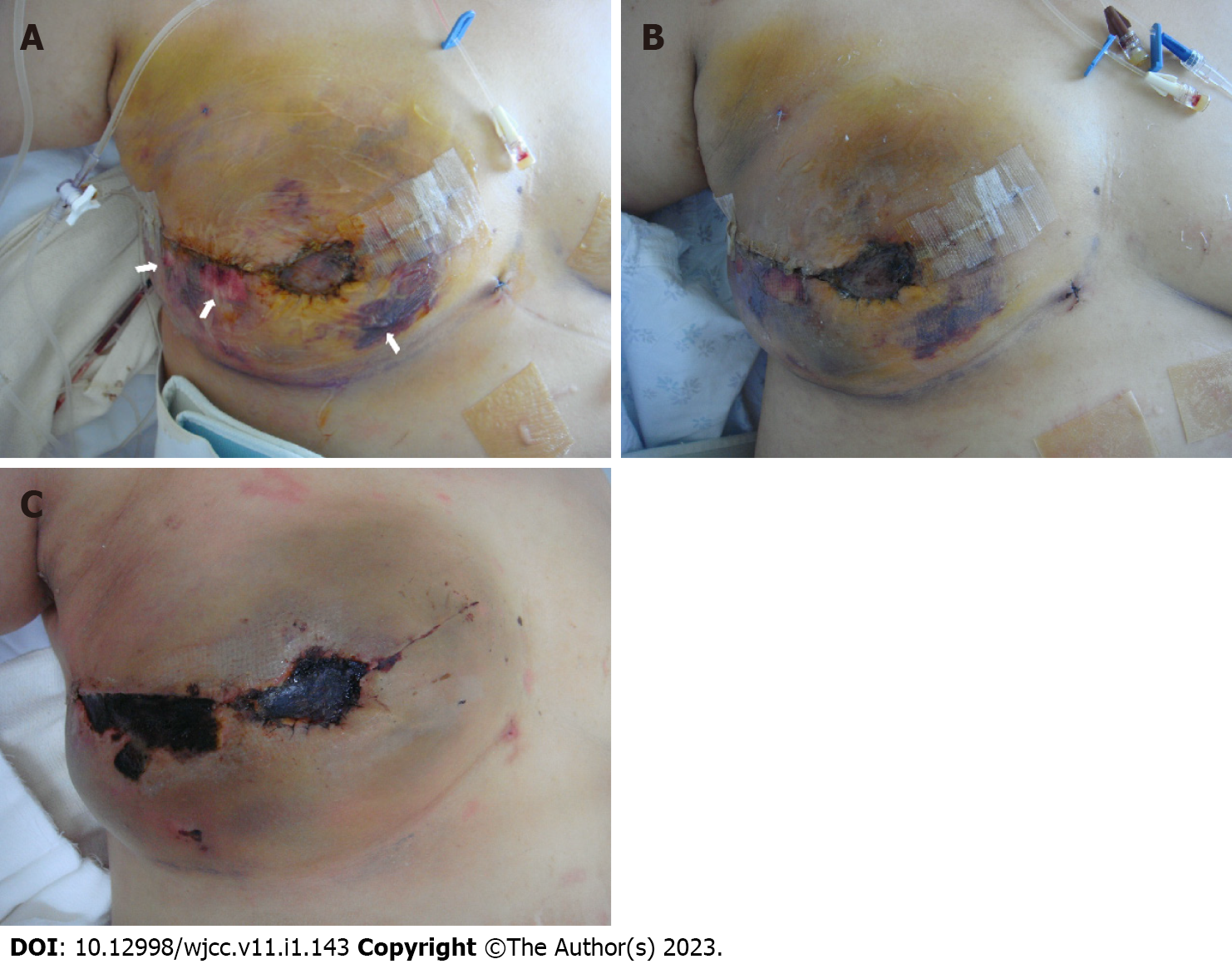
The burn wound was initially treated with a conventional dressing; however, the necrotic changes progressed with eschar formation (Figure 2). Therefore, we performed en bloc resection of the necrotic tissue at the 21st d postburn. During eschar resection, we confirmed flap survival by observing surviving glistening yellow adipose tissue in the subdermal layer (Figure 3A).
As the Doppler sound of the flap pedicle was also traceable, we applied a V.A.C. dressing (KCI International, San Antonio, TX, USA) to promote healing of the burn wounds. We sealed it entirely over the exposed area of the patient’s right breast under a pressure of -125 mmHg in continuous mode. However, after four days of V.A.C. application, the burn wound worsened, and the exposed adipose tissue changed to dry and brown-colored necrotic tissue (Figure 3B). Upon further debridement, we observed an obstructed vascular pedicle with multiple thrombi in the transferred DIEP flap tissue (Figure 3C). We also observed the presence of unviable adipose tissue. First, we performed daily dressing to demarcate the necrotic tissue.
The burned wound gradually reached total necrosis with a collapsed vascular pedicle of the DIEP; additionally, all of the necrotic tissue was removed with aggressive debridement.
The remnant defect was covered by bilateral advancement flap surgery.
No additional complications, such as necrotic changes, were found at the subsequent return visit (Figure 3D). This resulted in the same situation as flap failure.
Thermal injuries have been occasionally reported after replantation[3] and various free tissue transfers, including autologous breast reconstruction[1-5]. The majority of thermal injuries occur in the early postoperative period by the application of warming devices to increase flap viability[1],and debilitate dissipating heat through thermoregulatory responses, such as cutaneous vasodilation[4]. After thermal injury at the transferred flap site, progression of the wound must be prevented to avoid the devastating consequences of flap loss. Therefore, various studies have discussed the accurate assessment and appropriate treatment of burn wounds at the flap site[6-9].
Most reported thermal injuries at the flap site have been treated conservatively with conventional dressings or surgical interventions[2,4,5]. Conservative dressing methods include the removal of heat sources and standard burn dressing combined with topical chloramphenicol/collagenase applications, paraffin gauze, silver sulfadiazine, or topical antibiotics to for induce secondary intention. Surgical intervention implies the debridement of necrotic tissue, full thickness or split thickness skin graft, locoregional flaps accompanied with undermining dissection for flap advancement, additional pedicled flap or free tissue transfer[5]. However, the degree of the burn can be aggravated by excessive edema formation, inflammatory activation, and bacterial load to the open wound, which might require a more invasive surgical approach to salvage the damaged tissue[6]. Several studies have proved that NPWT application at the burn site has potential benefits in burn wound care[6-8]. The application of NPWT within 48 h on burn wounds promotes wound healing by stabilizing the wound environment, including cell proliferation, angiogenesis, and tissue oxygenation[7,8]. The potential ischemia caused by adequate negative tissue pressure stimulates vasodilator mediators[18]. In addition, the application of NPWT to the burn site increases microvascular perfusion, resulting in the reduction of edema and capillary congestion[8]. Those studies suggest that the use of NPWT may be applied extensively to various complicated flap wounds.
The efficacy of NPWT has been expanded to include various clinical trials of flap wounds. Borderline perfusion of the transferred flaps may also benefit from the application of NPWT to restore microcirculation by reducing capillary congestion and lowering interstitial tissue pressure[12-16]. Hanasono et al[12] showed that the uptake of skin grafts in pedicled and free muscle flaps was increased via a reduction in hematoma, seroma, and shear forces after the application of negative pressure. Goldstein et al[13] demonstrated that random local flaps with poor perfusion increased viability via the restoration of blood circulation under NPWT application. However, the use of NPWT on traumatized or skin-containing free flaps with impaired perfusion still carries a high risk of entering a state of irreversible ischemia caused by arterial insufficiency[18]. This phenomenon can be explained by the fact that the main vascular pedicle and the microcirculation of the subcutaneous and subdermal regions can be compressed under relatively high negative pressure[11,18]. If the main flap pedicle is within 1 cm of the absorbent foam, it can be compressed due to the proportional effect of the suction pressure[19]. In our case, the flap pedicle was located at the level of the anterior axillary line, which was sufficiently far from the NPWT application site. Therefore, it was assumed that the collapse of some perforators at the NPWT application site led to damage of the main pedicle.
To maximize the efficacy and safety of NPWT in free tissue transfers, various studies have used different negative pressure levels and application designs considering the condition and stability of the flap tissue[11]. Uygur et al[14] placed the NPWT device on only one side of the flap to prevent compression of the major pedicle. In addition, a canister-free disposable NPWT system (Pico Smith & Nephew, NJ) was also developed to compensate for the limitations of conventional NPWT devices. This system is easy to use for burn injuries and a variety of wounds, and increases patient mobility by reducing the size and weight of the system[20,21].
Closed-incision negative wound therapy represents as reliable tools to reduce surgical site complications on the abdominal donor-site in autologous free-flap breast reconstruction[22]. Kuo et al[23] presented successful cases of latissimus dorsi muscle flap and skin graft reconstruction with NPWT to large breast wounds. Complete resection of necrotic tissue preceded the application of NPWT, and most of the necrotic tissue resolved within five days according to previous research[16]. In contrast to previous successful reports, we were confronted with a devastating outcome after applying NPWT to the compromised perfusion site of the reconstructed breast due to thermal injury. Because there are few clinical cases on the application of NPWT to reconstructed breasts with thermal injury[2], this report may indicate several concerns that should be considered before NPWT is applied to a short-lasting free flap site after burn injury. The first concern with this unexpected event was thought to be the collapse of the exposed microvessels after debridement due to the increased tissue pressure caused by the high external negative pressure. Second, we believe that compression of the main pedicle could be induced by its proximity to the absorbent foam of NPWT. The final concern with the application of NPWT is the inability to monitor the flap conditions under complete sealing. The other possible reasons for flap failure are arterial insufficiency or venous congestion of the flap itself or mechanical compression induced by the patient’s position or involuntary activity. However, this is highly unlikely because of the postoperative period. Although the default setting of NPWT (-125 mmHg in the continuous mode) has been routinely applied regardless of the flap type, the level of pressure, mode, duration of NPWT, and monitoring methods for flap salvage should be considered before installation. Lance et al[16] recommended that -75 to -100 mmHg with an intermittent mode would be appropriate for the exposed fat tissue or muscles, especially in free flap cases with the prevention of vascular compression of the perfusion compromised structure. The usual duration of NPWT was less than a week, and daily change of the foam was necessary to monitor the free flap[15]. Most importantly, the location of absorbent foam is crucial because it must avoid the pedicle site. It is recommended that the foam be located on the margin of the wound, similar to the usual manner of Pico application.
In conclusion, NPWT effectively promotes survival in free tissue transfer; however, special attention is required in cases of burn injury at the transferred flap site. Microsurgeons should be cautious about the location of the pedicle, pressure, and mode when using NPWT at the burn site over the transferred free flap tissue.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Long P, China; Zhu L, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Faulkner HR, Colwell AS, Liao EC, Winograd JM, Austen WG Jr. Thermal Injury to Reconstructed Breasts from Commonly Used Warming Devices: A Risk for Reconstructive Failure. Plast Reconstr Surg Glob Open. 2016;4:e1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Mohanna PN, Raveendran SS, Ross DA, Roblin P. Thermal injuries to autologous breast reconstructions and their donor sites--literature review and report of six cases. J Plast Reconstr Aesthet Surg. 2010;63:e255-e260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Lee SH, Sim SH, Ki SH. Low-Temperature Burn on Replanted Fingers and Free Flaps in Hand. Ann Plast Surg. 2018;81:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Delfino S, Brunetti B, Toto V, Persichetti P. Burn after breast reconstruction. Burns. 2008;34:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Enajat M, Rozen WM, Audolfsson T, Acosta R. Thermal injuries in the insensate deep inferior epigastric artery perforator flap: case series and literature review on mechanisms of injury. Microsurgery. 2009;29:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns. 2004;30:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Kantak NA, Mistry R, Halvorson EG. A review of negative-pressure wound therapy in the management of burn wounds. Burns. 2016;42:1623-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Kantak NA, Mistry R, Varon DE, Halvorson EG. Negative Pressure Wound Therapy for Burns. Clin Plast Surg. 2017;44:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563-76; discussion 577. [PubMed] |
| 10. | Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51:301-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 313] [Article Influence: 28.5] [Reference Citation Analysis (2)] |
| 11. | Yu P, Yu N, Yang X, Jin X, Lu H, Qi Z. Clinical Efficacy and Safety of Negative-Pressure Wound Therapy on Flaps: A Systematic Review. J Reconstr Microsurg. 2017;33:358-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Hanasono MM, Skoracki RJ. Securing skin grafts to microvascular free flaps using the vacuum-assisted closure (VAC) device. Ann Plast Surg. 2007;58:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Goldstein JA, Iorio ML, Brown B, Attinger CE. The use of negative pressure wound therapy for random local flaps at the ankle region. J Foot Ankle Surg. 2010;49:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Uygur F, Duman H, Ulkür E, Ceiköz B. The role of the vacuum-assisted closure therapy in the salvage of venous congestion of the free flap: case report. Int Wound J. 2008;5:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Qiu SS, Hsu CC, Hanna SA, Chen SH, Cheong CF, Lin CH, Chang TN. Negative pressure wound therapy for the management of flaps with venous congestion. Microsurgery. 2016;36:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Lance S, Harrison L, Orbay H, Boudreault D, Pereira G, Sahar D. Assessing safety of negative-pressure wound therapy over pedicled muscle flaps: A retrospective review of gastrocnemius muscle flap. J Plast Reconstr Aesthet Surg. 2016;69:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Eisenhardt SU, Schmidt Y, Thiele JR, Iblher N, Penna V, Torio-Padron N, Stark GB, Bannasch H. Negative pressure wound therapy reduces the ischaemia/reperfusion-associated inflammatory response in free muscle flaps. J Plast Reconstr Aesthet Surg. 2012;65:640-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Bi H, Khan M, Li J, Pestana IA. Use of Incisional Negative Pressure Wound Therapy in Skin-Containing Free Tissue Transfer. J Reconstr Microsurg. 2018;34:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Kairinos N, Solomons M, Hudson DA. The paradox of negative pressure wound therapy--in vitro studies. J Plast Reconstr Aesthet Surg. 2010;63:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Payne C, Edwards D. Application of the Single Use Negative Pressure Wound Therapy Device (PICO) on a Heterogeneous Group of Surgical and Traumatic Wounds. Eplasty. 2014;14:e20. [PubMed] |
| 21. | Hudson DA, Adams KG, Van Huyssteen A, Martin R, Huddleston EM. Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, no-canister system. Int Wound J. 2015;12:195-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Siegwart LC, Sieber L, Fischer S, Maraka S, Kneser U, Kotsougiani-Fischer D. Influence of closed incision negative-pressure therapy on abdominal donor-site morbidity in microsurgical breast reconstruction. Microsurgery. 2022;42:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Kuo CY, Kan JY, Kao CN, Ou-Yang F, Wu CC, Shiau JP, Li CL, Hou MF, Huang SH. Utilizing NPWT improving skin graft taking in reconstruction for extended breast skin defects following mastectomy. Clin Case Rep. 2021;9:e04716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |