Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.127
Peer-review started: July 21, 2022
First decision: August 4, 2022
Revised: September 12, 2022
Accepted: November 28, 2022
Article in press: November 28, 2022
Published online: January 6, 2023
Processing time: 167 Days and 15.7 Hours
Approximately 65%-78% of patients with a spinal cord injury (SCI) develop any symptom of spasticity. The aim of this study was to investigate the tolerability and short-term effects of radial extracorporeal shock wave therapy (rESWT) on plantar flexor spasticity in a patient with incomplete SCI.
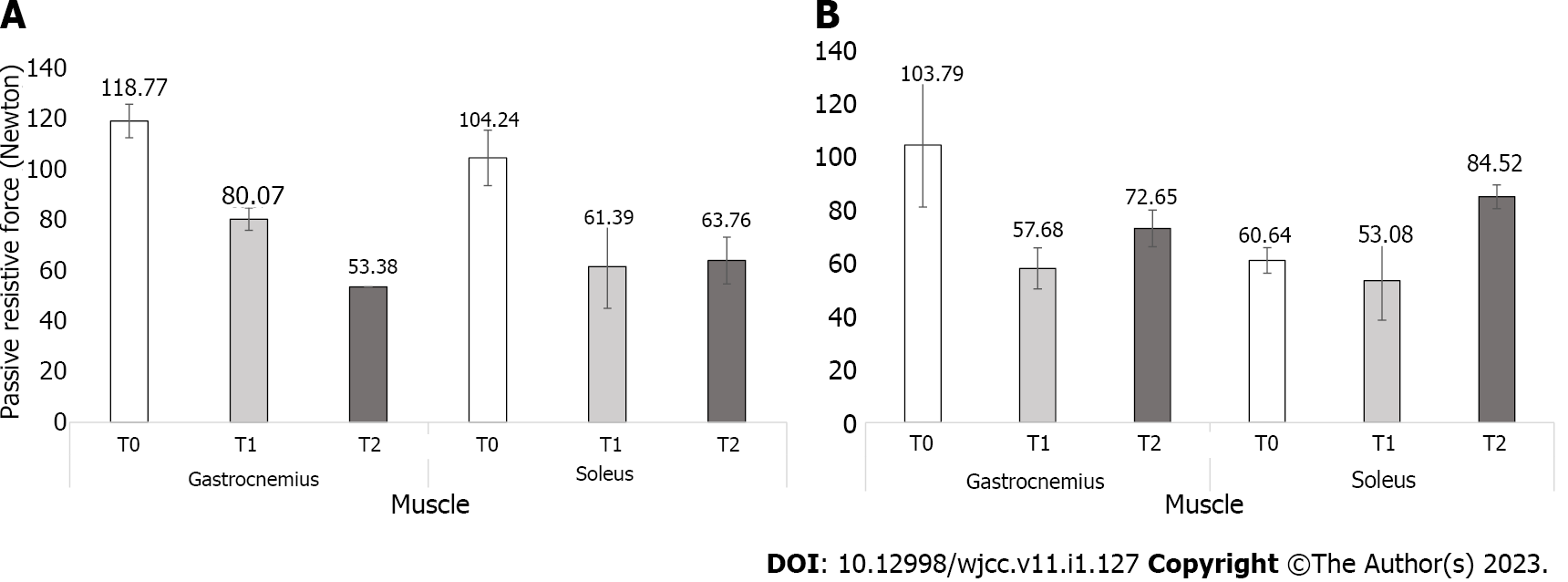
An 18-year-old man with an incomplete SCI completed five sessions of rESWT. The primary outcomes were the changes in ankle-passive range of motion (A-PROM) and passive resistive force to ankle dorsiflexion. The outcomes were assessed at baseline (T0), immediately after treatment (T1) and 1 wk after the end of treatment (T2). The A-PROM increased by 15 degrees at T1 and 25 degrees at T2 compared with T0. The passive resistive force to ankle dorsiflexion at low velocity decreased by 33% at T1 and 55% at T2 in the gastrocnemius muscle and by 41% at T1 and 39% at T2 in the soleus muscle compared with T0. At high velocity, it also decreased by 44% at T1 and 30% at T2 in the gastrocnemius muscle compared with T0. However, in the soleus muscle, the change was minor, with a decrease of 12% at T1 and increased by 39% at T2 compared with T0.
In this patient, the findings showed that rESWT combined with conventional therapy was well-tolerated and could be effective in improving A-PROM and passive resistive force to ankle dorsiflexion in the short-term. Further randomized controlled clinical trials with longer period of follow-up are necessary to confirm the results obtained in patients with SCI.
Core Tip: Spasticity is a major problem in the life of spinal cord injury (SCI), which can affect to their daily life activities. Extracorporeal shock wave therapy (ESWT) is defined as a sequence of mechanical pulses waves, characterized by a high peak pressure, fast pressure rises and short time duration. Previous studies have reported that ESWT is effective for pain relief and hypertonia in post-stroke, cerebral palsy, and multiple sclerosis patients. However, no clinical trials have investigated the effects of ESWT in the symptoms of spasticity in spinal cord injury patients. This is the first case report published which analysed the effects of ESWT in plantar flexor spasticity symptoms in a volunteer with incomplete SCI.
- Citation: Comino-Suárez N, Gómez-Soriano J, Ceruelo-Abajo S, Vargas-Baquero E, Esclarín A, Avendaño-Coy J. Extracorporeal shock wave for plantar flexor spasticity in spinal cord injury: A case report and review of literature. World J Clin Cases 2023; 11(1): 127-134
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/127.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.127
Spasticity may produce structural changes in the muscle properties and not only by hyperreflexia and alterations of the central processing of sensory input in the spinal cord[1]. Approximately 65%-78% of patients with a chronic spinal cord injury (SCI) (≥ 1 year after injury) develop any symptom associated with spasticity[2]. Problematic spasticity is characterized by pain and interference in daily life activities, such as walking; decreased range of joint motion; more contractures; stress; inability to sleep; and more complications and hospitalizations[3-5].
Numerous therapeutic options are available for the treatment of spasticity. Physiotherapy should be considered as a first option, which includes techniques such as thermotherapy/cryotherapy, stretching, positioning, and splinting to prevent contractures and physical modalities such as vibration, electrical currents, and extracorporeal shock wave therapy (ESWT). However, some individuals do not respond to conservative treatment, making necessary the prescription of medications or the application of other invasive techniques (i.e., injections and/or surgical interventions). Although pharmacological agents have been evidenced to be effective in the reduction of spastic symptoms, there are important adverse effects that affect the daily life activities and should be considered before their use (e.g., sedation, insomnia, fatigue, dry mouth, depression, and hallucinations), making it necessary to look for new effective therapies without adverse effects[2,6].
ESWT is defined as a sequence of mechanical pulse waves with high peak pressure (100 MPa), fast pressure rise (< 10 ns) and short duration (10 µs)[7]. Generally, ESWT is classified as focused extracorporeal shock wave therapy (fESWT) or radial extracorporeal shock wave therapy (rESWT). fESWT is based on the use of single pressure pulses of microsecond duration that are concentred in small focal areas, and these can be guided by ultrasound or radiographs. On the other hand, rESWT is generated pneumatically and is transmitted radially from the tip of the applicator to the target area[8,9]. Previous studies have reported that ESWT is effective for pain relief and hypertonia after stroke[10,11], cerebral palsy[12,13] and multiple sclerosis[14]. However, there are some pathophysiological differences between spasticity of cerebral or spinal origin, having important consequences for the therapeutic approach that is chosen. Until now, no clinical trials have been published to analyse the effects of rESWT on the hypertonia of patients with a SCI. Therefore, we report the case of a volunteer with an incomplete SCI to investigate the effects of rEWST on plantar flexor hypertonia as a preliminary study to perform a randomized controlled clinical trial in patients with a SCI.
The participant was an 18-year-old man from the National Paraplegic Hospital (Toledo, Spain) who suffered an accident on September 27, 2020. He was referred to department of rehabilitation medicine due an increased in the muscle tone of the right triceps surae, which hinders his ability to walk.
At the enrolment, 5 mo after the accident, the patient was clinically stable, without concomitant pathologies. The patient was diagnosed as having an incomplete SCI at the cervical level (C5) according to the American Spinal Injury Association (ASIA) Impairment Scale C. The patient had a considerable increase in the muscle tone of the right triceps surae, making the passive movement of the ankle difficult with a grade 3 in the Modified Ashworth Scale (MAS). The patient was classified as grade 2 by the Penn scale, reporting that spasms occurred less than once per hour. The patient was able to stand or walk with technical aids, with a score of 8 on the Walking Index for Spinal Cord Injury II (WISCI-II), 56 points on the Spinal Cord Independence Measure (SCIM-II), 0.391 index score on the quality of life with the European Quality of Life 5-Dimension 3-Level (EQ-5D-3L) questionnaire and 80 points in patient’s self-rated health on a vertical visual analogue scale (EQ-VAS). The antispastic medication and standard rehabilitation programme did not change the hypertonia in the plantar flexors during the 2 mo prior to enrolment. The patient had not received previous treatment of the affected leg with phenol, alcohol, botulinum toxin injection or surgical procedures. The skin and sensation were normal, without soreness in the target area. The patient signed written informed consent to receive the intervention.
There was no illness in previous medical history.
As primary outcomes, we assessed ankle-passive range of motion (A-PROM) and passive resistive force to ankle dorsiflexion. As secondary outcomes, we measured the changes in muscular tone, spasm frequency, quality of life, pain perceived during therapy, functionality, and possible adverse effects. The assessments were performed by the same assessor at baseline (T0), immediately after treatment (T1) and 1 wk after the end of treatment (T2).
A-PROM was measured with a plastic universal goniometer[15]. The patient was in the supine position with the knee extended to evaluate the extensibility of the gastrocnemius muscle. The measurement started with the ankle in the rest position, and then the assessor passively moved the joint to the maximum possible dorsiflexion. The mean of three consecutive measures was calculated.
For the passive resistive force to ankle dorsiflexion (N), a handheld dynamometer (MicroFET2® Hoggan Scientific, LLC) was employed. The patient was in the supine position with the knee in flexion (soleus) and then extended (gastrocnemius) with the ankle in approximately 35 degrees of plantar flexion. The assessor moved the ankle to 0 degrees of dorsiflexion at low velocity (approximately 3 s) and fast velocity (approximately 1 s). The mean of three consecutive performances of passive dorsiflexion for each velocity was recorded. The end of the position being 0 degrees of dorsiflexion was chosen because this angle has easy reliability and avoids reaching the maximum joint limit. The resistive force testing at low velocity evidenced the mechanical response to a muscle stretch that does not evoke a reflex response. On the other hand, fast velocity represents total muscle stiffness, including reflex-mediated resistance[16]. Previous studies have used a handheld dynamometer to assess the resistive torque, showing high reliability[17-19].
Plantar flexor hypertonia was measured with the MAS[20], with the patient in the supine position and the knee extended (gastrocnemius). The MAS was scored as follow: 0 = no increase in tone; 1 = slight increase in tone, manifested by a catch and release or by minimal resistance at the end of the ROM when the affected part(s) is moved in flexion or extension; 1+ = slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM; 2 = more marked increase in muscle tone through most of the ROM but affected part(s) easily moved; 3 = considerable increase in muscle tone, passive movement difficult; and 4 = affected limb rigid in flexion or extension.
The Penn scale[21] was performed to measure the self-reported spasm frequency. This scale has a score ranging from 0 to 4 as follows: 0 = no spasms; 1 = mild spasms induced by stimulation; 2 = less than one full spasm per hour; 3 = spasms occurring more than once per hour; and 4= spasms occurring more than 10 times per hour.
The quality of life was assessed with the EQ-5D-3L questionnaire[22]. This questionnaire is composed of five dimensions, each describing a different aspect of health: Mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with the value of 1 meaning full health and the value of 0 the worst health. The EQ-VAS is a vertical scale from 0 (the worst health imaginable) to 100 (the best health imaginable), where patients are asked to indicate their overall health today.
Functionality was measured with the WISCI-II, which evaluates the physical assistance needed and devices required for walking a 10-metre distance[23], and the SCIM-III, which measures the ability to perform routine daily tasks. The SCIM-III is a questionnaire divided in three subscales: Self-care (0-20 points), respiration and sphincter management (0-40 points) and mobility (0-40 points), with higher scores indicating more independence[24].
The possible adverse effects were registered after each session of treatment. The pain perceived during the intervention was also assessed with the VAS[25].
Blood investigation findings were normal.
Magnetic resonance findings were normal.
The final diagnosis was incomplete SCI at the cervical level (C5) according to ASIA Impairment Scale C. Hypertonia in the right triceps surae and limited ankle range of motion being the passive movement difficult (Grade 3).
rESWT was applied with the BTL- Power device (BTL Industries Ldt. Czech Republic). The patient received five sessions, with a frequency of one session per week. The intervention was administered by the same physiotherapist with the patient in the prone position and his feet suspended at the end of the bed. The transmission gel was applied between the applicator and target area. The head of the applicator was 15 mm and was moved slowly around the entire muscle belly and muscle-tendon junction with a pressure of 2 bar, frequency of 8 Hz and 1500 shots. The antispastic medication and the rehabilitation programme remained the same and did not change during the rESWT. Daily physical therapy sessions were performed. These sessions were based on passive and active mobilizations of the upper and lower limbs, strengthening exercises, static stretching and standing positions. The frequency of the physical therapy sessions was five sessions per week, and every session lasted 1 h.
The patient completed the five sessions of rESWT. Adverse effects (i.e., tingling, aching, redness, bruising or haematoma) were not observed. Treatment with rESWT was well tolerated, and the patient did not report pain (0 ± 0 VAS score) during the treatment. Pharmacological treatment was not modified during the experimental treatment. All the outcomes showed improvements after treatment, and these changes persisted 1 wk after treatment.
A-PROM was severely limited at T0 with -15 degrees of dorsiflexion, which was increased to 0 degrees at T1 and 10 degrees at T2. The passive resistive force to ankle dorsiflexion showed significant clinical differences when comparing T0, T1 and T2. Regardless of the velocity, the passive resistive force measured at low velocity decreased by 33% at T1 and 55% at T2 in the gastrocnemius muscle compared with T0. The passive resistive force in the soleus muscle decreased by 41% at T1 and 39% at T2 compared with T0. When measured at high velocity, the passive resistive force in the gastrocnemius muscle also decreased by 44% at T1 and 30% at T2 compared with T0, while the soleus muscle decreased by 12% at T1. However, there was increased of 39% at T2 compared with T0 (Figure 1).
The results of the clinical outcomes showed a reduction of muscle tone, with a value of 2 in the MAS at T1 and T2 and a decrease in the frequency of spasms. There were significant changes in functionality. The patient was able to ambulate 10 metres with two crutches, no braces and with the physical assistance of one person at T1 and T2. The results of the SCIM-III increased by 17 points at T1 and T2. Regarding the quality of life, the results of the EQ-5D-3L and EQ-VAS showed improvement, with better results in the anxiety/depression dimension and better punctuation in the overall health status in the EQ-VAS at T1 and T2 (Table 1).
| Outcome | Pre-Treatment | Post-Treatment |
| MAS | 3 | 2 |
| PENN | 2 | 1 |
| WISCI-II | 8 | 11 |
| SCIM-III | 56 | 73 |
| EQ-5D-3L | 0.77 | 0.783 |
| EQ-VAS | 80 | 90 |
This is the first documented case of a subject with a SCI who had persistent spasticity receiving rESWT. The applied protocol for rESWT was well tolerated and had no reported adverse effects. The results from this case report showed that five sessions of rESWT combined with the conventional rehabilitation programme significantly improved A-PROM and passive resistive force in the plantar flexors. There were also improvements in the clinical outcomes assessed, with reductions of spastic symptoms, decreasing the hypertonia of the ankle plantar flexors (MAS), and the frequency of spasms (Penn scale), as well as improvements in functionality and quality of life. These results are in accordance with the results obtained in previous studies in which lower limb spasticity was measured with the MAS and A-PROM in stroke patient[26-29]. We have always performed the MAS and A-PROM with the knee extended, and we did not compare the assessment with the knee extended and the knee flexed. Consequently, the extensibility of the gastrocnemius muscle could influence the results obtained. However, the study performed by Radinmehr et al[30] compared both positions and found positive results after ESWT, independent of the knee position. The improvements in the MAS and A-PROM may be explained by the effect of rESWT on structural muscle properties, changing the muscle extensibility and leading, as secondary result, to an increase in the articular ROM[31].
The positive findings regarding functionality and quality of life could be related to the reduction of the hypertonia. The gastrocnemius and soleus are lower limb muscles with prominent roles in walking and standing. Ankle dorsiflexion is a key factor in the gait cycle, being necessary during the swing phase[32]. The reduction of plantar flexor hypertonia could improve the position of the lower limb, giving more independence in the daily life activities and improving the patient's self-rated health.
The modulation of the muscle tone after rESWT can be used to perform antagonistic muscle exercises, ankle mobilizations or functional activities immediately after the treatment. It has been evidenced that rESWT added to the conventional therapy of patients with a stroke can be a beneficial option to enhance the effects of other interventions[33]. In one study, rESWT combined with botulinum toxin A injections[34] had a greater significant effect in the reduction of muscle tone compared with other therapies. Another study assessed hamstring tightness in healthy people. rESWT was performed in addition to hamstring stretching, showing significant changes in muscular flexibility compared with only stretching at the end of treatment and 4 wk of follow-up[35].
This case report found that passive resistive force to ankle dorsiflexion decreased after rESWT without differences in the knee position. However, the velocity had a significant effect on the passive resistive force. The changes did not persist and the resistance to ankle mobilization was increased especially with the knee extended position at T2 during the high velocity assessment. The velocity-sensitive ankle plantar flexor response suggests the presence of a hyperactive stretch reflex, as was found in the study by Radinmehr et al[30]. In contrast, the changes in passive resistive force measured at low velocity were longer-lasting effect one week after the end of treatment, which may corroborate that the effect of rESWT was focused on the fibrosis muscle component, improving the viscoelasticity of hypertonic muscles.
ESWT is a novel and effective method for improving spasticity. Nevertheless, the mechanism behind the positive effects of rESWT are not completely understood. Previous studies have suggested that the thixotropic effect of ESWT induces cellular metabolic effects. It may modulate endogenous nitric oxide (NO) production, which plays a crucial role in the reduction of hypertonia. NO can stimulate angiogenesis and neovascularization of tissues, which is related to the anti-inflammatory and analgesic effects of ESWT, thereby improving muscle stiffness[36,37]. NO also is involved in important physiological functions of the central nervous system, including neurotransmission, memory and synaptic plasticity.
To date, there is not enough evidence on ESWT decreasing the excitability of alpha motor neurons. According to the results obtained in previous studies, patients that ameliorated the MAS score did not show changes in the F-wave amplitude or Hmax/Mmax ratio after ESWT, which measures spinal cord excitability[7]. The published literature supports the hypothesis that the mechanism of action of ESWT in the reduction of spastic symptoms is related to changes in fibrosis and on the rheological components of the chronic hypertonic muscles rather than on a neural level[33].
Related with the treatment dosage used, previous studies have shown that 1500 shots reduced the spasticity of ankle plantar flexors in patients with stroke[26,27]. The results obtained in a meta-analysis concluded that there is no evidence that higher dosage (> 1500 shots) or higher treatment frequencies (> 12 Hz) had greater effectiveness in stroke spasticity[11].
This case report has some limitations that should be considered. Firstly, we used the MAS as a tool for muscle tone evaluation because it is widely used and easy to administer. However, it is a subjective scale with high variation in reliability, which measures the muscle tone at rest. Furthermore, this scale does not differ if the resistance during the stretch has a neural or mechanical component. Secondly, the short-term period of follow-up limited to 1 wk, occasioned by the patient being discharged from the hospital, was not enough to determine the durability effect of ESWT. Thirdly, the dosage and parameters employed were based on previous studies in patients with stroke. We do not know if higher dosages of rESWT or a greater number of sessions would have the same results in the outcomes assessed. This is a single case in a patient with an incomplete SCI, further studies with larger samples of patients, double-blind designs and long-term follow-ups that would detect the real therapeutic effects of rESWT are required.
The results obtained in this case report and the absence of adverse effects points to the possibility of rESWT becoming an alternative non-invasive method for improving spastic symptoms, A-PROM, passive resistive force in the plantar flexors, functionality, and quality of life in patients with a SCI in the short-term. Further formal controlled studies recruiting larger sample sizes with longer period of follow-up are required to analyse the effectiveness and long-term effects of rESWT on hypertonia of the plantar flexors in patients with a SCI.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chiu CD, Taiwan; Dragonieri S, Italy; Wang G, China S-Editor: Liu XF L-Editor: A P-Editor: Liu XF
| 1. | Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity--from a basic science point of view. Acta Physiol (Oxf). 2007;189:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 278] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 2. | Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 333] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Bravo-Esteban E, Taylor J, Abián-Vicén J, Albu S, Simón-Martínez C, Torricelli D, Gómez-Soriano J. Impact of specific symptoms of spasticity on voluntary lower limb muscle function, gait and daily activities during subacute and chronic spinal cord injury. NeuroRehabilitation. 2013;33:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Sköld C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: Nature, severity, and location. Arch Phys Med Rehabil. 1999;80:1548-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Westerkam D, Saunders LL, Krause JS. Association of spasticity and life satisfaction after spinal cord injury. Spinal Cord. 2011;49:990-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Gómez-Soriano J, Taylor J. Espasticidad después de la lesión medular: Revisión de los mecanismos fisiopatológicos, técnicas de diagnóstico y tratamientos fisioterapéuticos actuales. Fisioterapia. 2010;32:89-98. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Manganotti P, Amelio E. Long-term effect of shock wave therapy on upper limb hypertonia in patients affected by stroke. Stroke. 2005;36:1967-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Speed C. A systematic review of shockwave therapies in soft tissue conditions: Focusing on the evidence. Br J Sports Med. 2014;48:1538-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Yoo JI, Oh MK, Chun SW, Lee SU, Lee CH. The effect of focused extracorporeal shock wave therapy on myofascial pain syndrome of trapezius: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e19085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Guo P, Gao F, Zhao T, Sun W, Wang B, Li Z. Positive Effects of Extracorporeal Shock Wave Therapy on Spasticity in Poststroke Patients: A Meta-Analysis. J Stroke Cerebrovasc Dis. 2017;26:2470-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Xiang J, Wang W, Jiang W, Qian Q. Effects of extracorporeal shock wave therapy on spasticity in post-stroke patients: A systematic review and meta-analysis of randomized controlled trials. J Rehabil Med. 2018;50:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Amelio E, Manganotti P. Effect of shock wave stimulation on hypertonic plantar flexor muscles in patients with cerebral palsy: A placebo-controlled study. J Rehabil Med. 2010;42:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Gonkova MI, Ilieva EM, Ferriero G, Chavdarov I. Effect of radial shock wave therapy on muscle spasticity in children with cerebral palsy. Int J Rehabil Res. 2013;36:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Marinelli L, Mori L, Solaro C, Uccelli A, Pelosin E, Currà A, Molfetta L, Abbruzzese G, Trompetto C. Effect of radial shock wave therapy on pain and muscle hypertonia: A double-blind study in patients with multiple sclerosis. Mult Scler. 2015;21:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Nakhostin Ansari N, Naghdi S, Hasson S, Rastgoo M. Efficacy of therapeutic ultrasound and infrared in the management of muscle spasticity. Brain Inj. 2009;23:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Jiménez-Sánchez C, Ortiz-Lucas M, Bravo-Esteban E, Mayoral-Del Moral O, Herrero-Gállego P, Gómez-Soriano J. Myotonometry as a measure to detect myofascial trigger points: An inter-rater reliability study. Physiol Meas. 2018;39:115004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Boiteau M, Malouin F, Richards CL. Use of a hand-held dynamometer and a Kin-Com dynamometer for evaluating spastic hypertonia in children: A reliability study. Phys Ther. 1995;75:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | In T, Jung K, Lee MG, Cho HY. Whole-body vibration improves ankle spasticity, balance, and walking ability in individuals with incomplete cervical spinal cord injury. NeuroRehabilitation. 2018;42:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Lamontagne A, Malouin F, Richards CL, Dumas F. Evaluation of reflex- and nonreflex-induced muscle resistance to stretch in adults with spinal cord injury using hand-held and isokinetic dynamometry. Phys Ther. 1998;78:964-75; discussion 976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3491] [Cited by in RCA: 3397] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 21. | Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, Kroin JS. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320:1517-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 487] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | EuroQol Research Foundation. EQ-5D-3L User Guide. 2018. Available from: https://euroqol.org/publications/user-guides. [DOI] [Full Text] |
| 23. | Jackson AB, Carnel CT, Ditunno JF, Read MS, Boninger ML, Schmeler MR, Williams SR, Donovan WH; Gait and Ambulation Subcommittee. Outcome measures for gait and ambulation in the spinal cord injury population. J Spinal Cord Med. 2008;31:487-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Ackerman P, Morrison SA, McDowell S, Vazquez L. Using the Spinal Cord Independence Measure III to measure functional recovery in a post-acute spinal cord injury program. Spinal Cord. 2010;48:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2532] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 26. | Moon SW, Kim JH, Jung MJ, Son S, Lee JH, Shin H, Lee ES, Yoon CH, Oh MK. The effect of extracorporeal shock wave therapy on lower limb spasticity in subacute stroke patients. Ann Rehabil Med. 2013;37:461-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Sohn MK, Cho KH, Kim YJ, Hwang SL. Spasticity and electrophysiologic changes after extracorporeal shock wave therapy on gastrocnemius. Ann Rehabil Med. 2011;35:599-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Hasuk B, Jung Min L, Kyung Hwan L. The Effects of Extracorporeal Shock Wave Therapy on Spasticity in Chronic Stroke Patients. Ann Rehabil Med. 2010;34:663-669. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Santamato A, Micello MF, Panza F, Fortunato F, Logroscino G, Picelli A, Manganotti P, Smania N, Fiore P, Ranieri M. Extracorporeal shock wave therapy for the treatment of poststroke plantar-flexor muscles spasticity: A prospective open-label study. Top Stroke Rehabil. 2014;21 Suppl 1:S17-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Radinmehr H, Nakhostin Ansari N, Naghdi S, Olyaei G, Tabatabaei A. Effects of one session radial extracorporeal shockwave therapy on post-stroke plantarflexor spasticity: A single-blind clinical trial. Disabil Rehabil. 2017;39:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Mori L, Marinelli L, Pelosin E, Currà A, Molfetta L, Abbruzzese G, Trompetto C. Shock waves in the treatment of muscle hypertonia and dystonia. Biomed Res Int. 2014;2014:637450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Mohammadi R, Talebian S, Phadke CP, Yekaninejad MS, Hadian MR. Effects of Treadmill Incline and Speed on Ankle Muscle Activity in Subjects After a Stroke. Arch Phys Med Rehabil. 2016;97:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Cabanas-Valdés R, Calvo-Sanz J, Urrùtia G, Serra-Llobet P, Pérez-Bellmunt A, Germán-Romero A. The effectiveness of extracorporeal shock wave therapy to reduce lower limb spasticity in stroke patients: A systematic review and meta-analysis. Top Stroke Rehabil. 2020;27:137-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Santamato A, Notarnicola A, Panza F, Ranieri M, Micello MF, Manganotti P, Moretti B, Fortunato F, Filoni S, Fiore P. SBOTE study: Extracorporeal shock wave therapy vs electrical stimulation after botulinum toxin type a injection for post-stroke spasticity-a prospective randomized trial. Ultrasound Med Biol. 2013;39:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Kim YW, Chang WH, Kim NY, Kwon JB, Lee SC. Effect of Extracorporeal Shock Wave Therapy on Hamstring Tightness in Healthy Subjects: A Pilot Study. Yonsei Med J. 2017;58:644-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Mariotto S, de Prati AC, Cavalieri E, Amelio E, Marlinghaus E, Suzuki H. Extracorporeal shock wave therapy in inflammatory diseases: Molecular mechanism that triggers anti-inflammatory action. Curr Med Chem. 2009;16:2366-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 37. | Molina JA, Jiménez-Jiménez FJ, Ortí-Pareja M, Navarro JA. The role of nitric oxide in neurodegeneration. Potential for pharmacological intervention. Drugs Aging. 1998;12:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |