Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2931
Peer-review started: October 8, 2021
First decision: December 17, 2021
Revised: January 4, 2022
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: March 26, 2022
Processing time: 164 Days and 23.8 Hours
Turner syndrome (TS) with leukemia is a complicated clinical condition. The clinical course and outcome of these patients are poor, so the treatment and prognosis of TS with hematological malignancies deserve our attention.
Here, we report a case of a 20-year-old woman diagnosed with TS, primary myelofibrosis (PMF), cirrhosis, and an ovarian cystic mass. This is the first report on the coexistence of TS and PMF with the MPL and SH2B3 mutations. The patient was diagnosed with cirrhosis of unknown cause, splenomegaly and severe gastroesophageal varices. Additionally, an ovarian cystic mass caused the patient to appear pregnant. The patient was treated with the JAK2 inhibitor-ruxolitinib according to peripheral blood cells, although myelofibrosis was improved, the splenomegaly did not reduce. Moreover, hematemesis and melena occasionally occurred.
Ruxolitinib may clearly reduce splenomegaly. Though myelofibrosis was improved, cirrhosis and splenomegaly in this case continued to worsen. Effective treatment should be discussed.
Core Tip: A case of Turner syndrome (TS) with chronic myeloid proliferative neoplasm is very rare. Here we report a 20-year-old woman diagnosed with TS, primary myelofibrosis, cirrhosis, and an ovarian cystic mass. The level of myelofibrosis was reduced after ruxolitinib treatment, however, anemia, thrombocytopenia, cirrhosis and splenomegaly continued to worsen. This indicates that the deterioration of splenomegaly may be caused by portal hypertension. Other treatment options and special care for patients such as the one in this case should be discussed.
- Citation: Xu LW, Su YZ, Tao HF. Turner syndrome with primary myelofibrosis, cirrhosis and ovarian cystic mass: A case report. World J Clin Cases 2022; 10(9): 2931-2937
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2931.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2931
Turner syndrome (TS) is the most common X chromosome monosomy, affecting one in 2500 live births among females[1]. The clinical features of TS include a short stature, gonadal failure, cubitus valgus, micrognathia, short neck, limb edema, webbed neck or cystic hygroma, low posterior hairline, short fourth metacarpal, and multiple naev[2-4]. Patients with TS suffer progressive cardiovascular, metabolic, and autoimmune comorbidities, as well as dysgerminoma[5,6]. TS with leukemia is a complicated clinical condition. Although the correlation between TS and hematologic malignancies remains unclear, several cases of TS with acute myelocytic leukemia[1] or large granular lymphocyte leukemia[7] have been reported. The incidence of chronic myeloid proliferative neoplasm in TS patients is rare, especially coinciding with cirrhotic portal hypertension and gynecologic disease. We report a case of TS with primary myelofibrosis (PMF), cirrhosis, and an ovarian cystic mass.
In April 2020, a 20-year-old woman with fatigue and shortness of breath hospitalized at our clinic.
Six months prior, she had an abnormal menstrual period and was admitted to another hospital. She had elevated white blood cell (WBC) counts, anemia, and thrombocytopenia. She was diagnosed with PMF by bone marrow aspiration. Biopsy exhibited myelofibrosis (MF) grade 2. Since then, she has been treated with ruxolitinib (10 mg, per oral, twice daily) without regular follow-up. Magnetic resonance and enhancement revealed cirrhosis, splenomegaly, and a large cystic mass in the right ovary. However, no further details regarding cirrhosis and the cystic mass obtained.
The patient had no significant medical history.
The patient had no remarkable personal or family history. The rest of the patient’s relatives are very healthy and tall.
On admission, the patient presented with anemia and abdominal swelling, similar to that of a pregnant woman. On physical examination, she was 148 cm tall, which was not consistent with the other family members. She weighed 45 kg and had a body mass index of 20.5. She had a webbed neck, low posterior hairline, epicanthus, and sixth toe on her right foot. She was clinically diagnosed with TS.
The chromosome analysis of the bone marrow cells revealed 45, X/46, XX, confirming the diagnosis of TS[8]. The peripheral blood cell count revealed a WBC count of 1.93 × 109/L, hemoglobin (Hb) count of 38 g/L, and platelet (PLT) count of 84 × 109 /L. Bone marrow aspiration indicated the coexistence of megakaryocytic emperipolesis and immature megakaryocyte cluster formation, while biopsy exhibited MF grade 1. Gene mutation testing revealed MPL and SH2B3 mutations.
Laboratory tests reported a remarkably elevated glutamic oxalacetic transaminase of 77.95 U/L (normal: 13-35 U/L), and glutamic-pyruvic transaminase of 84.1 U/L (normal: 7-40 U/L), while cholesterol and glucose levels were in the normal range (Table 1). The hepatitis panel, human immunodeficiency virus test, and autoantibody screening results were negative (Table 2). The carbohydrate antigen level of CA125 was 78.5 U/mL (normal < 35 U/mL). Gonadal hormones such as serum β-HCG and luteinizing hormone were within the normal ranges (Table 1). However, liver fibrosis markers were significantly elevated (Table 3), the significantly decreased amount of ferritin, normal ceruloplasmin and ophthalmic testing without ophthalmic signs (Kayser–Fleischer rings) allowed us to exclude the diagnosis of hemochromatosis and Wilson’s disease. No structural defects observed in the heart and major vessels on echocardiography. Abdomen ultrasound showed a huge cystic mass measured as 30 mm × 148 mm on the right ovary, which is considered as a serous cystadenoma.
| Characteristics | Test value | Normal value range |
| Glutamic oxalacetic transaminase | 77.95 U/L | 13-35 U/L |
| Glutamic-pyruvic transaminase | 84.1 U/L | 7-40 U/L |
| Cholesterol | 5.86 mmol/L | 3.1-6.0 mmol/L |
| Glucose | 4.51 mmol/L | 3.89-6.11 mmol/L |
| CA125 | 78.5 U/mL | < 35 U/mL |
| β-Human chorionic gonadotropin | 0.33 mIU/mL | < 2 mIU/mL |
| Luteinizing hormone | 4.25 mIU/mL | 0.56-89.08 mIU/mL |
| Follicle-stimulating hormone | 4.1 mIU/mL | 1.38-16.69 mIU/mL |
| Estradiol | 314.7 pg/mL | 21-649 mIU/mL |
| Prolactin | 29.84 mIU/mL | 5.18-26.53 mIU/mL |
| Characteristics | Test value | Normal value range |
| Antinuclear antibodies | Negative | < 1:32 |
| nRNP/Sm | Negative | Negative |
| Sm | Negative | Negative |
| SSA | Negative | Negative |
| Ro-52 | Negative | Negative |
| SS-B | Negative | Negative |
| Scl-70 | Negative | Negative |
| Jo-1 | Negative | Negative |
| The centromere B | Negative | Negative |
| dsDNA | Negative | Negative |
| Nucleosome | Negative | Negative |
| Histone | Negative | Negative |
| Anti-ribosomal P protein antibody | Negative | Negative |
| Anti-mitochondrial antibody | Negative | Negative |
| Anti - liver and kidney microsomal antibodies | Negative | Negative |
| Anti-liver solute antigen antibody | Negative | Negative |
| Anti-hepatopancreatic antigen antibody | Negative | Negative |
| Characteristics | Test value (ng/mL) | Normal value range (ng/mL) |
| Collagen type IV | 172.87 | 0-95 |
| Laminin | 169.82 | 0-130 |
| N-terminal peptide of type III procollagen | 6.83 | 0-15 |
| Hyaluronic acid | 71.21 | 0-120 |
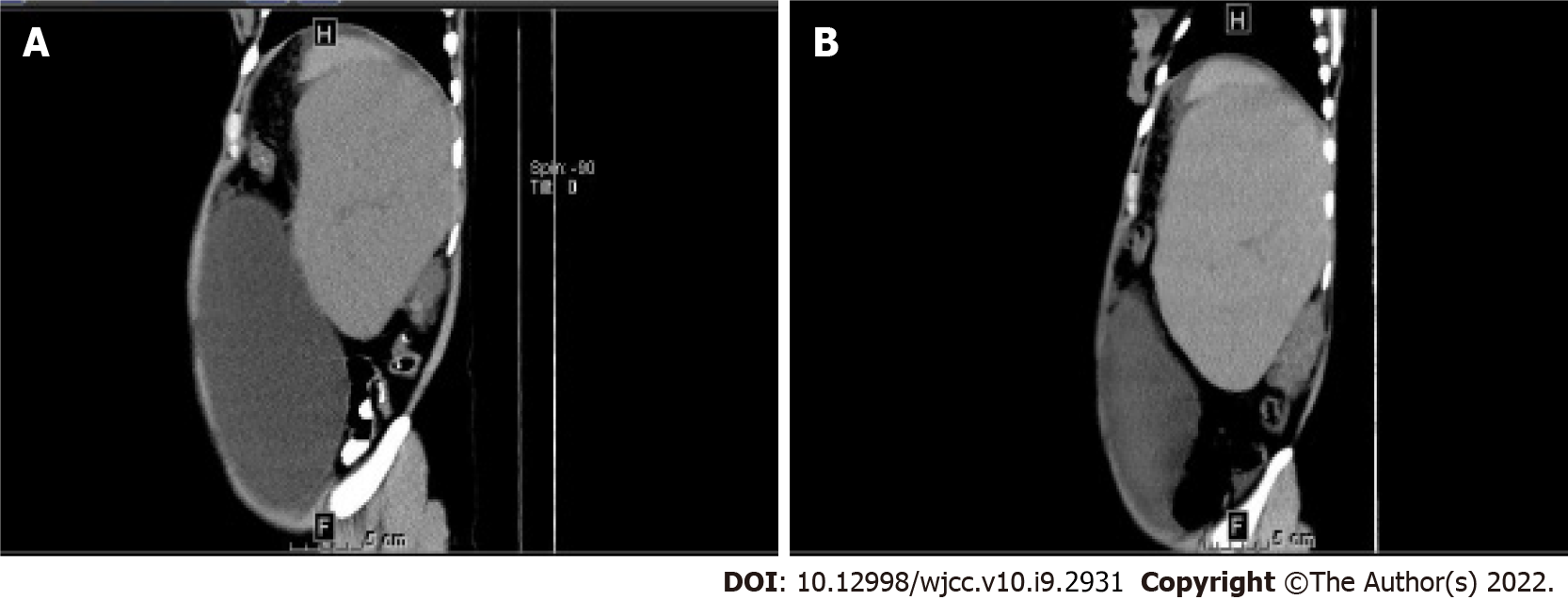
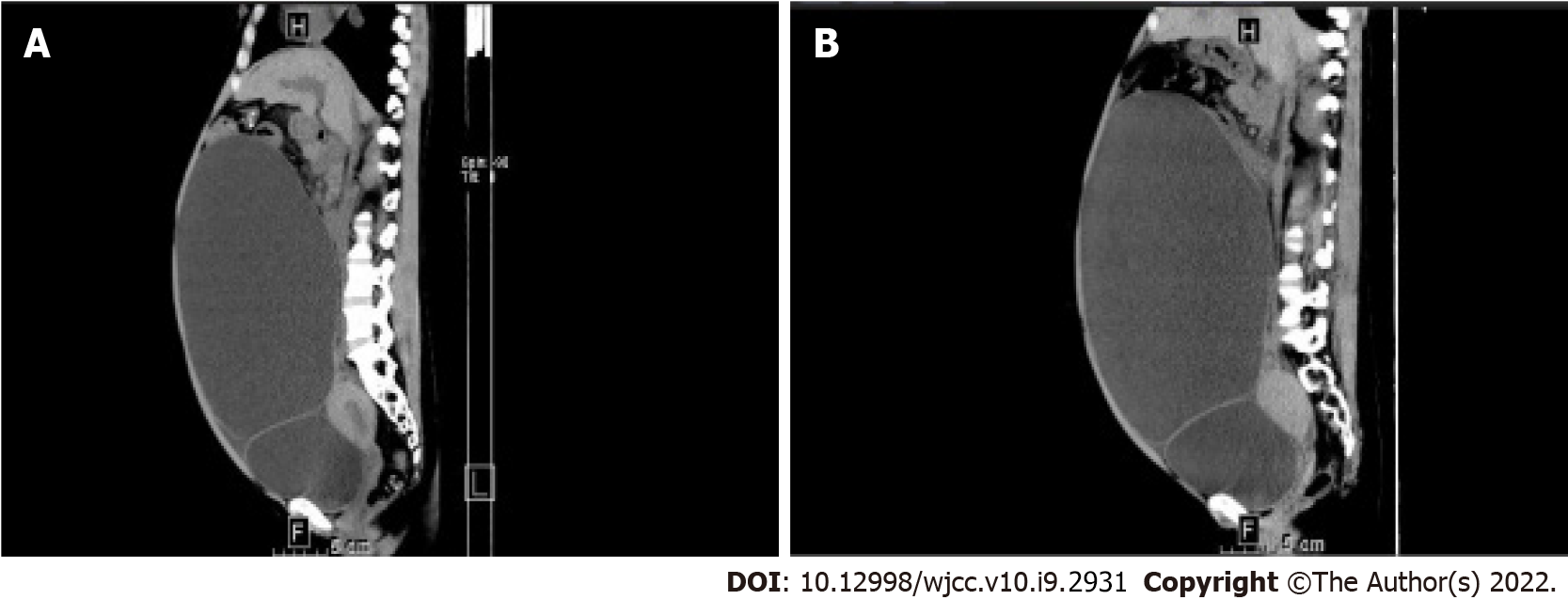
Abdominal computed tomography (CT) revealed splenomegaly (114 mm × 100 mm × 163 mm; Figure 1A), cirrhosis, and portal hypertension. On the CT scan, an ovarian cystic mass, measuring 236 mm × 143 mm × 255 mm (Figure 2A) was detected on the right side.
“The patient is diagnosed with TS, PMF, cirrhosis and large ovarian cystic mass. At present, she is facing the problem that her uterus is currently bleeding irregularly and the large ovarian cystic mass make her life inconvenient. Removal of the uterus and cystic mass is urgently needed, however, portal hypertension and splenomegaly make it extremely difficult, owing to the risk of excessive bleeding and unstable circulation.”
“The patient is diagnosed with cirrhosis with portal hypertension and splenomegaly. During the surgery, because a change in abdominal pressure can cause venous bleeding in the liver, splenectomy and splenic embolization should be considered to reduce portal pressure prior to surgery.”
“A large ovarian cystic mass has been found; removal of the ovarian cystic mass may easily lead to disorder of the internal circulation. Insufficient return blood can lead to cardiac arrest at any time, which can be life-threatening.”
TS, PMF with MPL and SH2B3 mutations, cirrhosis, and ovarian cystic mass were diagnosed.
Ruxolitinib was intermittently used based on the blood platelet count. The recommended starting dose is 5mg or 10 mg twice daily, depending on platelet counts (≥ 50 to < 75 × 109/L, 75-100 × 109/L, respectively) regardless of Hb level. When the blood platelet count < 50 × 109/L, ruxolitinib use is recommended to cease.
During her hospitalization, the patient had menorrhagia. Triptorelin (3.75 mg, monthly) was injected to control the abnormal bleeding. Due to the splenomegaly and ovarian cystic mass, she had abdominal swelling similar to that of a pregnant woman and suffered from low self-esteem. A multidisciplinary team was formed to discuss the possibility of surgical removal of the ovarian cystic mass. Due to the high risk of sudden cardiac arrest and uncontrollable bleeding after tumor removal, surgery was not performed.
Six months later, the patient experienced hematemesis and melena for 3 d and was admitted to our hospital. Severe esophageal and gastric fundus varices were observed on gastroscopy. An abdominal CT scan showed splenomegaly (100 mm × 76 mm × 213 mm; Figure 1B) and an ovarian cystic mass with the same size (Figure 2B) as before. Peripheral blood cell count revealed a WBC of 2.59 × 109/L, Hb of 47 g/L, and PLT count of 41 × 109 /L. Ruxolitinib was interrupted. Antacids and hemostatic agents were administered to control the bleeding. At present, the patient has remained well with moderate anemia and thrombocytopenia.
TS patients have an increased morbidity and mortality due to coronary diseases, congenital malformations, and endocrine diseases[9]. Cases of TS with hematologic malignancies are rare, with few reported cases. Down syndrome is closely related to leukemia because of trisomy 21[8,10]. However, the mechanisms behind the relationship between TS and hematologic malignancies remain unknown. We report the first case of TS with PMF, cirrhosis, and an ovarian cystic mass.
This patient was a 20-year-old female who presented with extensive splenomegaly and cirrhosis. Although liver and ovarian failure are typical features of TS, cirrhosis and portal hypertension rarely occur, especially in young patients[11]. However, the incidence of cirrhosis is reportedly increased by 6-fold in TS patients[12]. For this patient, low levels of ferritin and no iron overload in the liver and/or spleen on magnetic resonance imaging of the liver could exclude the diagnosis of hereditary hemochromatosis[13]. Wilson’s disease was also excluded because of normal range of ceruloplasmin and absence of ophthalmic signs (Kayser–Fleischer rings)[14]. Also alcoholic liver disease, non-alcoholic fatty liver disease and infective forms of hepatitis was excluded. However, a thorough genetic examination is needed to verify that the patient has no genetic disorders related to liver cirrhosis such as hereditary hemochromatosis, Wilson’s disease and alpha-1 antitrypsin deficiency. During her hospital stay, the PLT count was sometimes less than 50 × 109/L and transfusion effect is poor, so liver biopsy was not considered. According to Roulot et al[15], the liver abnormalities in TS patients are mainly caused by congenital vascular disorders. Considering the unknown origin of cirrhosis, genetic disease detection or liver biopsy is needed when it is permitted.
The patient was diagnosed with PMF with MPL and SH2B3 mutations by bone marrow aspiration and next-generation amplicon sequencing. She was treated with ruxolitinib (5-10 mg twice daily), depending on platelet counts (≥ 50 to < 75 × 109/L, 75-100 × 109/L, respectively) regardless of Hb level. Though it is well recognized that low red blood cell counts are observed as side effects of ruxolitinib, Cervantes et al[16] and Verstovsek et al[17] believe that it is unnecessary to delay or withhold ruxolitinib because of co-existent or treatment related anemia. Ruxolitinib is discontinued when the blood platelet count < 50 × 109/L. Most of patients with myeloproliferative neoplasms can achieve a ≥ 50% reduction in palpable spleen length at any time during the treatment with ruxolitinib[16]. The level of myelofibrosis was reduced after ruxolitinib treatment, however, significant splenomegaly was observed in this patient after 48 wk treatment with ruxolitinib, hypersplenism may be the main cause of pancytopenia instead of the side effect of ruxolitinib. Therefore, we consider the blood routine does not represent his true level, we make treatment plan of ruxolitinib which is correlate with Roulot et al[15].
Whether the mutations found in this patient, such as those involving MPL and/or SH2B3, have prognostic importance on the treatment of PMF is unknown. In essential thrombocythemia patients, mutation in SH2B3 was found to be an additional negative prognostic factor[18], which has not been clearly demonstrated in PMF. Though it is recognized that MPL is associated with higher risk of fibrotic progression in essential thrombocythemia[19], definitive conclusions regarding the impact of MPL and SH2B3 mutations on prognosis or other pathological changes are difficult due to the fact that less than 10% of patients with PMF harbor alternations in the MPL gene[20] and the rarity of SH2B3 mutations in PMF patients.
Our patient had intermittent hematemesis and melena due to portal hypertension, resulting in a decreased platelet count. Cryptogenic cirrhosis with upper gastrointestinal bleeding has been reported in TS patients[11]. The reduction of intrahepatic resistance by a transjugular intrahepatic portosystemic shunt is a viable option in patients who were not successfully treated by conservative medical treatment[21].
An ovarian cystic mass was discovered by CT scan. It maintained the same size for 6 mo, and there was no apparent tumor infiltration. Although pathologic examination was not conducted, the mass was likely a benign ovarian tumor. Mukerji et al[22]. established a close relationship between TS and germ cell tumors. Moreover, gonadoblastomas commonly occur during the second decade of life, which is consistent with the age of our patient. Gonadoblastoma is a pre-malignant neoplasm with a germ cell component, liable to malignant transformation into invasive dysgerminoma. Further pathological examination should be performed in our patient to determine the necessity of gonadectomy for preventing cancer.
Our case was limited because no pathological data of the ovarian cystic mass and liver were obtained due to the risk of surgery. To our knowledge, this is the first case of TS accompanied by MF. More research is needed to understand the concurrence of TS and leukemia, as well as other complications. Further studies on cirrhosis and PMF are necessary to clarify the pathological and physical changes in hepatosplenomegaly.
The authors thank all the investigators involved in this study, including the physicians, nurses, pathologists and laboratory technicians.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta T, Navarro-Alvarez N S-Editor: Guo XR L-Editor: A P-Editor: Guo XR
| 1. | Siddiqui N, Ali Baig MF, Khan BA. A case report of acute myelogenous leukemia with Turner Syndrome. J Pak Med Assoc. 2017;67:1438-1440. [PubMed] |
| 2. | Mondal S, Bhattacharjee R, Chowdhury S, Mukhopadhyay S. Karyotype-Phenotype Correlation in Turner Syndrome at a Single Center in Eastern India. Indian Pediatr. 2021;58:34-37. [PubMed] |
| 3. | Park EG, Kim EJ, Kim HY, Kim SH, Yang A. Coexistence of Growth Hormone Deficiency and Pituitary Microadenoma in a Child with Unique Mosaic Turner Syndrome: A Case Report and Literature Review. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kilinc S, Yildiz M, Guven A. Associated clinical abnormalities among patients with Turner syndrome. North Clin Istanb. 2020;7:226-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Dejonckheere C, Moyson C, de Zegher F, Antonio L, Van Buggenhout G, Decallonne B. Neoplasia in Turner syndrome: a retrospective cohort study in a tertiary referral centre in Belgium. Acta Clin Belg. 2020;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Dulac Y, Pienkowski C, Abadir S, Tauber M, Acar P. Cardiovascular abnormalities in Turner's syndrome: what prevention? Arch Cardiovasc Dis. 2008;101:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Manola KN, Sambani C, Karakasis D, Kalliakosta G, Harhalakis N, Papaioannou M. Leukemias associated with Turner syndrome: report of three cases and review of the literature. Leuk Res. 2008;32:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Xavier AC, Taub JW. Acute leukemia in children with Down syndrome. Haematologica. 2010;95:1043-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897-3902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 430] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 10. | Marlow EC, Ducore J, Kwan ML, Cheng SY, Bowles EJA, Greenlee RT, Pole JD, Rahm AK, Stout NK, Weinmann S, Smith-Bindman R, Miglioretti DL. Leukemia Risk in a Cohort of 3.9 Million Children with and without Down Syndrome. J Pediatr. 2021;234:172-180.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Idilman R, De Maria N, Colantoni A, Kugelmas M, Van Thiel DH. Cirrhosis in Turner's syndrome: case report and literature review. Eur J Gastroenterol Hepatol. 2000;12:707-709. [PubMed] |
| 12. | Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA; United Kingdom Clinical Cytogenetics Group. Mortality in women with turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab. 2008;93:4735-4742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Wu L, Zhang W, Li Y, Zhou D, Zhang B, Xu A, Wu Z, Wu L, Li S, Wang X, Zhao X, Wang Q, Li M, Wang Y, You H, Huang J, Ou X, Jia J. Correlation of genotype and phenotype in 32 patients with hereditary hemochromatosis in China. Orphanet J Rare Dis. 2021;16:398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Kasztelan-Szczerbinska B, Cichoz-Lach H. Wilson's Disease: An Update on the Diagnostic Workup and Management. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Roulot D, Degott C, Chazouillères O, Oberti F, Calès P, Carbonell N, Benferhat S, Bresson-Hadni S, Valla D. Vascular involvement of the liver in Turner's syndrome. Hepatology. 2004;39:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Cervantes F, Ross DM, Radinoff A, Palandri F, Myasnikov A, Vannucchi AM, Zachee P, Gisslinger H, Komatsu N, Foltz L, Mannelli F, Passamonti F, Gilotti G, Sadek I, Tiwari R, Zor E, Al-Ali HK. Efficacy and safety of a novel dosing strategy for ruxolitinib in the treatment of patients with myelofibrosis and anemia: the REALISE phase 2 study. Leukemia. 2021;35:3455-3465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, Talpaz M, Winton EF, Harvey JH Jr, Arcasoy MO, Hexner E, Lyons RM, Paquette R, Raza A, Vaddi K, Erickson-Viitanen S, Koumenis IL, Sun W, Sandor V, Kantarjian HM. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1559] [Cited by in RCA: 1642] [Article Influence: 126.3] [Reference Citation Analysis (0)] |
| 18. | Zhou A, Afzal A, Oh ST. Prognostication in Philadelphia Chromosome Negative Myeloproliferative Neoplasms: a Review of the Recent Literature. Curr Hematol Malig Rep. 2017;12:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Ross DM, Babon JJ, Tvorogov D, Thomas D. Persistence of myelofibrosis treated with ruxolitinib: biology and clinical implications. Haematologica. 2021;106:1244-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Piasecki A, Leiva O, Ravid K. Lysyl oxidase inhibition in primary myelofibrosis: A renewed strategy. Arch Stem Cell Ther. 2020;1:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Tefferi A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2021;96:145-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 205] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 22. | Mukerji B, Balshan E, Haderer R, Shertz W, Graebe R. Adolescent Female With Turner's Syndrome and 46,X,der(Y) del(Y)(p11.2)del(q11.2) Karyotype With Gonadoblastoma and Dysgerminoma. Pediatr Dev Pathol. 2017;20:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |