Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2916
Peer-review started: October 6, 2021
First decision: December 10, 2021
Revised: December 19, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 26, 2022
Processing time: 167 Days and 4.6 Hours
Undifferentiated pleomorphic sarcoma (UPS) is a type of soft tissue sarcoma, the histologic origin and differentiation direction of which are still unclear. There are few treatment options for UPS other than surgery. Herein we describe a patient who had multiple recurrences of UPS postoperatively, but R0 resection was achieved by local hyperthermia combined with chemotherapy, thus providing a new treatment approach for similar situations.
A 65-year-old man sought evaluation from a physician for a mass on his right back. After surgery, the pathologic diagnosis was fibrosarcoma. During the follow-up evaluations until 2021, the patient had four relapses of varying degrees. Postoperative pathology confirmed the recurrence of UPS on the right back. In March 2021, he underwent local hyperthermia combined with two cycles of chemotherapy for recurring lesions. After magnetic resonance imaging re-examination and preoperative examination, the patient chose surgery again. During the operation, the tumors were easy to excise, the amount of bleeding decreased significantly, and the pathologic evaluation confirmed that one of the specimens was an R0 excision.
Local hyperthermia combined with chemotherapy enables R0 resection to be achieved in patients with advanced UPS recurrence.
Core Tip: Undifferentiated pleomorphic sarcoma is a type of soft tissue sarcoma, but the histologic origin and direction of differentiation are still unclear. Patients with locally advanced, unresectable, or metastatic disease have a poor prognosis, and there are few treatment options available. Clinically, local hyperthermia combined with chemotherapy can be used to achieve surgical R0 resection in this type of patient.
- Citation: Zhou YT, Wang RY, Zhang Y, Li DY, Yu J. Local hyperthermia combined with chemotherapy for the treatment of multiple recurrences of undifferentiated pleomorphic sarcoma: A case report. World J Clin Cases 2022; 10(9): 2916-2922
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2916.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2916
Undifferentiated polymorphic sarcoma, once known as malignant fibroblastoma, has been officially replaced by Undifferentiated pleomorphic sarcoma (UPS) malignant fibrous histiocytoma according to the latest classification of related sarcomas in 2013 and is defined as a soft tissue sarcoma type however, the histologic origin and direction of differentiation direction of which are still unclear[1,2]. At present, there are many clinical treatment methods for UPS (primarily surgical resection with adjuvant radiotherapy and chemotherapy), but the postoperative recurrence and metastasis rates are high and the prognosis is poor. In addition to early detection and complete tumor resection, effective measures to reduce recurrence rates, facilitate surgery, and achieve R0 resection are still lacking, representing a significant challenge.
Herein, we report a patient with advanced UPS who was admitted to the Hyperthermia Center of Zhongshan Hospital affiliated with Dalian University and treated with local hyperthermia combined with chemotherapy. The patient then proceeded with surgery, and R0 resection was achieved.
A 65-year-old elderly male patient was admitted to the hospital 6 years after the first surgery to remove a pleomorphic sarcoma from the right side of his back, and he experienced recurrence 4 mo after the last surgery.
The patient underwent primary mass resection from the right back in October 2015. The pathologic evaluation of the surgical specimen revealed fibrosarcoma; the specific stage and immunohistochemical results were not available. In November 2017, December 2018, September 2019, and August 2020, the patient underwent extended resection of soft tissue masses from the right back due to recurrence. The pathologic diagnoses indicated UPS recurrence. After surgery, 2 cycles of doxorubicin + cisplatin chemotherapy, 1 cycle of ifosfamide chemotherapy (specific dose unknown), 2 cycles of gemcitabine + docetaxel chemotherapy (specific dose unknown), anlotinib hydrochloride anti-vascular generative therapy, and bevacizumab + pembrolizumab immunotherapy were administered, but the efficacy was poor. The patient was referred to our hospital for further treatment.
The patient was diagnosed with type 2 diabetes several years after radical resection of renal cancer.
No data were available.
The right back had several well-healed, old surgical scars with no erythema, swelling, or ulcerations. At the level of the 12th rib along the right posterior subaxillary line, a palpable, tender, round-like mass approximately 3 cm in diameter was noted. An ellipsoidal, tender mass, approximately 4 cm in diameter, was palpable at the lateral margin of the lower right scapula and the medial side of the scapula. There was no apparent limitation in thoracic mobility, and breath sounds were minimal in both lungs.
No abnormalities in tumor markers were found.
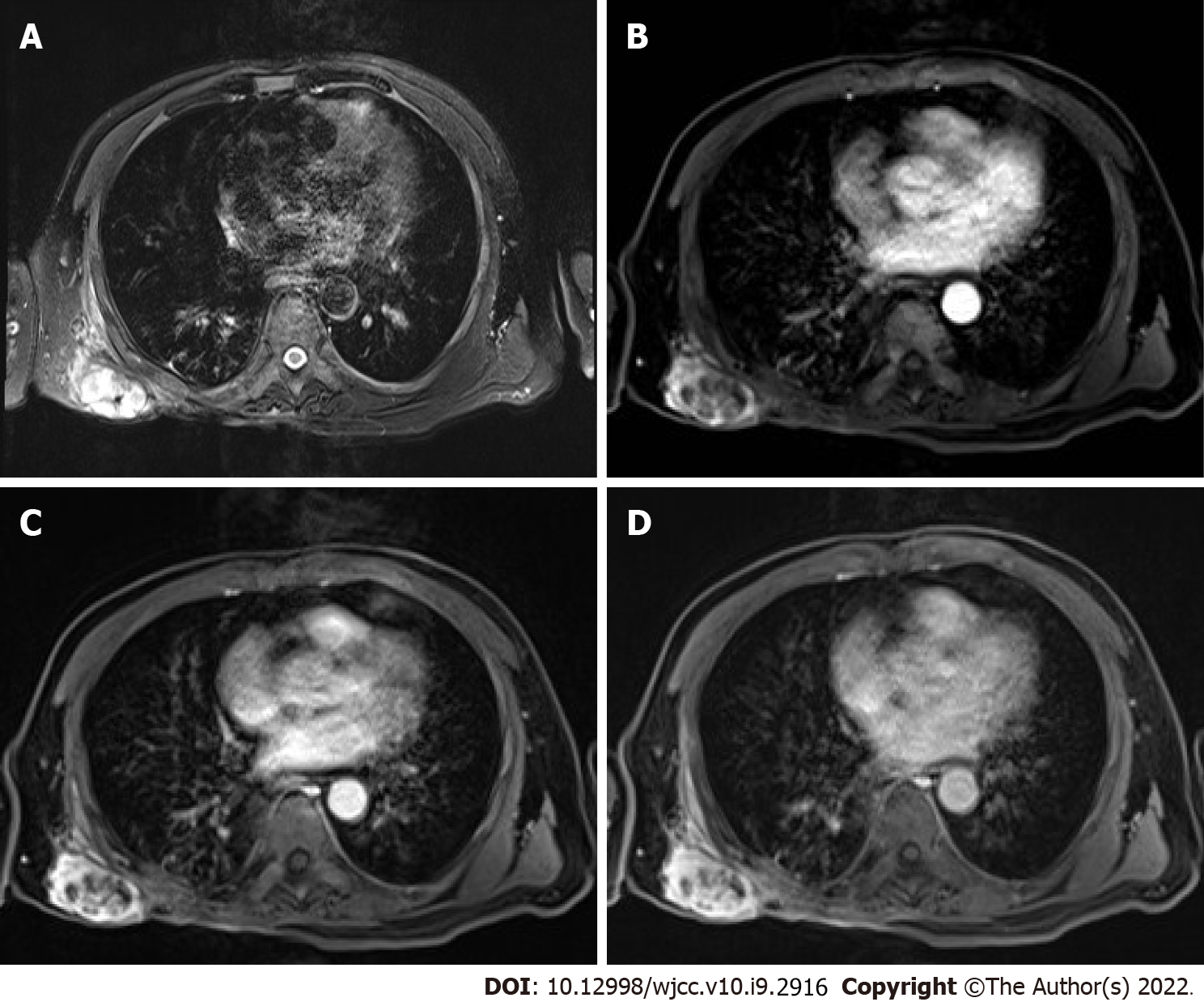
Magnetic resonance imaging (MRI) showed that the right subscapular muscle, infraspinatus muscle, teres major, and latissimus dorsi muscle had local structural disorder. The images revealed the following: Patchy and elliptical abnormal signals; equal or slightly low signals on T1-weighted images; high signals on T2-weighted images; slightly higher signals on DWI; and similar signals or a low signal on ADC. Part of the boundary was clear; and the largest lesion was approximately 5.9 cm in diameter.
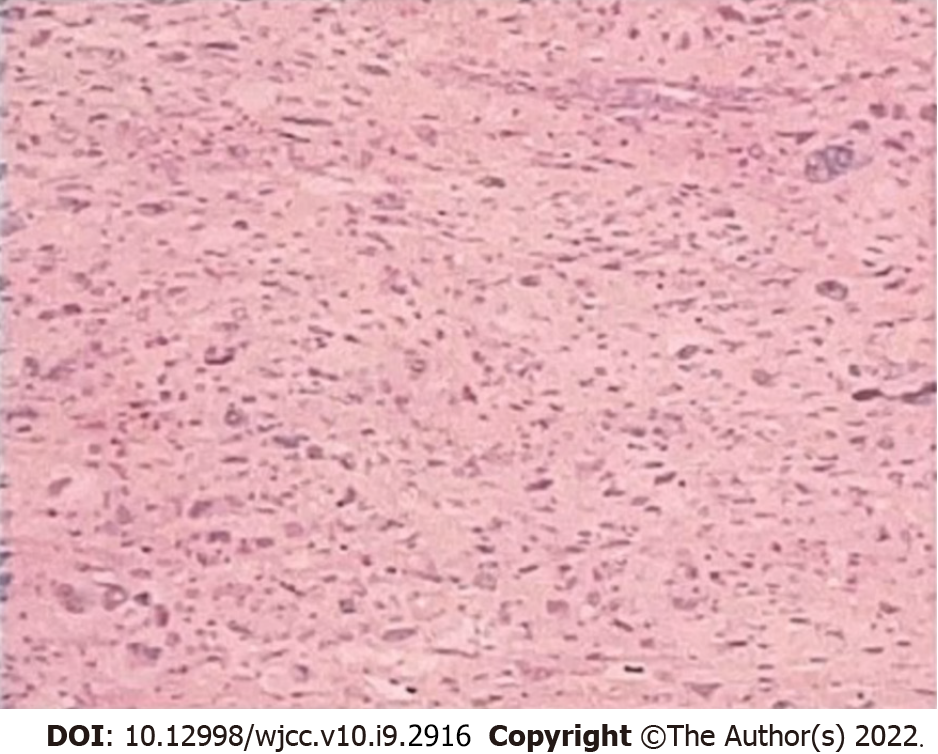
Based on both the clinical features and postoperative pathology (Figure 1), the patient was diagnosed with UPS recurrence.
Local hyperthermia (BSD-2000 deep hyperthermia; 420 Hz; 40 min/session; 3 times/wk) treatment was delivered to the area of recurrence on the back, combined with eribulin (2 mg on d1 and 8) chemotherapy for 2 cycles. After preoperative examination, enlarged resection of the mass on the right back was performed.
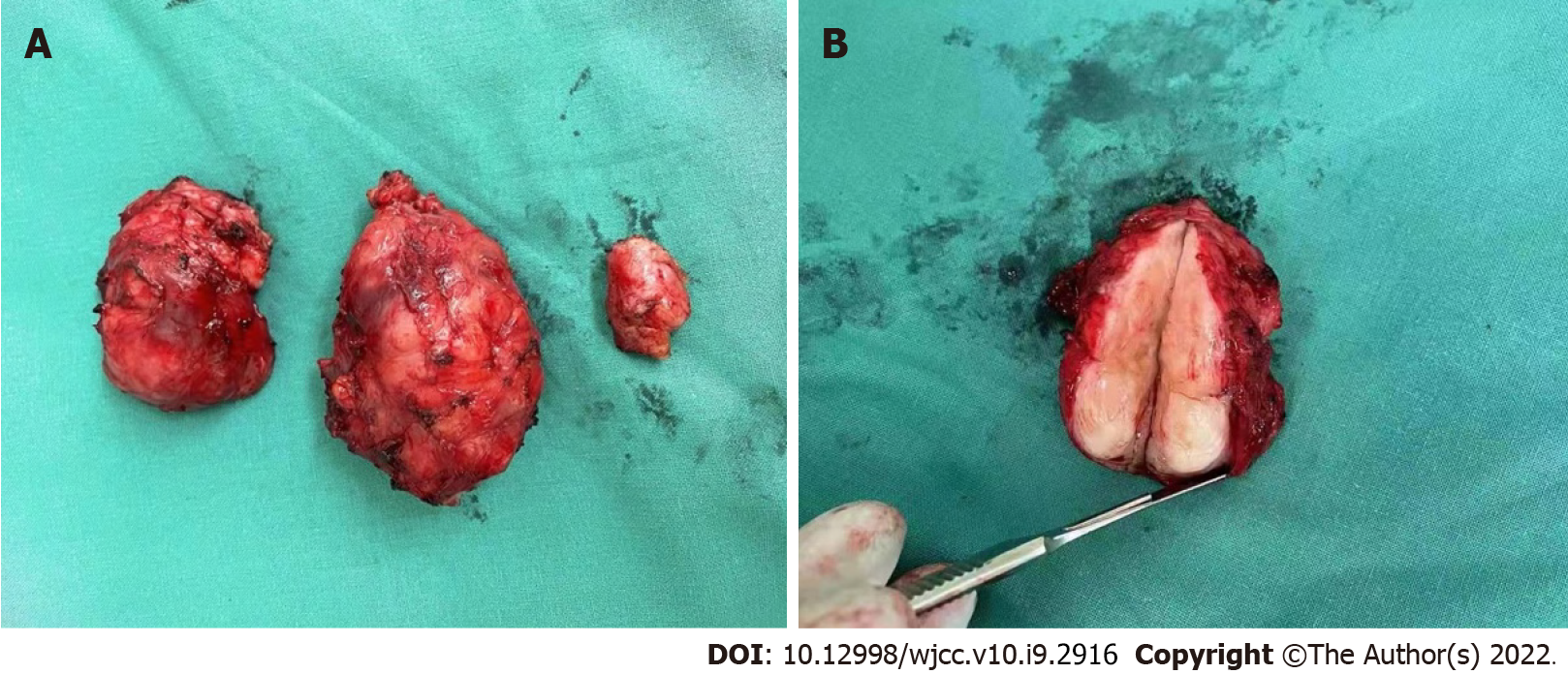
The findings from resection of the right dorsal recurrent lesion were as follows (Figure 2A): There was a mass approximately 3 cm × 3 cm × 2 cm in size at the level of the flat 12 ribs on the right back. The mass was opened, appearing red and white in color on the cut surface, with scar-like tissue and a tough texture. Intraoperative frozen pathology revealed the following: Heterotypic cells were noted in the lower cut edge of the right lower dorsal mass; no tumor involvement at the resection margins of the remaining (upper, inner, outer, and bottom) tumor on the right lower back; and no tumor residue at the incision; A mass approximately 5 cm × 4 cm × 3 cm in size was noted on the lateral edge of the right dorsal subscapularis. After the mass was opened, the cut surface appeared red and white in color with oval-shaped, fish-like tissue and a tough texture (Figure 2B). Intraoperative frozen pathology revealed the following: Heterotypic cells on the upper and bottom cut edges of the right dorsal mass but no tumor involvement at the lower, inner, and outer cut margins; A mass approximately 4 cm × 4 cm × 3 cm in size was noted in the right dorsal scapula within the internal border muscle. The mass was cut open, and the cut surface appeared red and white in color with ellipsoidal, fish-like tissue and a tough texture. Intraoperative frozen pathology revealed the following: No tumor tissue was seen in the upper, lower, outer, or bottom of the right dorsal mass.
The surgeons reported that the lesion had clear boundaries with normal tissue, which was easy to dissect, and the intraoperative bleeding volume was significantly reduced, at approximately 200 mL. The postoperative pathologic diagnosis revealed that the UPS had recurred. The patient’s condition was relatively stable. The last follow-up evaluation was 31 August, 2021.
UPS is derived from primitive undifferentiated mesenchymal cells and lacks specific immunohistochemical markers[3]. Immunohistochemically, Vim, CD68, AAT, and AACT are usually positive, while epithelial markers such as CK5/6 and SMA are generally negative[4]. It is a high-degree malignant tumor; detection and early treatment are key[5]. Complete surgical resection is the most important treatment for UPS, and it is also an effective treatment method to reduce recurrence and metastasis. The minimum resection margin should be at least 10 mm to minimize the risk of local recurrence and metastasis[6]. In patients who have resectable soft tissue sarcomas that are detected early, radical resection combined with chemotherapy or radiation improves the 5-year survival rate. For patients who have advanced soft tissue sarcoma but missed the opportunity for surgery or have repeated recurrences after surgery, comprehensive treatment can be considered, such as combined therapy with radiotherapy, chemotherapy, local hyperthermia, targeted therapy and immunotherapy. Nevertheless, the recurrence and metastasis rates of UPS remain high.
Local hyperthermia is a new, painless, noninvasive, green, and precise cancer treatment that can be administered after traditional treatment. The basic principle is to use all kinds of physical energy to produce a thermal effect in human tissues. The difference in temperature tolerance between normal cells and tumor cells can be used to kill tumor cells and avoid the destruction of normal tissue. As the fifth most common treatment after surgery, radiotherapy, chemotherapy, and biotherapy, local hyperthermia has been approved by the FDA and can be used either alone or in combination with radiotherapy and chemotherapy. During local hyperthermia treatment, blood vessels in the tumor dilate, blood circulation accelerates, and the cumulative concentration of internal chemotherapy drugs increases. Tumor hyperthermia also effectively inhibits DNA repair and the expression of multiple drug resistance, resulting in increased sensitivity of cancer cells to chemotherapeutic agents and reduced or reversed incidence of drug resistance in tumors; some chemotherapeutics can also achieve a synergistic antitumor effect[7]. Local hyperthermia focuses the antitumor effect of chemotherapy drugs in the heated tumor area. Issels et al[8] demonstrated for the first time in a phase 3 randomized trial that local hyperthermia combined with pre- and postoperative chemotherapy was clinically more effective than chemotherapy alone in a specific population of high-risk soft-tissue sarcoma patients. The use of local hyperthermia adjuvant therapy in tumor surgery can improve the local control rate and patient survival[9].
The patient presented herein had recurrent UPS postoperatively. The age, medical history, and site of onset were all consistent with the characteristics of UPS. This disease has a high degree of malignancy and can cause multiple recurrences after surgery. He had been treated with a variety of adjuvant chemotherapy regimens, all of which had poor efficacy, and serious adverse reactions were noted during treatment. Considering the actual situation of patients, local hyperthermia combined with Eribulin (a new chemotherapy drug) was given, which achieved good clinical effect. Eribulin is a synthetic analog of the soft seaweed B. Eribulin. It can inhibit microtubule polymerization through specific binding sites on β-tubulin, has antimitotic effects based on tubulin, prevents cells from transitioning to G2 and M stages, and disrupts the formation of mitotic spindles[10]. Kawai et al[11] reported a phase II study that compared pazopanib and trabectedin. Eribulin significantly improved the overall survival rate of patients with advanced sarcoma and has been gradually applied to the treatment of sarcoma. Although the lesion did not show significant shrinkage on MRI, the boundary of the lesion was clear, and there were multiple cystic necrotic areas inside the lesion, suggesting that the tumor activity in the lesion had been reduced (Figure 3). During the operation, it was found that the lesion could be easily removed, and the amount of bleeding was significantly reduced. Pathologic evaluation revealed that one of the sites was a successful R0 resection. This case shows that local hyperthermia combined with chemotherapy in UPS patients can cause tumor activity to decrease, can reduce the adhesions between the tumor and normal tissues, and can reduce intraoperative bleeding. It can provide a practical basis for increasing the R0 resection rate.
At the same time, this case also caused us to think more about the diagnosis, treatment and prognosis of UPS. At the 2021 Chinese Society of Clinical Oncology academic annual meeting, it was proposed that some types of soft tissue tumors show molecular changes, such as soft tissue tumors with PRDM10 rearrangement, which are mainly seen in low-grade UPS[12]. The study of Pazzaglia et al[13] proposed that the MET gene can predict the risk of metastasis in such patients at the genetic level. When their study divided the patients into prognostic subgroups, they found that the level of MET mRNA in metastatic patients was significantly higher than that in nonmetastatic patients. Regarding the treatment of UPS, in addition to the local hyperthermia combined with traditional chemotherapy strategy used for this patient, molecular targeted drugs and PD-1 and PD-L1 inhibitors have also gradually received attention. Molecular targeted drugs and PD-1 and PD-L1 inhibitors are also gradually becoming valued. For example, drugs such as apatinib, olazumab, lidafromus and anlotinib have shown significant efficacy in UPS treatment[14]. In 2021, a study on the efficacy of chidamine combined with the PD-1 inhibitor teriprizumab for the treatment of advanced soft tissue sarcoma (STS) conducted at Sun Yat-sen University found that after a follow-up of up to 40 wk, 3 of the 7 patients with advanced STS, 3 patients experienced partial remission, and 2 patients were in stable condition[15]. According to existing international studies, the synergistic effect of hyperthermia and immunotherapy on tumors such as pancreatic cancer and breast cancer has been confirmed[16-18]. However, there are no relevant reports for UPS treatment at present, so more basic and clinical research is needed to explore the synergistic effects of hyperthermia and immunotherapy in the future.
UPS has a high clinical misdiagnosis rate, and patients with locally advanced, unresectable, or metastatic disease have a poor prognosis and few treatment options[19]. Local hyperthermia combined with chemotherapy for multiple recurrences of undifferentiated sarcoma after surgery has been rarely reported in clinical practice. By reporting this case, we hope to provide new ideas for the treatment of postoperative recurrence of advanced UPS, which could help to optimize surgical opportunities and achieve R0 resection. Along with the application of molecular-pathological diagnosis, continuous prospective research on the use of hyperthermia combined with radiotherapy, chemotherapy, and immunotherapy will provide more options for the comprehensive management of UPS.
The authors would like to thank and acknowledge the patient and his family for taking part in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding J, Menendez-Menendez J S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Rosenberg AE. WHO Classification of Soft Tissue and Bone, fourth edition: summary and commentary. Curr Opin Oncol. 2013;25:571-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 671] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 3. | Zhu Y, Hao D, Tang X, Sun L. Undifferentiated high-grade pleomorphic sarcoma of ethmoid sinus: a case report and literature review. Braz J Otorhinolaryngol. 2018;84:389-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 4. | Jiang WH, Wen JM, Xu CW, Wang JC, Chen G. Clinicopathological analysis of undifferentiated pleomorphic sarcoma. Linchuang yu Bingli Za Zhi. 2020;40:83742-842. [DOI] [Full Text] |
| 5. | Ren HH, Wang XB. A case of undifferentiated pleomorphic sarcoma of the right scapula and literature review. Shiyong Yixue Za Zhi. 2020;37:530-2+77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Fujiwara T, Stevenson J, Parry M, Tsuda Y, Tsoi K, Jeys L. What is an adequate margin for infiltrative soft-tissue sarcomas? Eur J Surg Oncol. 2020;46:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | He HJ, He HF, Chen PD, Zhang YS, Huang CJ. High-power microwave hyperthermia combined with chemotherapy and radiotherapy for hip osteosarcoma. Shiyong Yixue Za Zhi. 2008;658-60. [DOI] [Full Text] |
| 8. | Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM, Vujaskovic Z, Wessalowski R, Jauch KW, Dürr HR, Ploner F, Baur-Melnyk A, Mansmann U, Hiddemann W, Blay JY, Hohenberger P; European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC-STBSG); European Society for Hyperthermic Oncology (ESHO). Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 454] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 9. | Fan Q, Ma B, Guo A. [Treatment of malignant or aggressive bone tumors with microwave induced hyperthermia]. Zhonghua Wai Ke Za Zhi. 1997;35:484-487. [PubMed] |
| 10. | Niu XH. Progress in the treatment of bone and soft tissue tumors. Cancer Research on Prevention and Treatment. 2020;47:1-5. [DOI] [Full Text] |
| 11. | Kawai A, Araki N, Naito Y, Ozaki T, Sugiura H, Yazawa Y, Morioka H, Matsumine A, Saito K, Asami S, Isu K. Phase 2 study of eribulin in patients with previously treated advanced or metastatic soft tissue sarcoma. Jpn J Clin Oncol. 2017;47:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Puls F, Pillay N, Fagman H, Palin-Masreliez A, Amary F, Hansson M, Kindblom LG, McCulloch TA, Meligonis G, Muc R, Rissler P, Sumathi VP, Tirabosco R, Hofvander J, Magnusson L, Nilsson J, Flanagan AM, Mertens F. PRDM10-rearranged Soft Tissue Tumor: A Clinicopathologic Study of 9 Cases. Am J Surg Pathol. 2019;43:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Pazzaglia L, Novello C, Conti A, Pollino S, Picci P, Benassi MS. miR-152 down-regulation is associated with MET up-regulation in leiomyosarcoma and undifferentiated pleomorphic sarcoma. Cell Oncol (Dordr). 2017;40:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Luo CL, Li ZP. Research progress in molecular targeted therapy of soft tissue sarcoma. Zhongguo Aizheng Za Zhi. 2019;29:824-31. [RCA] [DOI] [Full Text] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Que Y, Zhang XL, Liu ZX, Zhao JJ, Pan QZ, Wen XZ, Xiao W, Xu BS, Hong DC, Guo TH, Shen LJ, Fan WJ, Chen HY, Weng DS, Xu HR, Zhou PH, Zhang YZ, Niu XH, Zhang X. Frequent amplification of HDAC genes and efficacy of HDAC inhibitor chidamide and PD-1 blockade combination in soft tissue sarcoma. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer. 2018;4:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 17. | Elming PB, Wittenborn TR, Horsman MR. OC-0053: Combining hyperthermia and checkpoint inhibitors: a method of increasing tumour immunogenicity? Radiother Oncol. 2018;127:S22-S3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X, Sun M, Wen W, Dai X, Yang M, Lv Q, Lu B, Jiang J. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin Cancer Res. 2016;22:1173-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 19. | Emambux S, Italiano A. Clinical efficacy of eribulin mesylate for the treatment of metastatic soft tissue sarcoma. Expert Opin Pharmacother. 2017;18:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |