Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2938
Peer-review started: October 13, 2021
First decision: December 3, 2021
Revised: December 18, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 26, 2022
Processing time: 160 Days and 5.2 Hours
Myoepithelial carcinoma (MC) is a rare malignant neoplasm that mainly occurs in the salivary gland. MC can be confused with many other tumors when arising outside the salivary glands because it presents with a wide spectrum of cytomorphological and immunohistochemical features. To the best of our knowledge, esophageal MC has not been previously reported. The purpose of this study was to describe the imaging and clinicopathological features of esophageal MC to improve the understanding of the disease.
Three men and one woman diagnosed with esophageal MC were enrolled in this study. The primary clinical symptom was dysphagia. The mass was mainly located in the middle esophagus. Laboratory tests revealed that two patients who underwent tumor abnormal protein were positive. Radical resection was performed for all patients with no adjuvant therapy. Hematoxylin-eosin staining showed infiltrative growth of epithelial cells with hyperchromatic and pleomorphic nuclei toward the periphery. Immunohistochemistry showed that all patients were positive for P63, and most patients were positive for SOX-10, AE1/AE3, P40, and calponin. The Ki-67 values were all higher than 60%. Patient one died one month after discharge from an unknown cause. Patient two lost to follow-up. At patient three’s four-month review, enhanced computed tomography (CT) showed anastomosis recurrence and bilateral lung metastases. He abandoned treatment and lost to follow-up. Patient four attended review appointments regularly and remained in a good general condition.
Here, we present the first report of esophageal MC and review the relevant literature. Esophageal MC is more likely to occur in the middle esophagus in older patients with male dominance. A fungating type observed on CT scanning may help narrow down the differential diagnosis. Cystic change or necrosis may occur in larger lesions. The final diagnosis should be made according to the pathological examination. The treatment for MC is surgical resection, and the efficacy of chemotherapy needs to be determined with future studies.
Core Tip: Esophageal myoepithelial carcinoma is an aggressive malignancy that has not been reported. In this study, we describe the clinical, pathological, immunohistochemical, and imaging findings of four patients with esophageal myoepithelial carcinoma (MC) and report their outcomes. Deepening the understanding of esophageal MC can help us narrow down the differential diagnosis and aid clinical decisions.
- Citation: Lu H, Zhao HP, Liu YY, Yu J, Wang R, Gao JB. Esophageal myoepithelial carcinoma: Four case reports. World J Clin Cases 2022; 10(9): 2938-2947
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2938.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2938
Myoepithelial carcinoma (MC) is an aggressive tumor that occurs mainly in the salivary gland and was first reported by Stromeyer et al[1] in 1975. MC has a multinodular architecture, and is composed of epithelioid, clear, spindle, and/or plasmacytoid cells, frequently arranged in cords or trabeculae in a myxoid or hyalinized stroma[2]. The sex distribution was approximately equal, with a mean age of 38 years, and the primary complaint of MC was a painless mass located in the parotid gland, oral cavity, or neck[3]. MC can also originate in the chest, lungs, skin, and stomach[4-6]. Due to the low incidence of MC, the clinical and biological behaviors have not yet been fully elucidated. The diagnosis depends on pathology and immunohistochemistry[2]. To the best of our knowledge, esophageal MC has not been previously reported. In this study, we describe the clinical, pathological, immunohistochemical, and imaging findings of four patients with esophageal MC and report their outcomes. The relevant literature was also reviewed to deepen understanding of esophageal MC. Clinical and pathological factors are shown in Table 1. Immunohistochemistry results are shown in Table 2. Computed tomography (CT) features are shown in Table 3.
| Case | 1 | 2 | 3 | 4 |
| Sex | M | M | M | F |
| Age (year) | 57 | 60 | 79 | 81 |
| Complaint | Dysphagia | Dysphagia | Dysphagia | Retrosternal discomfort |
| Location | Middle | Middle | Middle | Lower |
| Depth | Muscle layer | Submucosa | Whole layer | Whole layer |
| Size (cm) | 4.5 × 4.0 × 1.2 | 3.0 × 2.0 × 1.5 | 3.5 × 2.8 × 2.0 | 2.9 × 1.7 × 0.7 |
| Tumor marker | TAP (+) | TAP (+) | Normal | Normal |
| Node involvement | + | – | – | – |
| Cytology | MC + SCC | MC + SCC | MC | MC |
| Therapy | R | R | R | R |
| Follow up | Died from unknown cause | Lost to follow-up | Anastomosis recurrence and lung metastases | NED |
| Case | SOX-10 | P63 | CK5/6 | CK8/18 | Ki-67 | AE1/AE3 | P40 | Calponin | S-100 | CK7 | CD56 |
| 1 | + | + | + | + | > 70% | + | + | - | - | - | + |
| 2 | + | + | + | - | > 60% | + | + | + | + | + | - |
| 3 | + | + | - | + | > 70% | - | - | + | + | - | - |
| 4 | - | + | + | + | > 70% | + | + | + | - | - | - |
| case | 1 | 2 | 3 | 4 |
| Morphological subtype | Medullary | Fungating | Ulcerative | Fungating |
| Length (mm) | 49 | 96 | 55 | 45 |
| Enhancement degree | Homogeneous | Heterogeneous | Heterogeneous | Homogeneous |
| Enhanced homogeneity | Mild | Marked | Mild | Mild |
| Enlarged lymph node | – | + | – | – |
| Cystic change /necrosis | – | + | + | – |
| Ulceration | – | + | – | – |
Case 1: A 57-year-man presented with dysphagia.
Case 2: A 60-year-man was referred to our clinic for dysphagia.
Case 3: A 78-year-old man presented with dysphagia.
Case 4: An 80-year-old woman presented with retrosternal discomfort.
Case 1: Approximately six months ago, the patient presented with dysphagia without regurgitation or hiccups.
Case 2: The patient presented with dysphagia that had been present for two months, with belching, heartburn, and regurgitation.
Case 3: The patient presented with dysphagia ten days prior.
Case 4: The patient had experienced spontaneously resolving nocturnal episodes of retrosternal discomfort with chest tightness, heartburn, and regurgitation for one month.
Case 1: The patient was diagnosed with chronic bronchitis 30 years prior and intermittently took oral aminophylline.
Case 2: The patient had hypertension and liver cirrhosis.
Case 3: The patient’s previous medical history was clear.
Case 4: The patient underwent a right hip replacement 16 years prior.
Only case 1 and case 2 occasionally smoked. Family members of the patients had no history of confirmed malignant tumors.
Case 1: The main finding on clinical examination was barrel chest.
Case 2: No abnormalities were discovered on physical examination.
Case 3: No abnormalities were discovered on physical examination.
Case 4: No abnormalities were discovered on physical examination.
Case 1: The patient underwent the tumor abnormal protein (TAP) exam to find positive results, and all other laboratory findings were within normal limits. None of the other laboratory [red blood cell (RBC), erythrocyte sedimentation rate (ESR), white blood cell (WBC), hemoglobin] values were considered clinically significant.
Case 2: The patient was hepatitis B virus-positive. He underwent the TAP exam to find positive. Hemoglobin, 93 g/L. None of the other laboratory values were considered clinically significant.
Case 3: The patient underwent a tumor marker exam [alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 72-4 (CA72-4)] to find that the levels were all within normal limits. None of the other laboratory values were considered clinically significant.
Case 4: The patient underwent a tumor marker exam (AFP, CEA, CA125, CA19-9, CA72-4) to find that the levels were all within normal limits. None of the other laboratory values were considered clinically significant.
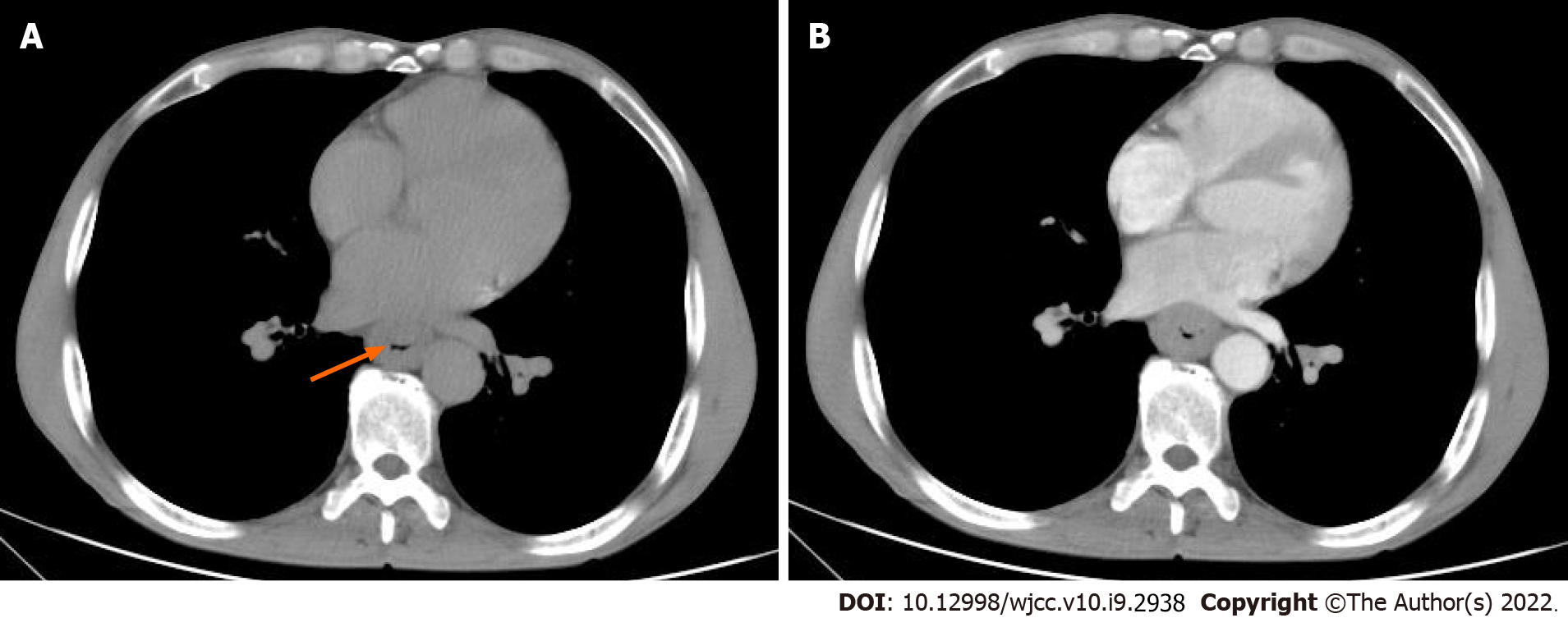
Case 1: Abdominal ultrasound showed no major abnormalities. Enhanced CT revealed a thickened wall and narrowed lumen of the lower esophagus, indicating a medullary-type tumor, with an evident fat layer between the lesion and surrounding tissues (Figure 1). The thickest part of the tumor was approximately 13 mm, and the length of the lesion was approximately 49 mm. The contrast scan showed uniform mild enhancement. Endoscopy showed irregular mucosal uplift in the esophagus 33-37 cm away from the incisors, accounting for half of the lumen.
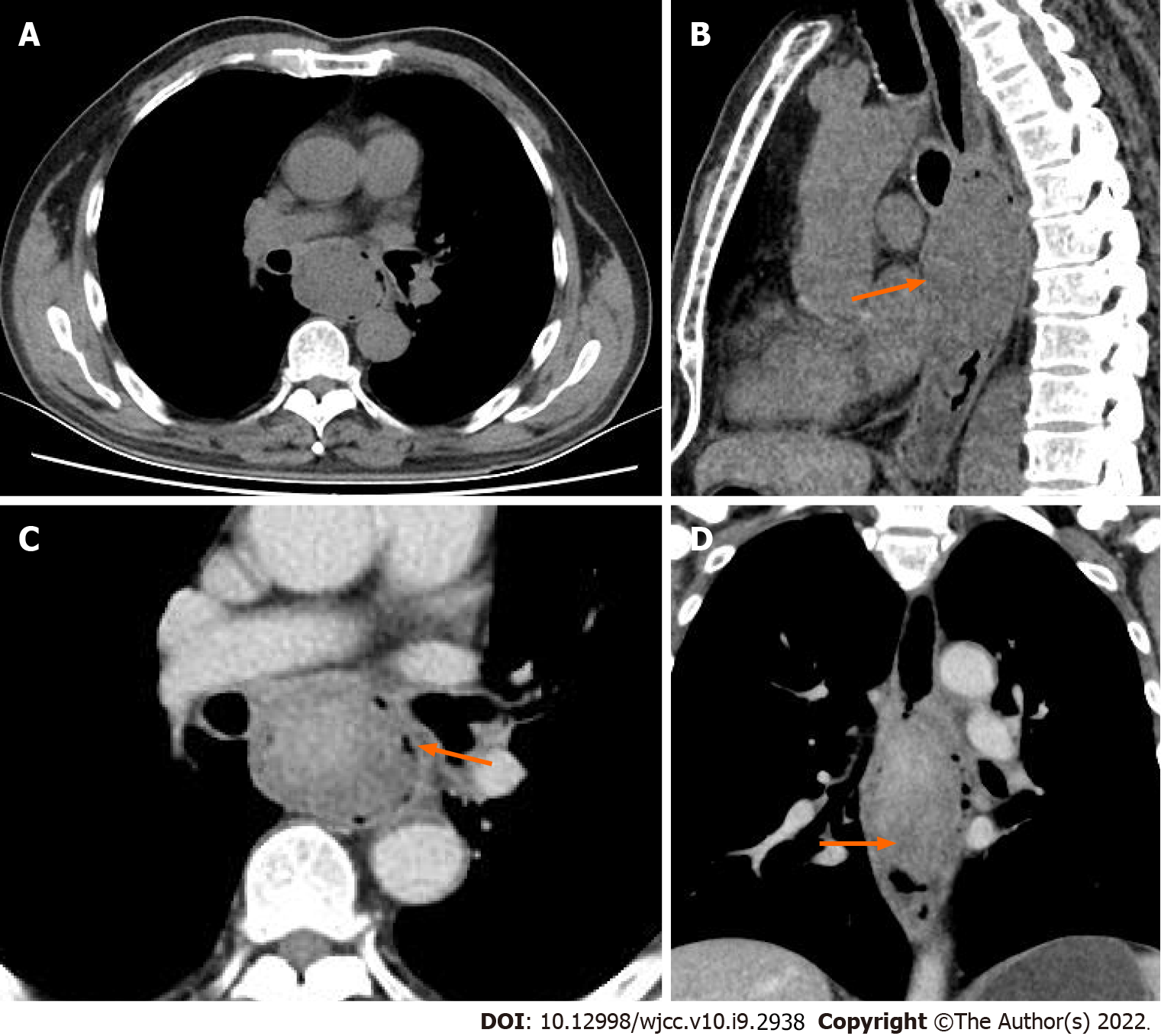
Case 2: Enhanced CT revealed a local thickened wall with a fungating-type mass with ulceration and cystic change or necrosis. The contrast scan showed obvious heterogeneous enhancement (Figure 2). The fatty spaces between the left main bronchus and the left ventricle disappeared, and the length of the lesion was approximately 96 mm.
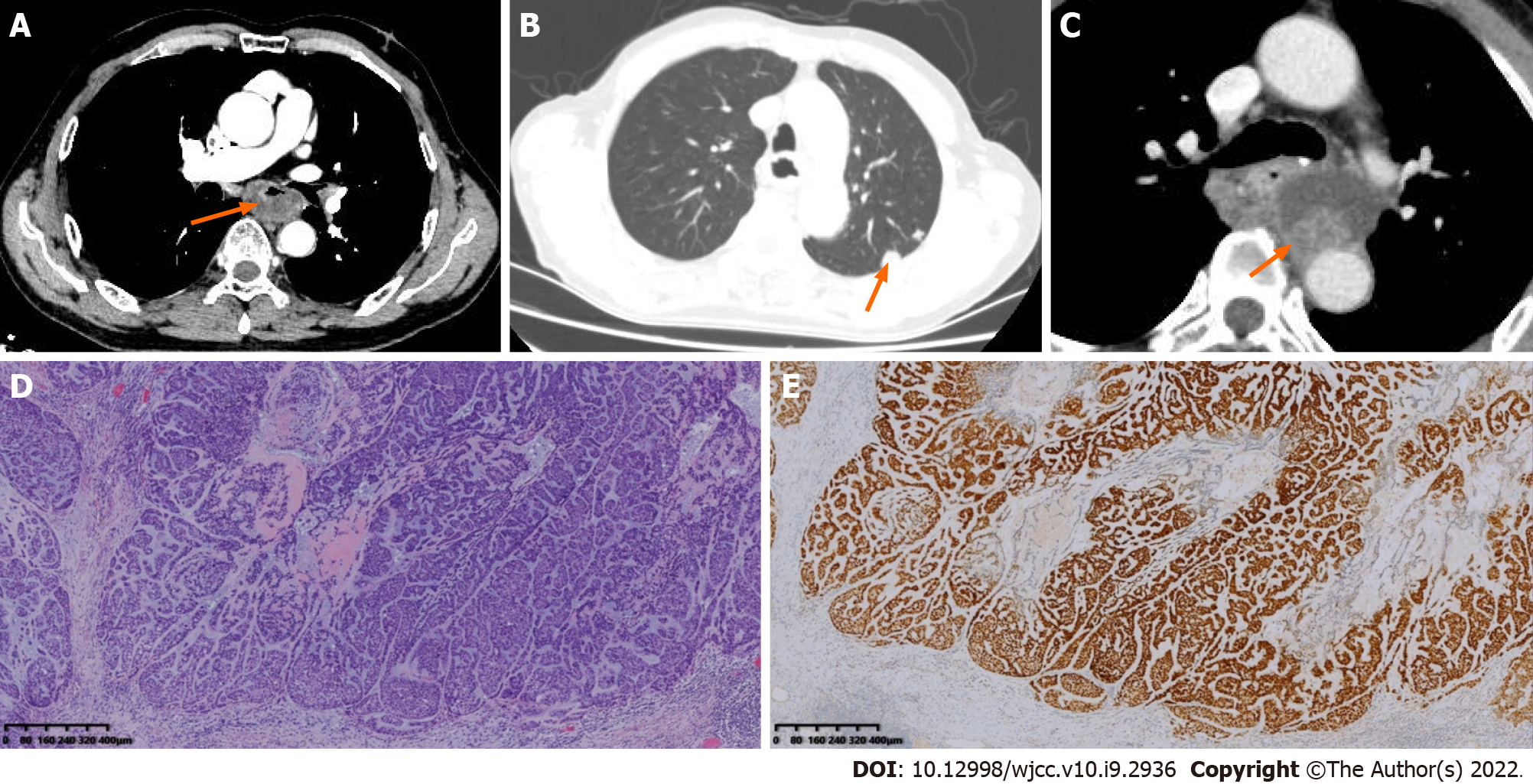
Case 3: Enhanced CT revealed a local thickened wall with a complete mucosal layer with cystic change or necrosis (Figure 3). The thickest part was approximately 18 mm, and the lesion length was approximately 55 mm.
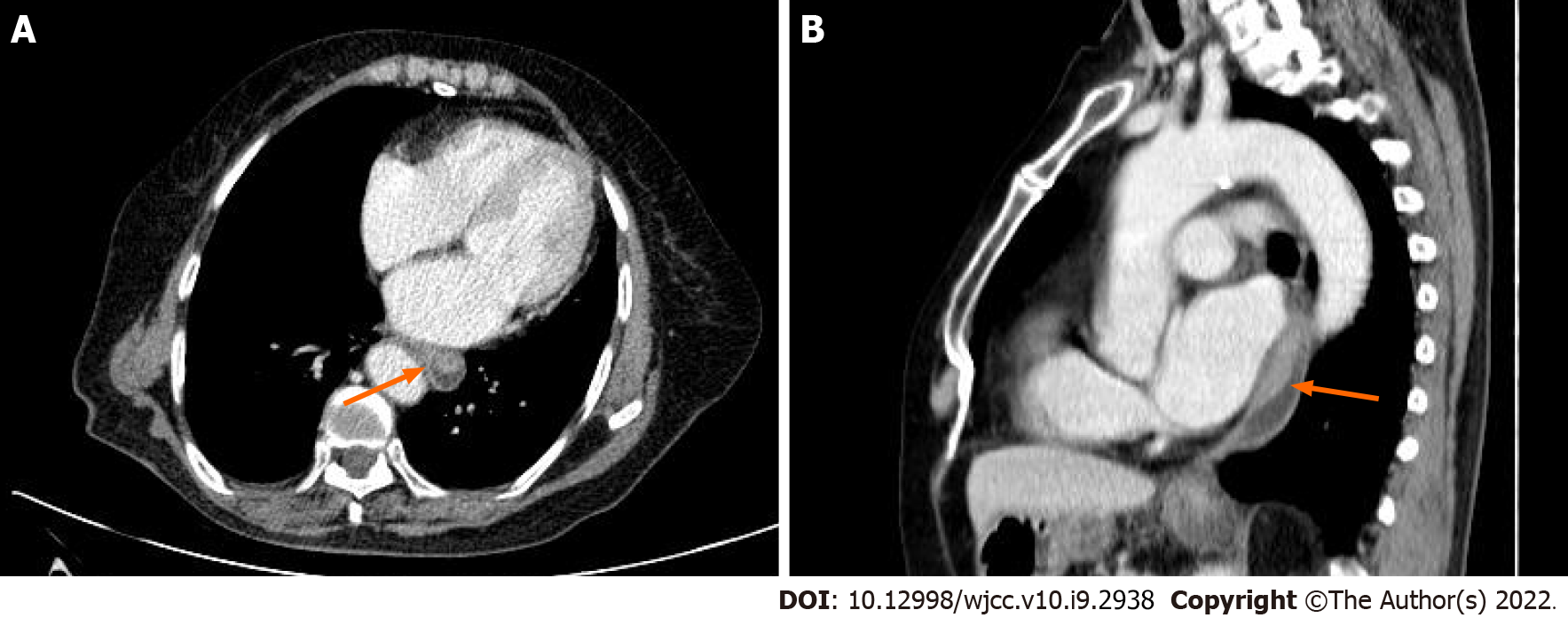
Case 4: Enhanced CT revealed a local thickened wall with a fungating-type mass with a complete mucosal layer, and no cystic change or necrosis was observed (Figure 4). Fat was evident between the lesion and the surrounding tissues, and the length of the lesion was approximately 45 mm. The enhanced scan showed homogeneous and mild enhancement. A soft-tissue nodule (28 mm × 15 mm) near the spinal column in the lower lobe of the right lung was also found, which was closely associated with the adjacent pleura and moderately enhanced. Bone scintigraphy revealed no abnormalities except for sparse distribution of the right acetabulum and proximal femur.
The immunohistochemistry results are presented in Table 2.
Case 1: Based on the pathological results, the final diagnosis was esophageal MC with basal squamous cell carcinoma, poorly differentiated, infiltrating the muscle layer with neither obvious vascular invasion nor perineural invasion or lymph node metastasis observed (4/22).
Case 2: The patient was diagnosed with esophageal MC with localized squamous cell carcinoma infiltrating the submucosa. There was neither obvious vascular invasion nor perineural invasion, and no lymph node metastasis was observed (0/21).
Case 3: The patient was diagnosed with esophageal MC infiltrating all layers, and neither obvious vascular invasion nor perineural invasion was observed. No lymph node metastasis was observed (0/13).
Case 4: The patient was diagnosed with high-grade esophageal MC infiltrating all layers, and no lymph node metastasis was observed (0/13). Moderately differentiated adenocarcinoma was observed in the lower lobe of the right lung.
Radical resection was performed for all patients. No adjuvant therapy was administered.
Case 1: The incision healed favorably, but the patient died one month after discharge from an unknown cause.
Case 2: The incision healed favorably. The patient recovered and was lost to follow-up after discharge.
Case 3: The patient recovered favorably and was discharged. At the four-month review, enhanced CT revealed anastomosis recurrence and bilateral lung metastases. The patient abandoned treatment and was lost to follow-up after discharge.
Case 4: The patient recovered favorably and was discharged. She attended review appointments regularly and remained in a good general condition.
To the best of our knowledge, MC was first reported by Stromeyer et al[1] in 1975. It is a rare malignant neoplasm that mainly occurs in the salivary gland[7]. Locations outside the salivary gland have rarely been reported, such as the bladder, skin, and gastrointestinal tract[5,6,8]. MC can be confused with many other tumors when arising outside the salivary glands because it presents with a broad spectrum of cytomorphological and immunohistochemical features[4]. The combination of histopathology and immunohistochemistry has diagnostic significance for myoepithelial carcinoma[9]. Specifically, myoepithelial differentiation and tumor infiltration into adjacent tissues are the currently accepted diagnostic criteria[10]. Here, we report the first pathologically confirmed cases of esophageal MC in four patients.
The incidence of MC increases with age, but no sex-specific differences exist[11]. In the present study, the average age of the patients was approximately 69 years, older than previous studies on MC[3,10]. A male-dominated was also observed. The primary complaint of most MC patients was a painless mass originating from the parotid gland and palate[10]. In this study, all patients presented an obvious sign of progressive dysphagia. The lesions all originated from the middle esophagus, which was similar to esophageal cell squamous carcinoma (ESCC). A mean tumor size of 3.5 cm was observed in our study, which was larger than the only previous report on gastric MC[6], but equal to the previous study on MC[11].
CEA examination of MC is usually negative, which can differentiate MC from adenoid cystic carcinoma[10]. In the present study, CEA exams were performed on two patients who turned out to be normal. TAP is increased in many carcinomas, such as colon, gastric, breast, ovarian, endometrial, and lung cancers, and plays a critical role in the development and progression of cancer, as well as the regulation of cell proliferation, apoptosis, differentiation, and development[12]. According to our study, TAP levels also increased in patients who underwent TAP exams. MC often demonstrates a low rate of neural invasion (8%) and angiolymphatic invasion (4%)[10], which was also observed in our study.
Studies have revealed that S-100, vimentin, and CK are more definitive markers of myoepithelial cells and help differentiate MC from other malignant tumors[9,10]. In this study, CK expression was observed in all tumors. SOX-10 can provide a basis for diagnosing salivary gland tumors based on tissue origin because it can specifically identify acinar and myoepithelial cells in salivary gland tissue[13]. Most tumors (3/4) in our study were observed to be positive for SOX-10. Ki-67 > 10% has diagnostic value in differentiating benign myoepithelioma from MC. Moreover, Ki-67 > 50% suggested that MC was more likely to recur or metastasize, indicating a poor prognosis[7,14,15]. In this study, all patients had a high Ki-67 level. One of two patients who had received regular examinations developed anastomosis recurrence and lung metastasis four months after the surgery.
Most literature on MC is focused on pathology and lacks detailed imaging data descriptions. Salivary gland MC showed an irregular lobulated or multinodular lesion with vague margins and inhomogeneous attenuation on unenhanced CT imaging. After contrast injection, it revealed moderate and intense inhomogeneous enhancement, including cystic and slit-like regions with no enhancement, small tortuous vessels in the arterial phase, and intense nodular enhancement[16]. Hassan et al[17] reported a liver MC showing a cystic tumor with a thick wall on ultrasonic echography. Tseng et al[6] reported a low-grade gastric myoepithelial carcinoma, but the report lacks detailed imaging data description. CT is a useful tool for evaluating original tumors of the esophagus, and knowledge of the imaging features of protruding esophageal lesions helps narrow down the differential diagnosis[18]. In this study, most of the lesions showed thickening of the esophageal wall or a soft-tissue mass with a complete mucosal layer. Cystic changes or necrosis are more likely to be observed in larger lesions. Most of them were not accompanied by enlarged lymph nodes. Unlike the findings of a big data study in which only 9.4% of esophageal cancer was the fungating type[19], this type was the most common in our study. This finding suggests that the fungating type may be more common in esophageal MC.
Most of the patients in this study were in the advanced stage at the time of admission, similar to ESCC[20]. Early detection and treatment of ESCC can improve prognosis[21]. As constantly improved and developed technology, endoscopic imaging techniques have been used to achieve early diagnosis and treatment of early esophageal cancer[21]. Endoscopic imaging techniques may also be used in the detection and treatment of early esophageal myoepithelial carcinoma in the future. CT is a noninvasive tool to evaluate recurrence[18], which is important due to the high recurrence rate of MC[22]. Anastomotic recurrence occurs infrequently in esophageal cancer (3%-9%), shown as soft-tissue masses or intramural nodular wall thickening of the stomach or esophagus at the anastomosis site on CT[22]. In the present study, we found that esophageal MC may have a higher anastomosis recurrence rate. Anastomosis recurrence exhibited a cystic-solid mass which is different from that of ESCC.
As the most common esophageal neoplasm, radical resection is the main surgical method of ESCC. The role of radiotherapy and chemotherapy in the postoperative treatment of ESCC has been widely recognized[23]. However, due to the lack of understanding of myoepithelial carcinoma, the main treatment is still surgical resection with no adjuvant[24]. Patients in this study all underwent surgical resection without chemotherapy or radiation. Although some studies have shown that conventional chemotherapy has some effects on MC[8,25], this still needs to be confirmed in a study with a large sample size. MC has a high recurrence and metastasis rate[24,26]. Regional and distant metastases mainly occur in end-stage disease, with distant metastases in the cervical lymph nodes and some organs, such as the lung, kidney, brain, and bone[27]. A retrospective study suggests that adjuvant radiation may reduce the rate of local recurrence[11]. Even though R0 resection was achieved in all patients in the present study, one patient still developed lung metastases and anastomosis recurrence.
The present study was a retrospective study with a small number of subjects. We first detailed reported the clinical, pathological, immunohistochemical, and imaging findings of four patients with esophageal MC, along with their outcomes. We also reviewed the relevant literature to deepen the understanding of esophageal MC.
Here, we presented the first report of the imaging and clinicopathological features of esophageal MC in four patients and reviewed the relevant literature. Esophageal MC is more likely to originate from the middle esophagus in elderly populations with male dominance. Esophageal MC should be included in the differential diagnosis of esophageal cancer. A fungating type observed on CT scanning may help narrow down the differential diagnosis. Cystic change or necrosis may occur in larger lesions. A characteristic anastomotic recurrence was observed on CT as a cystic-solid mass. The final diagnosis depends on pathological examination. The treatment for MC is surgical resection, and the efficacy of chemotherapy needs to be determined with future studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Hisada H, Liu T, Toyoshima O, Yu H S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Stromeyer FW, Haggitt RC, Nelson JF, Hardman JM. Myoepithelioma of minor salivary gland origin. Light and electron microscopical study. Arch Pathol. 1975;99:242-245. [PubMed] |
| 2. | Losito NS, Botti G, Ionna F, Pasquinelli G, Minenna P, Bisceglia M. Clear-cell myoepithelial carcinoma of the salivary glands: a clinicopathologic, immunohistochemical, and ultrastructural study of two cases involving the submandibular gland with review of the literature. Pathol Res Pract. 2008;204:335-344.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Yokose C, Asai J, Kan S, Nomiyama T, Takenaka H, Konishi E, Goto K, Ansai S, Katoh N. Myoepithelial carcinoma on the right shoulder: Case report with published work review. J Dermatol. 2016;43:1083-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Choi JW, Na SY. Image Gallery: Myoepithelial carcinoma involving the skin. Br J Dermatol. 2017;176:e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Tseng CE, Hsieh YH, Wei CK, Huang HY, Chi CL. Myoepithelial carcinoma of the stomach: a diagnostic pitfall. World J Gastroenterol. 2015;21:4391-4396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 7. | Nagao T, Sugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A, Kondo Y, Nagao K. Salivary gland malignant myoepithelioma: a clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998;83:1292-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Ordoñez-Tanchiva K, Guerra-Canchari P, Sueldo-Espinoza D. Myoepithelial Carcinoma of Urinary Bladder in a Pediatric Patient. A Case Report. Urology. 2020;144:202-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Kong M, Drill EN, Morris L, West L, Klimstra D, Gonen M, Ghossein R, Katabi N. Prognostic factors in myoepithelial carcinoma of salivary glands: a clinicopathologic study of 48 cases. Am J Surg Pathol. 2015;39:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Kane SV, Bagwan IN. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 51 cases in a tertiary cancer center. Arch Otolaryngol Head Neck Surg. 2010;136:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Giridhar P, Gupta P, Mallick S, Upadhyay AD, Rath GK. Impact of adjuvant therapy on survival in patients with myoepithelial carcinoma: A systematic review and individual patient data analysis of 691 patients. Radiother Oncol. 2019;140:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Cheng Y, Chen Y, Zang G, Chen B, Yao J, Zhang W, Wang H, Yu L, He P, Zhang Y, Wu H. Increased Expression of TAP Is Predictive of Poor Prognosis in Patients with Non-Small Cell Lung Cancer. Cancer Manag Res. 2020;12:1941-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, Watabe Y, Honda K, Yamada T, Yoshimoto S, Asai M, Okano H, Kanai Y, Tsuda H. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26:1041-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Brown DC, Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990;17:489-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 454] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Moul JW. Angiogenesis, p53, bcl-2 and Ki-67 in the progression of prostate cancer after radical prostatectomy. Eur Urol. 1999;35:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Yue D, Feng W, Ning C, Han LX, YaHong L. Myoepithelial carcinoma of the salivary gland: pathologic and CT imaging characteristics (report of 10 cases and literature review). Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:e182-e187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Hassan W, Nishi J, Tomiyasu S, Urakado T, Haraoka K, Yamanaka T, Fujiyama S, Ito T. Unusual biliary myoepithelial carcinoma in liver-case report and immunohistochemical study. Int J Clin Exp Pathol. 2014;7:2647-2653. [PubMed] |
| 18. | Tomita H, Miyakawa K, Wada S, Okamoto S, Morimoto T, Kishimoto K, Nakajima Y. The imaging features of protruding esophageal lesions. Jpn J Radiol. 2016;34:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Su M, Li XY, Tian DP, Wu MY, Wu XY, Lu SM, Huang HH, Li DR, Zheng ZC, Xu XH. Clinicopathologic analysis of esophageal and cardiac cancers and survey of molecular expression on tissue arrays in Chaoshan littoral of China. World J Gastroenterol. 2004;10:2163-2167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Fatehi Hassanabad A, Chehade R, Breadner D, Raphael J. Esophageal carcinoma: Towards targeted therapies. Cell Oncol (Dordr). 2020;43:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 21. | Yang H, Hu B. Recent advances in early esophageal cancer: diagnosis and treatment based on endoscopy. Postgrad Med. 2021;133:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Kim TJ, Lee KH, Kim YH, Sung SW, Jheon S, Cho SK, Lee KW. Postoperative imaging of esophageal cancer: what chest radiologists need to know. Radiographics. 2007;27:409-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Zhou Y, Hou P, Zha KJ, Wang F, Zhou K, He W, Gao JB. Prognostic value of pretreatment contrast-enhanced computed tomography in esophageal neuroendocrine carcinoma: A multi-center follow-up study. World J Gastroenterol. 2020;26:4680-4693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Chamberlain F, Cojocaru E, Scaranti M, Noujaim J, Constantinou A, Thway K, Fisher C, Messiou C, Strauss DC, Miah A, Zaidi S, Benson C, Gennatas S, Jones RL. Adult soft tissue myoepithelial carcinoma: treatment outcomes and efficacy of chemotherapy. Med Oncol. 2019;37:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Su YX, Roberts DB, Hanna EY, El-Naggar A, Saylam G, Frank SJ, Weber RS, Kupferman ME. Risk Factors and Prognosis for Myoepithelial Carcinoma of the Major Salivary Glands. Ann Surg Oncol. 2015;22:3701-3707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Wang C, Zhang Z, Ge Y, Liu Z, Sun J, Gao Z, Li L. Myoepithelial Carcinoma of the Salivary Glands: A Clinicopathologic Study of 29 Patients. J Oral Maxillofac Surg. 2015;73:1938-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |